Abstract
Watching an actor make reaching movements in a perturbing force field provides the observer with information about how to compensate for that force field. Here we asked two questions about the nature of information provided to the observer. Is it important that the observer learn the difference between errant (curved) movements and goal (straight) movements by watching the actor progress in a relatively orderly fashion from highly curved to straight movements over a series of trials? Or is it sufficient that the observer sees only reaching errors in the force field (FF)? In the first experiment, we found that observers performed better if they observed reaches in a FF that was congruent, rather than incongruent, with the FF used in a later test. Observation-trial order had no effect on performance, suggesting that observers understood the goal in advance and perhaps learned about the force-field by observing movement curvature. Next we asked whether observers learn optimally by observing the actor's mistakes (high-error trials), if they learn by watching the actor perform with expertise in the FF (low-error trials), or if they need to see contrast between errant and goal behavior (a mixture of both high- and low-error trials). We found that observers who watched high-error trials were most affected by observation but that significant learning also occurred if observers watched only some high-error trials. This result suggests that observers learn to adapt their reaching to an unpredictable FF best when they see the actor making mistakes.
INTRODUCTION
Humans can learn how to perform specific movements by observing the movement of others. What information is present in the visual display of actions that allows the observer to learn a new motor skill or modify an existing skill? One piece of information that may be learned by observing is the movement target. Electrophysiological recording studies of awake, behaving monkeys suggest that the mirror-neuron system codes information about the actor's goal. Mirror neurons are defined as those neurons that fire both when a primate reaches to grasp a three-dimensional target and when the primate observes the same action performed by another monkey or human (Di Pellegrino et al. 1992; Gallese et al. 1996, 2002; Gentilucci et al. 1988; Rizzolatti et al. 1996). These neurons, documented in the ventral premotor (F5) region of the primate frontal lobe and in the Brodmann area (BA) 7b of the parietal lobe (Fogassi et al. 2005), do not respond to the object alone, nor do they respond to pantomimed or “fake” grasping. This pattern of response suggests that mirror neurons code the actor's movement target; in other words, the understanding that the observed movement is aimed to interact with a target object (Rizzolatti et al. 2001).
Human studies have shown that the cortical motor system is engaged by observing actors who 1) perform simple, single-joint movements (Stefan et al. 2005), 2) learn to compensate reaching for a complex force-field pattern (Mattar and Gribble 2005), and 3) learn the steps of a complex spatial sequence of actions (Petrosini 2007; Torriero et al. 2007). Within the realm of motor learning, Stefan et al. (2005) reported that transcranial magnetic stimulation (TMS) pulses applied to the motor cortex can induce the execution of a previously observed thumb movement and that this induction is reduced after subjects observed thumb movements in the opposite direction. These results suggest that the threshold for executing a movement with a specific effector in a specific direction was lowered by observing an actor perform the same movements. Concurrent observation of a unidirectional thumb action can also enhance or interfere with TMS pulse–induced performance of a physically learned action (Stefan et al. 2008). Together these studies suggest that by observing others' actions, humans learn both about the nature of the expected action (a goal) and about the differences between the expected action and the observed action (errors).
The effects of observational learning on performance of more complex movements, like reaching, also suggest direct links between observational learning and cortical motor system engagement. Participants who were asked to perform reaching movements in a robotic-manipulandum-imposed (clockwise) force field were better able to produce movements that compensated for the force field if they had previously observed an actor making reaching movements in the same force field, whereas other participants were less able to compensate for the force field if they previously observed reaching in the opposing force field (Mattar and Gribble 2005). Importantly, Mattar and Gribble showed that participants in their study (a study that used the same movie stimuli and manipulandum test as are used in this one) had very little insight into the nature of the force field after observation. In one demonstration, although participants were distracted during observation by a backward-counting task, effects on postobservation performance persisted. In the other, participants were asked on exit if the force field they saw was the same or different from the one they felt, and participants' responses were at chance. Together these factors suggest that the observed effects of observation on later performance in the force field cannot be attributed to the acquisition of simple decision rules. In a follow-up study, Brown et al. (2009) reported that repetitive TMS applied to the motor cortex after observation interfered with the effect of motor learning by observing. Finally, Malfait et al. (2010) used functional imaging techniques to show that observation of movement error (movement curvature) recruits the same brain regions that are engaged when participants process self-produced movement curvature errors in real time (Diedrichsen et al. 2005). These multiple sources of evidence converge to suggest a clear link between the observation of movement error (movement curvature) and the engagement of the cortical motor system.
To learn to perform reaching movements that compensate for a complex force field by observing, the observer needs to know that the goal of the task is to make relatively straight movements (Flash and Hogan 1985; Morasso 1981) and that there is a force pushing the hand in accord with a certain pattern. How does the observer know these two pieces of information? One possibility is that observers learn to treat curved movements as erred and relatively straight movements as the goal by watching an orderly progression of curved-to-straight movements. Under this hypothesis, participants must assume that movements made at the end of observation are consistent with the goal. Another possibility is that observers have a prior understanding, perhaps from experience, that reaches are typically straight and smooth (Morasso 1981). Under this hypothesis, the goal is known before observation begins and observed deviations from the goal, regardless of when they are observed in the sequence, are treated as error information whose pattern is learned by the motor system.
