Abstract
We report here the first direct measurements of persistent inward currents (PICs) in rat hindlimb motoneurons, obtained from ketamine–xylazine anesthetized rats during slow voltage ramps performed by single-electrode somatic voltage clamp. Most motoneurons expressed PICs and current–voltage (I–V) relations often contained a negative-slope region (NSR; 13/19 cells). PICs activated at −52.7 ± 3.89 mV, 9 mV negative to spike threshold. NSR onset was −44.2 ± 4.1 mV. PIC amplitudes were assessed by maximum inward currents measured relative to extrapolated leak current and to NSR-onset current. PIC conductance at potentials just positive to activation was assessed by the relative change in slope conductance (gin/gleak). PIC amplitudes varied widely; some exceeded 5 and 10 nA relative to current at NSR onset or leak current, respectively. PIC amplitudes did not vary significantly with input conductance, but PIC amplitudes normalized by recruitment current decreased with increasing input conductance. Similarly, gin/gleak decreased with increasing input conductance. Currents near resting potential on descending limbs of I–V relations were often outward, relative to ascending-limb currents. This residual outward current was correlated with increases in leak conductance on the descending limb and with input conductance. Excluding responses with accommodation, residual outward currents matched differences between recruitment and derecruitment currents, suggesting a role for residual outward current in frequency adaptation. Comparison of potentials for PIC activation and NSR onset with interspike trajectories during discharge demonstrated correspondence between PIC activation and frequency–current (f–I) range boundaries. Contributions of persistent inward and outward currents to motoneuron discharge characteristics are discussed.
INTRODUCTION
Persistent inward currents (PICs) are a critical component of motoneuron function. Persistent Na+ currents are essential for repetitive firing and the combination of persistent Na+ and L-type (CaV1.3) Ca2+ channels amplify the effects of both excitatory and inhibitory synaptic inputs as well as support self-sustained discharge and bistability in some motoneurons. PICs also influence the frequency–current (f–I) relations, as shown by the association between their activation and initiation of the secondary range of the f–I relation in cat (Schwindt and Crill 1982) and rat (Li et al. 2004) motoneurons.
Application of somatic voltage clamp has provided direct assessments of net PIC magnitudes and their distribution in cat (Lee and Heckman 1998b) and turtle (Svirskis and Hounsgaard 1997) hindlimb motoneurons and in rat hypoglossal (Powers and Binder 2003) and sacral (Li and Bennett 2003; Li et al. 2004) motoneurons. These studies provide direct estimates of net PIC amplitude, demonstrate negative-slope regions (NSRs) in some motoneurons capable of supporting voltage plateaus or bistability, and provide for comparisons of PIC characteristics with discharge properties. Estimates of PICs in rat hindlimb motoneurons have as yet been indirect, based on the difference between the injected current required for recruitment and that sufficient to support continued discharge as current is reduced (Button et al. 2006, 2007, 2008). These studies provide evidence that some ketamine–xylazine anesthetized rats produce PICs, as do unanesthetized decerebrate rats, and that the incidence of PIC-producing motoneurons increases following spinal transection. However, this indirect approach does not provide the information on PIC amplitudes and characteristics provided by voltage clamp as needed to assess the contribution of these currents to discharge characteristics.
The intention of the study presented here was to obtain direct measurements of net PICs produced in rat hindlimb motoneurons using the method of somatic voltage clamp. Our investigation of firing characteristics of rat hindlimb motoneurons (Turkin et al. 2010) provides evidence of PIC activation not only in motoneurons with self-sustained discharge, but also in many motoneurons with adapting patterns of discharge, suggesting that outward currents complement PICs in setting discharge characteristics. We also found that most rat hindlimb motoneurons had a high-gain region, preceding the primary range in the f–I relation, and that discharge properties were strongly size dependent, as indicated by correlations with input conductance. Consequently, our goals were to examine and compare PICs and outward currents evident during slow voltage ramps, examine the distribution of these currents in relation to input conductance, and compare these currents with the discharge characteristics in the same motoneurons in which they were recorded.
We found PICs in most motoneurons, some substantial, in addition to evidence of outward currents that contribute to discharge frequency adaptation. Moreover, the distribution of these currents was associated with input conductance. A preliminary report of some of these findings was previously presented (Turkin et al. 2009).
