Abstract
Motor neurophysiologists are placing greater emphasis on sensory feedback processing than ever before. In line with this shift, a recent article by Ostry and colleagues provided timely new evidence that force-field motor learning influences not only motor output, but also proprioceptive sense. In this Neuro Forum, the merits and limitations of Ostry and colleagues are explored in the context of recent work on proprioceptive function, including several recent studies from this journal.
Over the past several decades, an important shift has occurred in the fields of motor neuroscience and rehabilitation. In contrast to the once predominant focus on how individuals generate and control motor output, contemporary work has evolved to include a greater emphasis on sensory feedback processing. This fundamental change is likely the result of our growing understanding regarding the role sensory information plays in promoting neural plasticity through use-dependent mechanisms. Indeed, it is now well accepted that a strong relationship exists between massed sensorimotor practice and the expression of neural representations within a number of sensorimotor areas of the brain (Nudo 2006).
Arguably the most important source of sensory feedback for promoting neural plasticity is our sense of proprioception (Xerri et al. 1998). Proprioception can be defined as an individual's ability to perceive body segment positions and movements in space and is derived from complex somatosensory signals provided by multiple muscle, joint, and skin receptors. Studies involving individuals who lack proprioceptive sense due to large fiber neuropathy have taught us important lessons regarding the role of proprioceptive feedback during the performance of ecologically valid activities of daily living. Despite having motor systems that remain well intact, “deafferentation” resulting in a lack of proprioceptive feedback leads to profound deficits in most aspects of motor ability (Rothwell et al. 1982).
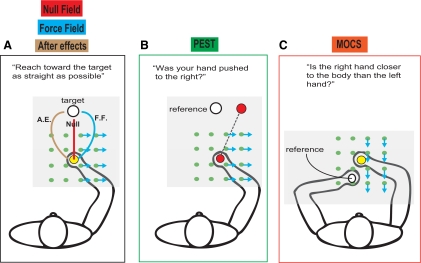
The focus of this Neuro Forum is a recently published article by Ostry and colleagues (2010), in which compelling evidence was provided that a motor learning paradigm can influence not only motor output, but also proprioceptive estimates of hand position in space. Specifically, these authors quantified the perception of subject hand location at various time points before and after adaptation to a velocity-dependent force field (FF) generated by a robot (see Fig. 1 for schematic of tasks). Testing time points included 1) baseline (Null), 2) following 150 movements (∼10 min) of motor adaptation within the FF environment, 3) following 50 movements made after motor adaptation within a non-FF environment (i.e., “washout” or aftereffect period), 4) following passively experienced movements similar to those generated by participants during learning of the FF environment, and 5) 24 h after learning. The main results of this study showed consistent, group biases in the estimation of hand position following the motor learning task. Importantly, these changes in hand proprioception persisted beyond the washout period immediately after testing and at 24 h following adaptation.
Fig. 1.
Schematic demonstrating the experimental tasks used by Ostry et al. (2010). A: participants reached toward a visual target while holding the handle of a robot that could generate velocity-dependent force fields. Visual feedback of target positions and a cursor representing hand position were provided by a computer-generated display on the mirror. A horizontal semisilvered mirror (transparent gray box) was placed just above the hand to block vision of the arm during the task. Reaching was performed before adapting to the force field (Null), in the force field (force field) and after force-field adaptation (After effect). B: parameter estimation by sequential testing (PEST) test of proprioceptive hand position sense, in which a threshold was determined indicating participants' estimates of left/right hand location with respect to the target (reference) position. C: method of constant stimuli (MOCS) test of proprioceptive hand position sense, in which a threshold was determined estimating participants' awareness of whether their right hand was closer or further from the body than their left hand, which served as a reference.
There were two unique methods used by Ostry et al. (2010) to assess participants' perception of hand location in space. During experiment 1, a thresholding technique (see their Fig. 1, B and C, our Fig. 1B) known as parameter estimation by sequential testing (PEST) was used that involved participants having their unseen hand systematically displaced to the left or right of subjective midline using force channels. The displacement value representing the point at which participants could no longer distinguish with high probability a rightward movement of the hand (see their Fig. 2A) was then determined. In experiment 2, hand position perception was quantified according to the method of constant stimuli (MOCS; see our Fig. 1C). This method required participants to judge whether their passively moved reaching hand was further or nearer to their body than a proprioceptive reference location provided by the opposite hand.
The tests of hand perception used by Ostry et al. (2010) represent significant innovation in terms of quantifying the proprioceptive sense of an end-effector (i.e., the hand), compared with more traditional assessments of proprioception that have focused on position and movement sense for single joints. This methodological advancement is important because estimation of end-effector position is likely to have greater behavioral relevance for an individual than any single joint (Van Beers et al. 1998). Indeed, the significance of end-effector representations of the hand location in proprioceptive space was recently underscored by Fuentes and Bastian (2010) in this journal. These authors demonstrated that the precision by which an individual can estimate a single joint (i.e., elbow) angle is enhanced when the task is performed relative to the end-effector location rather than a single joint itself. That is, individuals tested were more precise when asked to proprioceptively estimate the fingertip location (presumably relying on knowledge of elbow angle) than when they attempted to estimate elbow angle alone.
Despite the ecological value of proprioceptive assessments of end-effector location, Ostry et al. (2010) may have benefited from also exploring the consequences of FF motor learning on each individual arm joint. In this case, single-joint proprioceptive testing of the elbow, shoulder, and wrist might have revealed 1) some predictable combination of biases dependent on the direction of the robot-generated forces or 2) susceptibility of a particular joint to the adaptation paradigm. To accomplish this, the authors could have supplemented PEST and MOCS assessments with a single-joint position-matching paradigm. This test quantifies the ability of subjects to replicate target joint angles in the absence of vision.