Our initial study was designed to distinguish between these two ideas. We predicted that if observers learn about the movement goal by evaluating how performance changes over time, we would find effects of motor learning by observing only when participants are shown reaching movies in which trajectory error (movement curvature) gradually decreases in magnitude over trials (the fixed sequence condition) and not when trajectory error is shown varying randomly trial to trial (the random sequence condition). By contrast, if observers have prior knowledge of reaching goals and they learn by observing error without regard for order, we would find that learning by observing is equally effective in both fixed and randomly ordered sequence conditions. To preview, the results of the first study were consistent with the latter idea.
The idea that one can learn new motor skills by observing an actor dealing with movement errors is at odds with the commonly held notion that one can learn a new motor skill by observing the performance of an expert. In our second study, we asked what information is optimal for learning by observing? Are observers better able to learn by 1) observing the actor's mistakes (high-error trials), 2) observing the actor perform well in the force field (FF; low-error trials), or 3) do they need to see both high-error and low-error trials to observe the difference between good and poor performance? We hypothesized that in the specific case presented here—a force-field learning task in which the performance of a highly learned movement (reaching) is perturbed by an unpredictable force field—only erred performance can provide the information necessary to learn to predict and then plan and execute movements that compensate for the force field. Although the alternative outcome (that observers learn by observing the actor perform well) may seem unlikely, if participants learn by observing expert performance in all situations, observing low-error trials should also result in measurable differences in subsequent performance. Indeed, we still know little about what information participants attend to or garner during observation, and so we feel that this possibility needs to be tested.
METHODS
Participants
One hundred eleven undergraduates participated in the two studies described here. Forty-five participants completed the first experiment and 66 participants completed the second experiment (Tables 1 and 2). All participants reported being right-handed, with normal or corrected-to-normal vision, and no history of any neurological or musculoskeletal disorders. All gave written informed consent before participation. No participant had previous experience with the robotic manipulandum, and each was assigned randomly to one experimental condition. The UWO Research Ethics Board approved all procedures.
Table 1.
Learning conditions and design for experiment 1
| Sequence of Events |
||||
|---|---|---|---|---|
| Learning Condition Group | N | Training | Observation | Test |
| Control: random field fixed sequence | 9 | Null field | Random alternation between CCWFF and CWFF | CWFF |
| CCWFF-fixed | 9 | Null field | CCWFF-fixed sequence | CWFF |
| CCWFF-random | 9 | Null field | CCWFF-random order | CWFF |
| CWFF-fixed | 9 | Null field | CWFF-fixed sequence | CWFF |
| CWFF-random | 9 | Null field | CWFF-random order | CWFF |
CWFF, clockwise velocity-dependent perturbing force field; CCWFF, counterclockwise velocity-dependent perturbing force field.
Table 2.
Learning conditions and design for experiment 2
| Sequence of Events |
||||
|---|---|---|---|---|
| Learning Condition Group | N | Training | Observation | Test |
| CCWFF-high | 11 | Null field | CCWFF-high error trials only | CWFF |
| CCWFF-hi-lo | 11 | Null field | CCWFF-randomly sequenced high and low error trials | CWFF |
| CCWFF-low | 11 | Null field | CCWFF-low error trials only | CWFF |
| CWFF-high | 11 | Null field | CWFF-high error trials only | CWFF |
| CWFF-hi-lo | 11 | Null field | CWFF-randomly sequenced high and low error trials | CWFF |
| CWFF-low | 11 | Null field | CWFF-low error trials only | CWFF |
See Table 1 for abbreviations.
Video recording and presentation
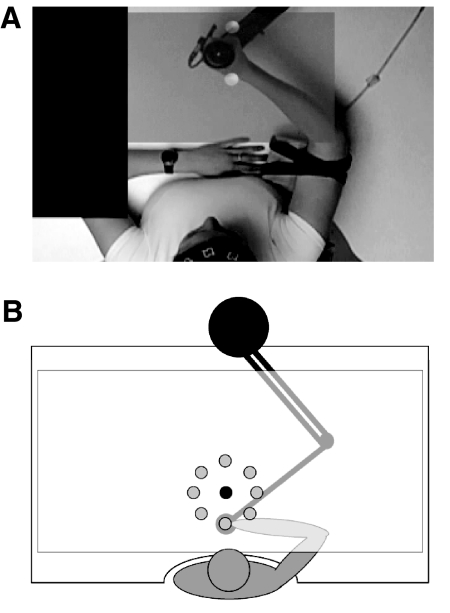
The video recordings have been described in detail elsewhere (Mattar and Gribble 2005). Briefly, participants observed video recordings of healthy naïve undergraduates making targeted reaching movements as the robotic manipulandum applied either a clockwise or counterclockwise velocity-dependent perturbing force field (CWFF and CCWFF, respectively) to the actor's hand. The videos provided participants with a top-down view of the head and shoulders of the actor, superimposed by a translucent image of the visual targets and the cursor showing the position of the actor's hand (Fig. 1A). Each recording was ∼6 min in duration and showed a series of 96 movements, 12 movements to each of eight targets.