METHODS
The experiments were conducted on adult Long–Evans rats (350–500 g) of either sex. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at St. Joseph's Hospital and complied with principles from the Guide for the Care and Use of Laboratory Animals. Somatic voltage-clamp recordings were made in hindlimb motoneurons, following several tests, to determine basic parameters of the motoneurons and their responses to ramp current injection. These responses are presented in the companion article (Turkin et al. 2010). Voltage-clamp recordings were attempted if recording conditions did not deteriorate and the current-injection performance of the electrode was acceptable, with rapid settling and capacitance that could be adequately compensated. Single-electrode discontinuous voltage clamp was performed with an Axoclamp 2A amplifier, with adjustments to capacitance compensation and phase control to achieve suitable electrode performance and attain the largest gain consistent with stable voltage-clamp performance. Rates for the current injection cycle were set at a minimum of 8 kHz and ranged from 8 to 10 kHz. Potentials on the monitor output of the amplifier during current-injection cycles were inspected continuously throughout the recordings. Breakthrough spiking was observed in some motoneurons as depolarization reached threshold. Under voltage-clamp control, breakthrough spikes were attenuated and lacked afterhyperpolarization (AHP) currents, indicating their generation at sites separate from the soma. Current–voltage (I–V) relations obtained with breakthrough spiking had characteristics similar to those without and were accepted for analysis (cf. Lee and Heckman 1998b). Voltage and current records were low-pass filtered at 4.8 kHz, digitized at 20.8 kHz, and saved in files for subsequent analysis.
Typically, two or three voltage-clamp trials were recorded, each trial consisting of a 5-s ramp depolarization starting between −70 and −60 mV and a 5-s ramp repolarization to the starting membrane potential. The peak depolarization of the ramp usually was increased slightly in successive trials to achieve sufficient depolarization to activate PICs while minimizing activation of strong outward currents. Trials were separated by ≥20 s. Voltage ramps usually depolarized to peak potentials between −35 and −30 mV. Responses of the trials were checked for consistency, although the peak inward currents often decreased in successive trials. Consequently, results of the first trial most often were used for analysis. In seven motoneurons, the peak of the depolarization command was increased in steps of 5 to 7 mV from approximately −45 to −30 mV, to examine the voltage dependence of residual outward currents that were observed in many motoneurons (see results).
Following completion of voltage-clamp recordings, a final determination was made of the antidromic action potential and the electrode was backed out of the motoneuron in steps to determine the resting potential. Membrane potentials recorded during voltage clamp were adjusted according to this determination.
Analyses were performed using standard and custom-written Matlab functions and scripts. Current and voltage records were digitally filtered (low-pass fourth-order Butterworth at 31 Hz, forward and backward to avoid phase shifts) to minimize breakthrough spikes and noise associated with single-electrode voltage clamp. Several parameters were measured to assess the amplitudes of persistent inward and outward currents, their associated conductances, and the voltages at which they were activated. The I–V plots of most motoneurons contained NSRs (Fig. 1A); the membrane potential at the start of this region on the ascending voltage ramp and its termination on the descending voltage ramp were determined as Vonset and Voffset, respectively. Both values were determined by inspection; potentials were selected at which the I–V slope was zero and which were flanked by positive- and negative-slope regions. In such records peak PIC amplitude was determined as the inward current measured relative to the current at Vonset, as shown in Fig. 1A. Measurements were made of the following slope conductances (Fig. 1B): gleak, at potentials negative to activation of PICs; gin, at potentials just positive to the membrane potential (Vstart) at which the I–V plot made an inward deviation from gleak; and g2, the slope conductance at potentials with full PIC activation and accompanying outward currents (Li and Bennett 2007). The leak conductance gleak was determined by selecting points on the most linear segment of the ascending limb of the I–V plot between resting membrane potential and about −55 mV and fitting a regression line to this segment. Inward deviation from this line exceeding the level of recording noise was then selected as the first point in a regression for gin. A second point was selected about 5 mV positive to this inward deviation point and gin was determined from the regression slope on this interval. The net inward current in the I–V relation after subtraction of currents expected from the extrapolated leak conductance was also measured (Fig. 1C). Comparisons of membrane potential during voltage clamp and during discharge evoked by current injection were limited to cells in which these comparisons could be fairly made; two cells were excluded because membrane potential at gin threshold could not be accurately determined because of small size and/or noise in the I–V relation (gin/gleak >0.8) and another two were excluded because of a shift of membrane potential between current-injection and voltage-clamp tests.