Converging results from the two behavioral assessments of hand position perception (i.e., PEST and MOCS) are reassuring in Ostry et al. (2010), demonstrating the robustness of their main findings. However, the underlying neural mechanisms driving the shift in hand location perception following FF learning remains unclear from the data. In the passive control condition under which participants experienced movements similar to those in the learning condition (without actually learning), no change in hand perception was observed. This result led the authors to speculate that the effects seen were likely due to alterations at the level of the CNS, rather than being driven by changes in the neural firing or gain of mechanoreceptors within the peripheral nervous system.
At the central level, our knowledge of the brain areas that are active during processing of movement-related proprioceptive feedback has dramatically increased over the past decade via the use of neuroimaging techniques. In this journal, Naito and colleagues (2005) used functional magnetic resonance imaging to show that a distributed network of brain regions is active in response to the stimulation of muscle spindles via tendon vibration. Interestingly, this work has shown that beyond conventional “proprioceptive areas,” such as the primary somatosensory cortex and secondary somatosensory cortex, the primary motor cortex is a key region for the perception of proprioceptive illusions of joint movement. Given the obvious importance of the motor cortex in the motor learning task of Ostry et al. (2010), this brain area may serve as an important “hub” that mediates the effects of motor learning on proprioception and somatosensation. Future work involving the use of transmagnetic stimulation to disrupt or potentiate the motor cortex may be of particular value for exploring this hypothesis.
The FF motor learning task by Ostry et al. (2010) had a surprisingly enduring effect on proprioception. The behavioral change in hand perception was not completely “washed out” following the aftereffect trials and remained evident 24 h after adaptation had taken place. A logical concern in this case might be that an insufficient number of washout trials led to the observed effect, given that previous FF adaptation studies specifically used an equal number of washout trials to adaptation trials to properly ensure a washout (Taylor and Thoroughman 2008). However, Ostry and colleagues (2010) demonstrated that there was no difference in aftereffect performance following the first quartile of trials. This finding suggests that a trial number imbalance did not influence the results.
In light of the fact that length of training given to subjects in Ostry et al. (2010) was relatively short (∼10 min), there appears to be a hysteresis to the long-lasting plastic changes in proprioception resulting from FF motor learning. In this case, a seemingly important question for future work is: Why were estimates of hand position biased so quickly and, yet, lasted for such a long duration? Exploration of this “sensory aftereffect” seems particularly important in determining whether the induction of proprioceptive biases following exposure to FF training might have positive or negative consequences for the participants tested. To this point, it is also unclear to what extent such changes in hand position perception are specific to the context of interacting with a robotic manipulandum or representative of a complete recalibration of the proprioceptive system. Previous work addressing the transfer of learning from robot to nonrobot movement situations would suggest that robot-based training has some degree of context dependence. Specifically, the aftereffects of robotic training are reduced when subjects are no longer required to interact with the robotic system (Cothros et al. 2006; Kluzik et al. 2008).
Further insight into the underlying nature of the proprioceptive bias induced by Ostry et al. (2010) may potentially be gleaned from the comparison of the mean lateral forces measured during the first 100 ms of the first perceptual test (see their Fig. 5). The force curves underlying these mean forces appear to show different trajectories and starting points, with the “24 h Left” curve showing its initial deviation nearly twice as late as the “Left” curve. Subsequent exploration of this phenomenon may be critical for teasing apart the mechanisms underlying the induction of proprioceptive biases following motor adaptation learning. This may be especially pertinent for those populations who show impairments in the timely generation of appropriate forces/movements.
Goble and Brown (2008) proposed a sensory modality-specific hypothesis of handedness in this journal, whereby the left and right arms of right-handed individuals were more specialized for proprioceptive versus visual target reaching. In the article by Ostry et al. (2010), adaptation was limited to the right arm of right-handers and proprioceptive testing was performed with only the right arm (PEST) or with the right arm relative to the left (MOCS). Given the asymmetric nature of proprioceptive ability between the two arms, one wonders whether force-field motor learning may have differential effects for the two upper limbs. One hypothesis might be that the less proprioceptive-dependent right arm would be less susceptible to biases in proprioceptively determined hand location than the more dependent left arm. Alternatively, it might be that the enhanced proprioceptive abilities of the left arm allow for a quicker, more adequate, recalibration of the proprioceptive biases induced by force-field motor learning.
In summary, the report by Ostry et al. (2010) has demonstrated an important relationship between FF motor learning and proprioception that is present immediately after adaptation and 24 h afterward. As described here and by the authors, future work determining the extent that related brain structures contribute to observed effects is necessary to elucidate how these seemingly disparate processes interact. Such exploration may reveal valuable mechanisms to aid in the treatment and rehabilitation of proprioceptively impaired populations.
GRANTS
This work was supported by a Canadian Institutes of Health Research, Institute of Aging fellowship to D. Goble and a National Institutes of Health postdoctoral supplement for underrepresented minorities to J. Anguera.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Cothros N, Wong JD, Gribble PL. Are there distinct neural representations of object and limb dynamics? Exp Brain Res 173: 689–697, 2006 [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 164–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol 99: 3063–3074, 2008 [DOI] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, Zilles K, Ehrsson HH. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol 93: 1020–1034, 2005 [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Plasticity. NeuroRx 3: 420–427, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain 105: 515–542, 1982 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS One 3: e2485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 367–377, 1998 [DOI] [PubMed] [Google Scholar]
- Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol 79: 2119–2148, 1998 [DOI] [PubMed] [Google Scholar]