Fig. 1.
Experimental set-up. A: 1 frame from a video displayed during observation. The video showed the actor moving the manipulandum handle from the start position to the target. A synchronized, transparent view of the start position, target, and cursor that was viewed by the actor was layered over the video of arm movements. B: the entire set of targets and the central start position used in both experiments.
Participants (observers) were instructed to watch the videos while keeping their arm still. To ensure that observers were attending to the video, we instructed subjects to verbally report trial numbers on which the actor made a movement timing error (indicated by the actor's target turning red or green).
We manipulated the order in which observers viewed individual trials performed by the actor. We divided the original 6 min recording described above into 96 short (3-s) movies, each portraying the actor's performance on one trial. Two groups of participants viewed trials performed by the actor in the order that they were recorded (i.e., trial 1–96; CCWFF–fixed sequence and CWFF–fixed sequence). The fixed sequence groups viewed the trials in a sequence consistent with what the actors experienced as they learned to compensate for their respective force fields, and in this way, the fixed trial sequences portrayed learning. In general, high-error trials were present early in the sequence and low-error trials were viewed later in the sequence. Two additional groups of participants viewed the same trials sequenced randomly (CCWFF–random and CWFF–random). A new random sequence was generated for each participant in these two groups. Because the appearance of high- and low-error trials was randomly sequenced, these movies did not portray the negative exponential reduction of hand-path error over the series of trials that is typical of learning. Finally, a control group of participants observed a movie in which the actor was exposed randomly to either the CWFF or the CCWFF.
The videos created for experiment 2 were identical to those for experiment 1 except that they were prepared as follows. The videos of individual trials were evaluated such that, for each target direction, the trials showing the highest and lowest trajectory curvature were selected. From these, three observation videos were assembled. In the high-error condition, participants viewed the actor performing movements with large errors only. The eight high-error trials (one for each movement direction) were repeated and presented pseudorandomly such that the observer saw 12 movements in each direction. In the high-low (hi-lo) error condition, participants viewed the actor performing both the high- and low-error movements. The eight high-error and eight low-error trial videos were repeated and presented pseudorandomly such that the observer saw 12 movements in each direction. In the low-error condition, participants viewed the actor performing movements with low errors only. The eight low-error trial videos were repeated and presented pseudorandomly such that the observer saw 12 movements in each direction.
Motor learning task
The motor learning task was identical for experiments 1 and 2. Each participant was seated at a table at chest height. A custom-made air-sled supported the participant's right arm against gravity and allowed the arm and hand to move over the surface of the table with little friction. The participant grasped the handle of an InMotion2 robotic manipulandum (InMotion Technologies, Cambridge, MA) and used the robot to reach to visually displayed targets.
Computer-generated displays were projected by an LED projector (Sony VPL-CS1) onto an angled mirror suspended above the workspace. The angled mirror reflected the display onto a horizontal screen (105 × 60 cm) and horizontal semi-silvered mirror (107 × 75 cm) that were supported above the table surface. The robotic arm was positioned below the surface of the mirror and moved parallel to the table surface. This arrangement gave participants the impression that the visual display appeared in the same horizontal plane in which the movements were performed (Fig. 1B).
Reaching movements were made from a central start location to one of eight peripheral targets positioned at a constant radial distance (10 cm) at 45° intervals (Fig. 1B). The position of the hand was represented in real time by a cursor. When the participant used the manipulandum to place the cursor in the central start location, a peripheral target appeared. The presentation of targets was pseudorandomized over series of eight movements. This eight target cycle was repeated 12 times per block, for a total of 96 movements per block.
The robotic manipulandum was programmed to produce forces that acted at the hand, proportional to the velocity of movement. These forces acted in a clockwise (CWFF) direction defined by the following equation
| (1) |
where Fx and Fy are forces generated by the manipulandum in the left/right and forward/backward directions, respectively, xY′ and yY′ denote hand velocities, and k = 15 Ns/m. In some parts of the experiment, the manipulandum produced no forces (a null field, k = 0).
Robot handle positions (x, y) were sampled at 500 Hz and stored on a digital computer. We measured maximum movement curvature: the maximum deviation of the hand path from the straight line connecting the spatial location of start of the movement with the location of the end of the movement. Positive curvature values indicated a deviation in the CW direction and negative values indicated a deviation in the CCW direction. We also measured movement time, where movement initiation was defined as the time at which tangential velocity first exceeded 0.05 ms−1, and movement end was defined as the first time after peak velocity that tangential velocity fell below 0.05 ms−1. Peak tangential velocity was also recorded.