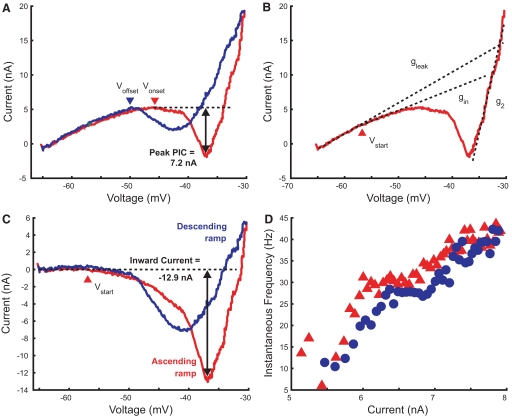
Fig. 1.
Measurements made from current–voltage (I–V) plots obtained during somatic voltage clamp. The red lines show the I–V relation obtained during the ascending limb of the voltage ramp; the blue lines show the relation during the return to resting potential. The horizontal dashed line in A shows the current at the start of the negative-slope region (NSR), marked by the arrowhead (Vonset); net inward current relative to this current was measured as peak persistent inward current (PIC), as indicated. Termination of the PIC on the descending voltage ramp is indicated by Voffset at the second arrowhead. Dashed diagonal lines in B mark regression lines used to determine the following slope conductances: leak conductance (gleak); the initial inward conductance (gin); and the outward conductance at depolarized potentials (g2). C shows the I–V relation after subtraction of the leak current (i.e., the gleak regression line). The arrowhead indicates the potential at which PIC is first activated (Vstart), corresponding to the inward deviation from the leak current. Net inward current relative to the leak current was measured as the negative peak in the I–V relation as shown. The frequency–current (f–I) plot obtained for this motoneuron (tibial, GN = 0.38 μS) is shown in D.
The dependence of persistent currents on motoneuron size was explored using input conductance as a measure of size. Input conductance was determined by injecting a series of current pulses using the discontinuous-current-clamp mode of the Axoclamp amplifier and calculating the regression slope of current pulse amplitude versus peak amplitude of the resulting change in membrane potential. Input conductance was used, rather than leak conductance, to provide more direct comparison to results of Turkin et al. (2010). Values of input conductance matched well those of leak conductance determined in voltage clamp (Gleak = −0.007 + 1.17GN, r = 0.94, P < 0.001) and choice of either conductance as a size measure did not affect the significance of the correlations that were tested.
RESULTS
Successful voltage-clamp recordings were made from 19 hindlimb motoneurons following determination of their responses to ramp current injection. Sixteen of these motoneurons responded to ramp current injection with repetitive discharge and were included in the set of motoneurons described by Turkin et al. (2010). We also studied another 3 motoneurons that were either incapable of regular discharge (2) or did not produce regular, sustained repetitive discharge, although brief (0.5 ms) and long (50 ms) depolarizing pulses elicited single action potentials in all 3 cells. Seven of the motoneurons belonged to the gastrocnemius-soleus (GS) motoneuron pool, 8 were innervated by more distal branches of the tibial nerve (Tib), and 3 by common peroneal (CP); 1 motoneuron was unidentified.
Characteristics of I–V relations in rat hindlimb motoneurons
Currents produced by rat hindlimb motoneurons in response to somatic voltage ramps were similar in most respects to those described previously for cat hindlimb and rat tail motoneurons (Lee and Heckman 1998b; Li and Bennett 2003, 2007; Li et al. 2004), although considerable variability was observed in the strength of the inward currents produced during depolarization. Examples of the I–V plots obtained during voltage clamp and corresponding f–I relations obtained during current ramps (Turkin et al. 2010) are shown in Figs. 1 and 2. Nearly all cells displayed an inward deviation from the leak conductance. In addition some cells showed strong PICs with NSRs and large peak PICs, as shown in Figs. 1 and 2A. Some of these cells were associated with self-sustained discharge (i.e., ΔI <0), such as the motoneuron with a prominent counterclockwise f–I relation shown in Fig. 2A, which continued to discharge after injected current was removed. However, self-sustained discharge was not observed in all cells with large PICs, as found for the cell illustrated in Fig. 1 (see also Fig. 5 in the following text). The initial peak PIC on the ascending ramp usually was larger than the sustained peak on the descending ramp (cf. Lee and Heckman 1998b). The somatic membrane potential at the end of the NSR on the descending voltage ramp (Voffset) was consistently more negative than the potential at the start of the NSR on the ascending ramp (Vonset), as reported previously for cat motoneurons (Lee and Heckman 1998b), consistent with a dendritic location for PICs and/or depolarization-induced facilitation of the channels that produce these currents (Moritz et al. 2007). Motoneurons with prominent NSRs (peak PICs ≥2 nA) were found in all motoneuron pools investigated.
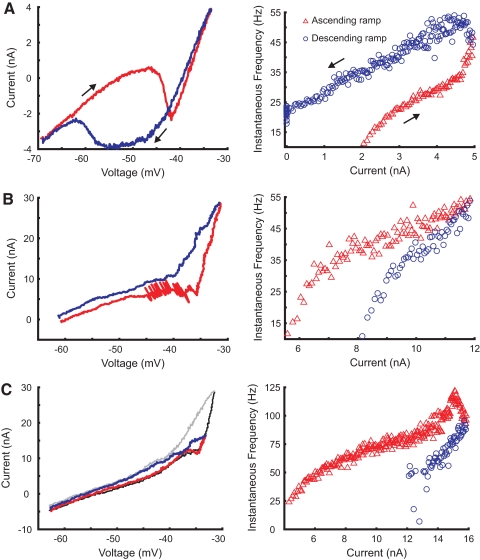
Fig. 2.
Examples of I–V relations and corresponding f–I relations. Recordings from ascending voltage and current ramps are represented by red lines and triangles, respectively; recordings from descending ramps are indicated by blue lines and circles. A shows plots for a motoneuron (gastrocnemius-soleus [GS], GN = 0.17 μS) with prominent PICs and self-sustained discharge following the end of injected current. The motoneuron whose plots are presented in B (GS, GN = 0.47 μS) produced an adapting pattern of discharge and small PICs. Breakthrough spikes are evident in this I–V plot. The plots in C were obtained from a motoneuron (tibial [Tib], GN = 0.43 μS) with weak net PICs and an acceleration in discharge at larger currents, before rates fell with accommodation and adaptation. Two overlapping I–V plots from successively larger voltage ramps are shown in C. Ascending limb of I–V plot in second trial is thin black line; descending limb is gray.
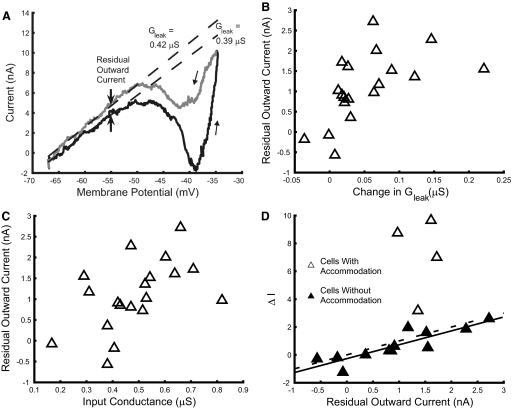
Fig. 5.
Residual outward currents in hindlimb motoneurons. Voltage-clamp records often showed evidence of an outward current that persisted as membrane potential returned to resting levels, as shown in A. This residual outward current was measured as the difference between regression lines (arrows) fit to determine leak conductance on the ascending and descending limbs of the voltage ramp. Leak conductance was often greater on the descending limb of the voltage ramp; residual outward current was correlated with these changes in leak conductance as shown in B (r = 0.57, P = 0.01). C shows that residual outward current was dependent on input conductance (r = 0.52, P = 0.023). The relation between residual outward current and ΔI is shown in D. Filled symbols represent cells that did not accommodate appreciably during ramp current discharge. ΔI varied directly with residual outward current (Ires) in these motoneurons, as indicated by the regression line (solid; ΔI = −0.272 + 0.996Ires, r = 0.88; P < 0.001). A line of identity (ΔI = residual outward current; dashed) is shown for comparison.