Procedure
The designs for the first and second experiments are presented in Tables 1 and 2, respectively. All participants initially performed the reaching task (96 trials) in a null (0 force) field. In experiment 1, depending on the condition to which they were assigned, each participant watched either the CWFF–fixed sequence, CCWFF–fixed sequence, CWFF–random sequence, CCWFF–random sequence, or random FF movie. Likewise, in experiment 2, participants watched either the CCWFF–high-error, CCWFF–high-low (mixed) error, CCWFF–low error, CWFF–high-error, CWFF–high-low (mixed) error, or CWFF–low error movie. Participants were instructed to watch the videos and report the trial number on which they saw the actor make an error. The videos were played twice in succession. Finally, all participants performed 192 reaching trials in the CW force field. Participants were instructed to make fast, accurate movements to targets. To encourage consistent movement times, participants were informed that they moved too fast if movement time was <420 ms and too slow if movement time was >520 ms. These criteria were used only to provide participants with feedback about the consistency of their movements and were not used to exclude data from the analysis.
Data analysis
All data analyses were conducted using MATLAB (The Mathworks) and SPSS software. We measured the extent to which the CWFF perturbed participants' reaching by measuring the maximum perpendicular distance between the hand and the straight line defined by the central start position and the current target. Peak hand tangential velocity was also assessed to confirm that the magnitude of the velocity-dependent perturbation did not differ between groups. For each of the dependent variables, mean values were computed over successive and exclusive windows of 16 movements. Changes in performance over trials were tested by submitting movement curvature measures to repeated-measures ANOVAs with trial bin as the only factor. We were also interested in the differences in movement curvature immediately after observation, and these differences were evaluated by submitting the mean movement curvature from the initial 16-trial bin to between-subject ANOVAs. For experiment 1, the data were submitted to a 2–force field (CWFF, CCWFF) × 2–trial sequence (fixed, random) between-subject ANOVA, and for experiment 2, the data were submitted to a 2–force field (CWFF, CCWFF) × 3–level of error information (high, hi-lo, low) between-subject ANOVA. Given the results of previous experiments from our laboratory (Brown et al. 2009; Cothros et al. 2006; Mattar and Gribble 2005), we were confident about our ability to predict the direction of differences between our CCWFF and CWFF observation groups. Consequently, we felt that Tukey's Honest Significant Difference (HSD) was a suitably conservative test for evaluating the differences between learning condition means.
Portions of this research were presented at the 2007 meeting of the Society for the Neural Control of Movement, Seville, Spain, and the 2007 meeting of the Society for Neuroscience, San Diego, CA.
RESULTS
Experiment 1
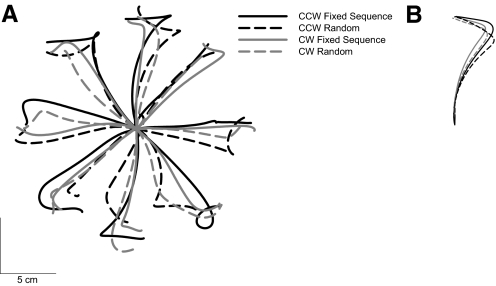
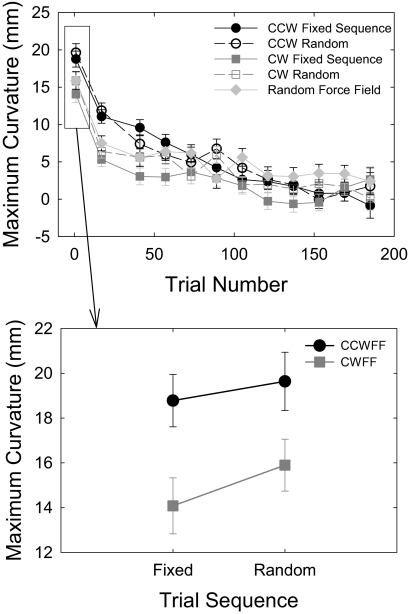
Initial trajectories are shown from representative subjects in each group (Fig. 2A), and mean trajectories averaged over the first eight trials in each condition are shown in Fig. 2B. Over the course of the CWFF test, all participants adapted their reaching to compensate for the force field applied to the hand. This adaptation is evident in Fig. 3A, which shows mean maximum movement curvature as a function of trial number (binned into groups of 16 trials) for each condition in the experiment. To test for changes in performance with practice, we submitted these means to a repeated-measures ANOVA and found a significant main effect of movement trial bin [F(11,99) = 65.13, P < 0.001]. Early in training, hand paths were curved in a manner consistent with the application of the CWFF, and late in training, mean hand paths were significantly straighter, indicating that all participants learned to compensate for the CWFF.
Fig. 2.
Trajectories from experiment 1. A: a representative subject from each experimental condition. B: the mean of trials 1–8 (all directions, no catch trials) and for all subjects in each experimental condition.
Fig. 3.
Results from experiment 1. A: learning over bins of 16 trials. Although there were differences in performance initially, these differences were no longer significant by the end of training. Error bars represent SE. B: participants' initial response to the clockwise velocity-dependent perturbing force field (CWFF) was significantly influenced by whether they previously observed a counterclockwise velocity-dependent perturbing force field (CCWFF) or a CWFF movie, but it was not significantly affected by whether they observed the fixed or random trial sequence.
OBSERVATION SEQUENCE DOES NOT INFLUENCE MOTOR LEARNING BY OBSERVING.