Despite the large PICs found in some motoneurons, PICs and NSRs often were small or absent, as shown in Fig. 2, B and C. The I–V relation shown in B, although partially obscured by current produced by breakthrough discharge, was characterized by a broad plateau and a small NSR during the ascending ramp; this characteristic was present, but reduced on the descending ramp. In this case, the corresponding f–I relation shows adaptation and no indication of discharge acceleration or a secondary range. The f–I relation shown in C does contain a secondary range and discharge acceleration at the high currents used in this trial. At this current level this motoneuron also had a strongly adapting pattern of discharge accompanied by accommodation (indicated by rise in spike threshold and decrease in spike amplitude; not shown), contributing to a large, positive value of ΔI. I–V relations for this cell were determined in trials with successively larger voltage ramps; two of these I–V relations are shown in C. A small NSR is evident in the earlier trial with a smaller depolarizing ramp; this NSR is replaced by a plateau before the slope increases sharply in the subsequent trial with greater depolarization. The region of the I–V plot in this cell with negative slope or plateau follows a zone of increased slope conductance (relative to gleak), suggesting that the I–V relation following the initial inward current is determined by a combination of inward and outward currents, with some outward currents in this case activated at somatic membrane potentials negative to those at which the inward currents responsible for the secondary range are evident in the f–I plot. One other motoneuron had an I–V relation, which suggested this combination of inward and outward currents.
All I–V plots included a region with large slope conductance at the most depolarized membrane potentials, including an outward current “wall” near −30 mV, as noted in previous reports. We also noted that the I–V relation on the descending ramp was often positive to that on the ascending ramp, suggesting the presence of a net outward current sustained near resting membrane potential following depolarization. Examples of this current are shown in Figs. 2, B and C and 5A. Overall, the characteristics of the I–V relations in rat hindlimb motoneurons were similar to those described previously for cat motoneurons and rat tail motoneurons, but we also noted features consistent with additional outward currents. Comparison of I–V and f–I relations suggested that both inward and outward currents influenced responses to somatic current injection.
Inward current amplitudes and activation of PICs
Persistent inward currents were activated with depolarization at membrane potentials below the threshold for discharge. Spike threshold at the start of discharge during ramp current injection was −43.7 ± 4.40 mV in cells in which we were able to compare discharge with currents measured during somatic voltage clamp. The membrane potential at which the I–V relation diverged in the negative direction from the regression line for leak conductance Vstart was −52.7 ± 3.89 mV. Vstart was very similar to the membrane potential at which the prespike trajectory diverged from the voltage expected from leak conductance during ramp current injection; the mean difference between these values was 0.645 ± 2.38 mV (paired t = 0.98, P = 0.35).
The size of this initial PIC was estimated by the relative change in slope conductance of the I–V relation at potentials just positive to Vstart. This change in conductance could be substantial, as much as 46% of leak conduction, and was correlated with input conductance. Figure 3 shows values of this slope conductance relative to leak conductance gin/gleak plotted against input conductance. Values of gin/gleak increased with input conductance (r = 0.51, P = 0.027) as the change in slope conductance produced by these initial PICs lessened, indicating that this PIC component relative to leak conductance is relatively smaller in large motoneurons with high-input conductance. In motoneurons without repetitive discharge the value of gin/gleak approached 1, whereas cells with self-sustained discharge (ΔI <0) had smaller values of gin/gleak. However, small values of gin/gleak were not sufficient to produce self-sustained discharge because similar values were found in cells with adapting patterns of discharge.
Fig. 3.
Variation of initial PIC conductance with input conductance. The conductance of this PIC was assessed as the slope conductance of the I–V relation at membrane potentials just positive to Vstart (cf. Fig. 1) normalized by leak conductance, gin/gleak. Black-filled symbols indicate cells with self-sustained discharge (ΔI <0), whereas motoneurons that were incapable of repetitive discharge are indicated by gray-filled symbols.