The degree of initial movement curvature, however, depended on whether participants observed the congruent CWFF or incongruent CCWFF video, as indicated by a main effect for learning condition [F(4,619) = 3.21, P < 0.013]. Figure 3B shows mean maximum curvature during early exposure to the CWFF (averaged over the 1st 16 trials) as a function of experimental condition. We submitted these values to a 2–force field (CWFF, CCWFF) × 2–trial sequence (fixed, random) between-subject ANOVA. There was a main effect for force field [F(1,509) = 11.85, P < 0.001]. Participants who observed CCWFF movies produced movements with significantly higher average curvature (mean = 19.21 ± 0.88 mm) than participants who observed CWFF movies (mean = 14.99 ± 0.86 mm). In contrast, there was no main effect (P = 0.276) or interaction (P = 0.696) involving trial sequence. To determine whether these observation factors influenced the final outcome of training, we submitted the curvature data collected during the last 16 trials of the CWFF training to the same two-way ANOVA. At the end of training, neither observation force field (P = 0.748) nor trial sequence (P = 0.882) nor their interaction (P = 0.230) significantly influenced movement performance in the force field.
One possibility is that there was greater initial trajectory error after watching CCWFF movies than CWFF movies because participants who observed the CCWFF movie moved more quickly and achieved greater peak velocities (and therefore experienced higher perpendicular forces) than participants who observed the CWFF movies. We addressed this concern by measuring and analyzing movement time, peak velocity, and maximum force applied by the robotic manipulandum to the hand. For each of these dependent measures, average performance over the initial 16 trials was submitted to a 2–force field (CWFF, CCWFF) × 2–trial sequence (fixed, random) ANOVA. For movement time, we found no main effects for force field (P = 0.977) and no interaction between force field and trial sequence (P = 0.569). There was a small but significant main effect for trial sequence [F(1,509) = 7.367, P = 0.007], such that movement times for participants who watched the random sequence movies (797 ± 10 ms) were longer than movement times for participants who watched the fixed-sequence movies (760 ± 10 ms). This difference in movement time, however, did not translate into a significant difference in achieved peak velocity or peak force applied to the hand. For each of the latter measures, there were no significant main effects force field (P > 0.1), trial sequence (P > 0.3), or interactions (P > 0.10). Therefore it is highly unlikely that this difference in movement time can account for finding that random-sequence error presentations induced learning by observing just as effectively as fixed-sequence presentations.
Experiment 2
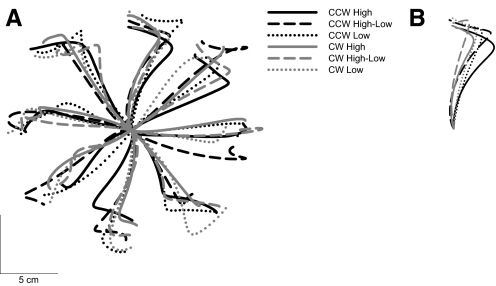
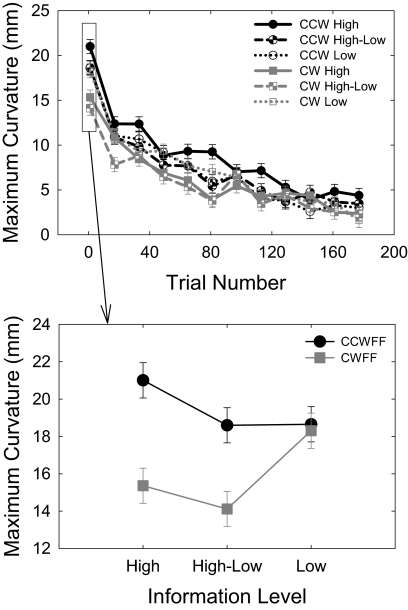
Initial trajectories are shown from representative subjects in each group (Fig. 4A), and mean trajectories averaged over the first eight trials in each condition are shown in Fig. 4B. Over the course of the CWFF test, all participants adapted their reaching to compensate for the force field applied to the hand. This adaptation is evident in Fig. 5A, which shows mean maximum movement curvature in the CWFF test as a function of trial number (binned into groups of 16 trials) for each condition in the experiment. To test for changes in performance with practice, we submitted these means to a repeated-measures ANOVA with movement trial bin (12 bins of 16 trials) as the only factor. We found a significant main effect of movement trial bin [F(11,715) = 223.02, P < 0.001]. Early in training, hand paths were curved in a manner consistent with the application of the CWFF, and late in training, mean hand paths were significantly straighter, indicating that participants learned to compensate for the CWFF.
Fig. 4.
Trajectories from experiment 1. A: a representative subject from each experimental condition. B: the mean of trials 1–8 (all directions, no catch trials) and for all subjects in each experimental condition.
Fig. 5.
Results from experiment 2. A: learning over bins of 16 trials. Although there were differences in performance initially, these differences were no longer significant by the end of training. Error bars represent SE. B: participants' initial response to the CWFF was significantly influenced by an interaction of force-field observation direction (CCWFF, CWFF) and information level (high error, high and low error, and low error).
OBSERVERS LEARN BEST BY WATCHING HIGH-ERROR TRIALS.
As in experiment 1, we determined the degree to which initial movement curvature depended on whether participants observed the congruent CWFF or incongruent CCWFF video and on the level of error information presented in the video. Figure 5B shows mean maximum curvature during early exposure to the CWFF (averaged over the 1st 16 trials) as a function of the force field observed in the video and the size of the errors viewed in the video. We submitted these values to a 2–force field (CWFF, CCWFF) × 3–level of error information (high, hi-lo, low) ANOVA. As in experiment 1, there was a main effect for force field [F(1,918) = 20.55, P < 0.001]. Participants who observed CCWFF movies produced movements with significantly higher average curvature (mean = 19.41 ± 0.54 mm) than participants who observed CWFF movies (mean = 15.92 ± 0.54 mm). Although there was no main effect of level of information (P = 0.052), there was a force field by level of information interaction [F(2,918) = 4.37, P = 0.013]. Simple main effects analyses were conducted on each level of information. These analyses showed that, when the video displayed only high levels of error, there were significant differences in performance after viewing the CCWFF (mean = 21.00 ±.94 mm) and CWFF [mean = 15.36, ± 0.93 mm; F(1,306) = 18.59, P < 0.001]. Likewise, when the video randomly displayed both high and low error trials, there were significant differences in performance after viewing the CCWFF (mean = 18.60 ± 0.97 mm) and CWFF [mean = 14.11 ± 0.94 mm; F(1,306) = 10.80, P = 0.001]. When the video displayed only low levels of error, however, there was no significant difference in performance after viewing the CCWFF (mean = 18.65 ± 0.94 mm) and CWFF (mean = 18.30 ± 0.94 mm; P = 0.796). These results show that one's ability to glean information about the force field from the video depends on the level of error information portrayed.
To ensure that these differences are caused by learning and not simply to differences in kinematics, we measured and analyzed movement time, peak velocity, and maximum force applied by the robotic manipulandum to the hand. For each of these dependent measures, average performance over the initial 16 trials was submitted to a 2–force field (CWFF, CCWFF) × 3–information level (high, high-low, and low) ANOVA. For movement time, we found no main effect for force field (P = 0.911), information level (P = 0.098), and no interaction between these two factors (P = 0.940). For peak velocity, we found no main effect for information level (P = 0.217) and no interaction between force field and information level (P = 0.124), but we did find a small but reliable difference between CCWFF (0.355 ± 0.03 ms−1) and CWFF [0.347 ±.03 ms−1; F(1,918) = 4.778, P = 0.029]. This difference in peak velocity did not translate into a significant difference in maximum force at the hand, however. For maximum force, we found no main effect for force field direction (P = 0.053) or information level (P = 0.192) and no interaction between these two factors (P = 0.218). It is unlikely that differences in kinematics reported here can account for the differences exerted by information level on learning reported above.
DISCUSSION
When observers watch others learn to compensate for a complex force field, do they learn by observing the actor's goal movement or do they learn by observing the actor's errors? In the first experiment reported here, we questioned whether the observer learns the difference between the movement goal and movement error by watching an orderly progression of curved-to-straight movements. Our strategy was to present both a fixed trial sequence (the negative exponential reduction of hand-path error over the series of trials) and a random trial sequence of hand-path errors for observation and to measure how well these movies supported motor learning by observing. The results clearly indicated that shuffling trial viewing order had no consequence on motor learning by observing, leaving open the possibility that observers have a prior understanding that the reach goal is to move smoothly along a relatively straight path. In the second experiment, we asked whether the observer learns best when viewing the actors' high-error trials or low-error trials, or if the viewer needs to see a mix of high- and low-error (goal) movements. We found that observers who watched high-error movies were most affected by observation but that significant learning also occurred if observers watched movies containing a mix of high- and low-error trials. These results suggest 1) that observers have a prior understanding about the goal of the movement and that they learn by observing movement errors and 2) that, within experiment 2, the more error information they receive (the more consistently they observe large errors), the better they learn to adapt their reaching to an unpredictable force field.
Observers know the goal in advance and use error information to update their performance
The results indicate that when participants observed actors making reaching movements in force-field environments that were either congruent or incongruent with the CWFF, this period of observation significantly influenced participants' initial ability to compensate for the CWFF force field, replicating Mattar and Gribble (2005). These results of experiment 1 extend these findings to show that the order in which participants viewed the actor's errors did not influence learning by observing. Our interpretation of this result is that participants do not need to glean information about the movement goal by watching error diminish over time. Given that the task is reaching, a highly learned movement, it is likely that they understand the movement goal in advance.
In our second study, we found that for this force-field learning task in which the performance of a highly learned movement (reaching) is perturbed by an unpredictable force field, observers learned by observing movement errors. The results of our second study are also consistent with the idea that observers had some knowledge of the goal in advance. The high-error movies contained only high-curvature movements and no low-error (goal-like) movements. Despite the fact that they provided the observer with little information about the implicit movement goal to perform a relatively straight trajectory, the high-error movies were the most effective at providing observers with the information necessary to learn to predict and then plan and execute movements that compensate for the force field.