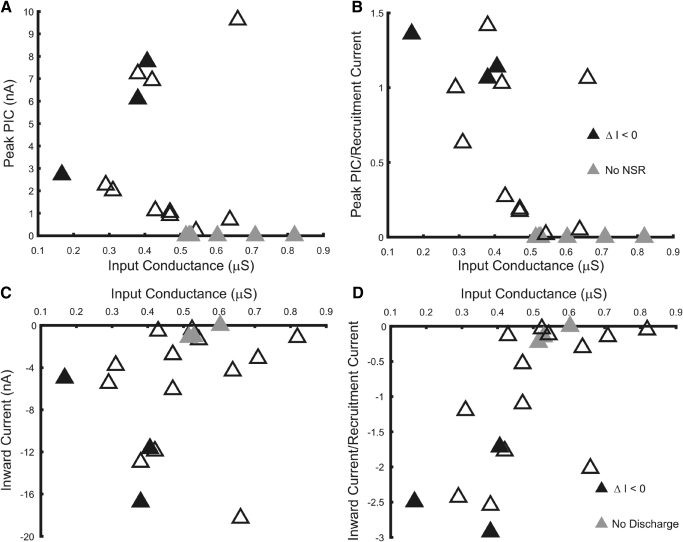
Figure 4 shows the distribution of PIC amplitudes in relation to input conductance. PIC amplitudes were assessed as the net inward current relative to both leak current (net inward current; Fig. 4C) and relative to the current at the start of the NSR (peak PIC; Fig. 4A; see Fig. 1 and methods). The former provides a measure of the overall inward current produced relative to that expected from the resting membrane properties of the motoneuron and is available for all cells, whereas the latter provides a measure of the net inward current available to produce a plateau and support self-sustained discharge in cells with NSRs. Of the 16 motoneurons with repetitive discharge, 13 had NSRs, although some of these were negligible and 3 cells with NSRs lacked a Voffset on the descending voltage ramp. The mean potential for Vonset was −44.2 ± 4.1 mV, whereas mean Voffset was −49.9 ± 5.6 mV. The smallest values of net inward current and peak PIC occurred in high-input conductance cells and all cells lacking NSRs had input conductances >0.5 μS. However, values in low-input conductance cells varied widely and large PIC values were observed in one unidentified motoneuron whose input conductance exceeded 0.5 μS. Overall, there was no association between input conductance and either net inward current amplitude or peak PIC.
Fig. 4.
Two measures of PIC amplitude vs. input conductance. Peak PIC amplitudes shown in A plot inward current relative to current at start of NSR, whereas inward currents plotted in C indicate net inward current relative to leak current (see Fig. 1). Black symbols indicate cells in which discharge during ramp current injection persisted at currents below the recruitment current (ΔI <0). Gray symbols in A indicate cells without a NSR in the I–V relation (no NSR), plotted with a value of 0; gray symbols in C indicate cells that did not discharge repetitively. B and D show these measures of PIC amplitude following normalization by recruitment current.
To assess the size of the PICs relative to the current required for motoneuron discharge, we normalized the PIC amplitudes by the current required to initiate discharge during ramp current injection. These normalized values, shown in Fig. 4, B and D, show that PIC amplitudes relative to recruitment current tend to be smaller in larger motoneurons. Normalized net inward current decreased with input conductance (r = 0.65; P = 0.0027), as did normalized peak PICs (r = −0.65, P = 0.0025). Although cells with self-sustained discharge all had substantial net inward currents and peak PICs, this characteristic alone was insufficient to produce self-sustained discharge, suggesting the contribution of other factors to discharge patterns.
Outward currents in hindlimb motoneurons
I–V characteristics with strong depolarization were consistent with activation of outward currents, as reported for cat hindlimb (Lee and Heckman 1998b) and rat tail motoneurons (Li and Bennett 2003, 2007; Li et al. 2004). These were usually evident as a steep increase in the slope of the I–V relation at the most depolarized potentials, as evident in Figs. 1, 2, 5, and 6. Also, as noted earlier, some motoneurons provided evidence of outward currents activated at more negative potentials. The magnitude of outward currents activated by depolarization, as indicated by the ratio of slope conductances G2/Gleak, varied from 1.15 to 19.1, with a mean of 7.98 (±5.08). These values were not associated with motoneuron input conductance (r = −0.14, P = 0.59). The large values of G2/Gleak are consistent with activation of large outward currents, produced at least in part by Ca2+-activated K+ currents following activation of Ca2+ L-channels (Li and Bennett 2007).
Fig. 6.
Dependence of residual outward current on amplitude of voltage ramp. A shows I–V plots from 3 successive trials in one motoneuron (tibial motoneuron, GN = 0.71 μS), shifted along the current axis for clarity. B plots residual outward current vs. peak membrane potential during successive voltage ramps. Each symbol represents a set of recordings from a different motoneuron. Input conductance ranged from 0.43 to 0.82 μS in this group of motoneurons.
Current was often more positive on the return of membrane potential to the linear portion of the I–V relation and resting potential, as noted earlier. Measures of this residual outward current were made by fitting regression lines to linear regions of the ascending and descending I–V relations to determine leak conductance before and after depolarization; the value of residual outward current was taken as the current separating regression lines at the depolarized end of these linear regions (usually about −55 mV). The leak conductance was often greater on the descending limb of the I–V relation, as in the example presented in Fig. 5A. Residual outward current amplitudes were correlated with the increase in leak conductance (Fig. 5B), suggesting that these outward currents were generated by an outward conductance activated during depolarization that was slowly deactivated and persisted during repolarization. Residual outward current was also correlated with input conductance (Fig. 5C), being most prominent in cells with larger input conductances.