The idea that one can learn new motor skills by observing an actor dealing with movement errors is consistent with current empirical findings and theoretical views about how humans use error information to update movement planning and execution (Anguera et al. 2009; Diedrichsen et al. 2005; Haruno et al. 2001; Kawato and Wolpert 1998; Kording and Wolpert 2004; Tseng et al. 2007; Wolpert et al. 2001), but it is at odds with the more commonly held idea—an idea that has inspired a host of instructional sport videos—that one can learn new motor skills by observing the performance of an expert. This finding is likely strongly tied to the fact that the movement under study here is a perturbation to a highly learned task, reaching.
Error information is used to update an internal model of reaching
One way to characterize motor learning is that it involves the use of sensory feedback to modify an internal model of a previously learned action or action routine, including those actions that, like reaching, take advantage of available synergies (d'Avella and Bizzi 2005; Debicki and Gribble 2004, 2005). According to this account, an inverse model of the limb is used to transform planned movement trajectory information into a motor command: the precisely timed muscle contractions that are required to propel the hand to the reaching target. When the motor command is generated, a forward model of the reaching movement is used to predict the sensory outcomes of that motor command. The predicted sensory outcomes are compared with the obtained feedback, both proprioceptive and visual sensory information about the trajectory of the hand, and the resulting error signal is used to update both the forward model and the inverse model (Kawato and Wolpert 1998).
This mechanism may also operate during observation (Mattar and Gribble 2005). When the observer sees the target presented to the actor, the observer generates but does not execute motor commands for a straight and smooth reaching action to the target and therefore generates a prediction about the sensory information that action would produce. The deviation of the observed hand path from the predicted hand path shows the error induced by the force field, and this error signal is used to update how subsequent motor commands are generated. When the observer is exposed to the force field, his initial reaching performance is consistent with what was previously learned about the force field pattern by observation. If the observer observed a CWFF, he generates reaches that compensate for the CWFF. If the observer watched reaching in a CCWFF, he generates reaches that fail to compensate for the CWFF.
This mechanism for motor learning by observing can be linked to mirror neurons, discovered in the ventral aspect of the premotor cortex (PMv) in monkeys (Di Pellegrino et al. 1992; Gallese et al. 1996; Gentilucci et al. 1988; Rizzolatti et al. 1996) and the inferior parietal cortex (Brodmann area 7b) (Fogassi et al. 2005; Gallese et al. 2002), which increase their activity both when a monkey performs a goal-directed action and observes the same or similar actions (Gallese et al. 1996). Importantly, in both monkeys and humans, the PMv is directly connected both to the spinal cord and to the primary motor cortex (M1), and it is these cortico-cortical connections that have been linked to changes in the neural excitability of M1 when human observers watch or listen to another person performing a goal-directed action, as measured by the amplitude of motor evoked potentials elicited by single pulse TMS applied to M1 (Baldissera et al. 2001; Fadiga et al. 1995; Gangitano et al. 2001; Patuzzo et al. 2003; Strafella and Paus 2000; Watkins et al. 2003). Functional brain imaging studies have also shown that brain regions associated with motor planning and execution are activated during action observation (Buccino et al. 2001; Malfait et al. 2010; Shmuelof and Zohary 2005, 2006).
Another possible mechanism is that observing others' performance motivates the observer to select one course of action over others (Bandura 1986; Bandura and Jeffrey 1973; Heyes 1994; Heyes and Dawson 1990). In this situation, the observer is thought to be influenced by the understanding that the actor's intentions are the same as his own. Indeed, one intriguing electrophysiological study shows that mirror neurons will fire when the monkey observes another reaching and grasping for a known object that is hidden behind an occluder (Umilta et al. 2001). This result suggests that mirror neurons can code the observer's understanding that the goal or intention of the actor is to interact with the target object (Rizzolatti et al. 2001). The link between action observation, the mirror neuron system, and how people understand the intentions of others, however, is not straightforward. Understanding intentions demands more than simply anticipating the end result of a single act (Rizzolatti et al. 2002); often we need to observe several acts in sequence before we can decipher the intention of the actor. Although it is conceivable that the mirror-neuron system contributes to this process and that understanding the actor's intentions is important for motor learning, it is unclear that it is the crucial component for knowing how one needs to move to satisfy a movement goal.
Summary
To summarize, we replicated previous findings by showing that observing others reaching in a perturbing force field influences how the observer later plans and executes reaching movements. We also extend these findings by showing, in two experiments using the perturbed reaching paradigm, that observers do not need to see low-error (goal) performance to benefit from observation. Indeed, the more error information they receive (the more consistently they observe large errors), the better they adapt their reaching to an unpredictable force field.
GRANTS
L. E. Brown was supported by a Canadian Institutes for Health Research (CIHR) Fellowship and a grant from National Sciences and Engineering Research Council of Canada. E. T. Wilson was supported by a National Science and Engineering Research Council Undergraduate Student Research Award. P. L. Gribble was supported by a CIHR New Investigator award. This research was funded by grants from CIHR (Canada), NSERC (Canada), and the National Institutes of Health (United States).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank the late D. Pulham for equipment construction and R. Billing and K. Trewartha for help with data collection.
REFERENCES
- Anguera JA, Seidler RD, Gehring WJ. Changes in performance monitoring during sensorimotor adaptation. J Neurophysiol 102: 1868– 1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Craighero L, Fadiga L. Modulation of spinal excitability during observation of hand actions in humans. Eur J Neurosci 13: 190– 194, 2001 [DOI] [PubMed] [Google Scholar]
- Bandura A, Jeffrey RW. Role of symbolic coding and rehearsal processes in observational learning. J Pers Soc Psychol 26: 122– 130, 1973 [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall, 1986 [Google Scholar]
- Brown LE, Wilson E, Gribble PL. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J Cogn Neurosci 21: 1013– 1022, 2009 [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activations premotor and parietal areas in a somatotopic manner: an fMRI study. 2001. [PubMed] [Google Scholar]
- Cothros N, Kohler S, Dickie EW, Mirsattari SM, Gribble PL. Proactive interference as a result of persisting neural representations of previously learned motor skills in primary motor cortex. J Cogn Neurosci 18: 2167– 2176, 2006 [DOI] [PubMed] [Google Scholar]
- d'Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci USA 102: 3076– 3081, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debicki DB, Gribble PL. Inter-joint coupling strategy during adaptation to novel viscous loads in human arm movement. J Neurophysiol 92: 754– 765, 2004 [DOI] [PubMed] [Google Scholar]
- Debicki DB, Gribble PL. Persistence of inter-joint coupling during single-joint elbow flexions after shoulder fixation. Exp Brain Res 163: 252– 257, 2005 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919– 9931, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res 91: 176– 180, 1992 [DOI] [PubMed] [Google Scholar]
- Fadiga l, Fogassi l, Pavesi G, Rizzolatti G. Motor facilitation during action observation. J Neurophysiol 73: 2608– 2611, 1995 [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Vetter P, Johannson R, Wolpert D. Prediction precedes control in motor learning. Curr Biol 13: 146– 150, 2003 [DOI] [PubMed] [Google Scholar]
- Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5: 1688– 1703, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science 308: 662– 667, 2005 [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain 119: 593– 609, 1996 [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G. Action representation and the inferior parietal lobule. In: Attention and Performance XIX. Common Mechanisms in Perception and Action, edited by Prinz W, Hommel B. Oxford, UK: Oxford University Press, 2002, p. 247–266 [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport 12: 1489– 92, 2001 [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71: 475– 490, 1988 [DOI] [PubMed] [Google Scholar]
- Haruno M, Wolpert DM, Kawato M. Mosaic model for sensorimotor learning and control. Neural Comput 13: 2201– 2220, 2001 [DOI] [PubMed] [Google Scholar]
- Heyes CM. Social learning in animals: Categories and mechanisms. Biological Reviews of the Cambridge Philosophical Society 69: 207– 231, 1994 [DOI] [PubMed] [Google Scholar]
- Heyes CM, Dawson GR. A demonstration of observational learning in rats using a bidirectional control. Q J Exp Psychol B 42: 59– 71, 1990 [PubMed] [Google Scholar]
- Kawato M, Wolpert D. Internal models for motor control. Novartis Found Symp 218: 291– 307, 1998 [DOI] [PubMed] [Google Scholar]
- Kording KP, Wolpert D. Bayesian integration in sensorimotor learning, Nature 427: 244– 247, 2004 [DOI] [PubMed] [Google Scholar]
- Malfait N, Valyear KF, Culham JC, Anton JL, Brown LE, Gribble PL. fMRI activation during observation of others' reach errors. J Cogn Neurosci 22: 1493– 1503, 2010 [DOI] [PubMed] [Google Scholar]
- Mattar AAG, Gribble PL. Motor learning by observing. Neuron 46: 153– 160, 2005 [DOI] [PubMed] [Google Scholar]
- Morasso P. Spatial control of arm movements. Exp Brain Res 42: 223– 227, 1981 [DOI] [PubMed] [Google Scholar]
- Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia 41: 1272– 1278, 2003 [DOI] [PubMed] [Google Scholar]
- Petrosini L. “Do what I do” and “Do how I do”: different components of imitative learning are mediated by different neural structures. Neuroscientist 13: 335– 348, 2007 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn Brain Res 3: 131– 141, 1996 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661– 70, 2001 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12: 149– 154, 2002 [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron 47: 457– 470, 2005 [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Zohary E. A mirror representation of others' actions in the human anterior parietal cortex. J Neurosci 26: 9736– 9742, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Classen J, Celnik P, Cohen LG. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci 27: 730– 738, 2008 [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci 25: 9339– 9346, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport 14: 2289– 2292, 2000 [DOI] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Caltagirone C, Petrosini L. The what and how of observational learning. J Cogn Neurosci 19: 1656– 1663, 2007 [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54– 62, 2007 [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Kaisers C, Rizzolatti G. “I know what you are doing”: a neurophysiological study. Neuron 32: 91– 101, 2001 [DOI] [PubMed] [Google Scholar]
- Watkins KE, Strafella AP, Paus T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia 41: 989– 994, 2003. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci 5: 487– 494, 2001 [DOI] [PubMed] [Google Scholar]