The tendency of motoneurons to adapt or sustain discharge in response to ramp current injection is also associated with input conductance; discharge adaptation, as indicated by ΔI, increases with input conductance (Turkin et al. 2010). We examined the relation between residual outward current and ΔI to determine whether this outward current contributed to discharge adaptation. For this analysis, residual outward current was determined from I–V relations with sufficient depolarization to include PICs, if present, and the beginning of the outward current wall. As shown in Fig. 5D, ΔI tended to increase with residual outward current. Inspection of records during ramp current injection showed evidence of accommodation in some cases, indicated by decreases in spike amplitude and increases in threshold at similar potentials on the ascending and descending ramps (e.g., Fig. 7B in Turkin et al. 2010). Accommodation in these cells likely contributed to their large positive values of ΔI. In motoneurons without signs of accommodation, ΔI was strongly correlated with residual outward current. The regression line for this relation matched the line of identity (Fig. 5D). Although this match suggests that the residual outward current was sufficient to account for values of ΔI in motoneurons with adapting patterns of discharge, differences between the conditions of voltage-clamp determinations of residual outward current and current-evoked discharge must be considered (see discussion).
Fig. 7.
Comparison of afterhyperpolarization (AHP) trajectories, PIC activation potentials, and discharge pattern in motoneurons with different patterns of discharge. Top and middle: selected trajectories of membrane potential following discharge during ramp current injection. Records start at termination of previous spike and end at threshold of following spike. The f–I relation determined during current injection is shown at bottom; only every third point is shown for clarity. Triangles indicate instantaneous frequency (inverse of interspike interval) during rising phase of current injection; circles indicate frequencies during falling phase of current injection. Filled symbols mark intervals whose trajectories are shown in top and middle, as indicated by number. The dashed lines in the top panel show membrane potentials at which departure from leak conductance occurs (Vstart), start of NSR (Vonset), and end of NSR on descending ramp (Voffset) determined from I–V relations during somatic voltage clamp in this motoneuron. A shows results from a GS motoneuron (GN = 0.17 μS), B from a GS motoneuron (GN = 0.41 μS), and C from a common peroneal (CP) motoneuron (GN = 0.42 μS).
In several motoneurons we performed voltage clamps of increasing amplitude to examine the dependence of residual outward current on the level of depolarization achieved during the ramp. Figure 6A shows three I–V relations obtained from voltage ramps with progressively larger depolarizations in one motoneuron. Residual outward current grows with depolarization, as indicated by the upward displacement of the down-ramp currents in each I–V relation. Results from all motoneurons with substantial outward currents subjected to ramps of increasing size are shown in Fig. 6B. Small levels of residual outward current were measured during all depolarizing ramps, but somatic depolarizations in excess of about −38 mV were required for residual outward currents of >1 nA.
Residual outward current was not associated with the presence of PICs. In the group of motoneurons shown in Fig. 6, values of persistent inward current were small, ranging from −3.1 to 0 nA (one cell did not discharge repetitively); only two cells of this group had NSRs. One motoneuron that was tested in this manner that expressed large net inward currents (−11.7 nA) and had a prominent NSR (peak PIC of 7.8 nA) did not produce residual outward current despite depolarizing ramps that advanced well into the outward current wall (to −32 mV; not illustrated). Overall, there was no association between residual outward current and either net persistent inward current (r = 0.2, P = 0.42) or peak PIC amplitude (r = −0.19, P = 0.54).
Comparison of PIC activation and discharge patterns
The voltage-clamp measurements demonstrate that rat hindlimb motoneurons produce a mixture of persistent inward and outward currents. Similarly, responses to ramp current injection suggest contributions by both PICs and outward currents, with several signs of PIC activation despite discharge patterns that are predominantly adapting. The f–I relations of motoneurons often have a high-gain secondary range at larger currents in addition to a primary range of moderate gain. Rat hindlimb motoneurons also include a “subprimary range,” an initial high-gain region preceding the primary range in their f–I relations (Turkin et al. 2010). We compared membrane potentials during trajectories between spikes and membrane potentials associated with PIC activation and deactivation (Vstart, Vonset, and Voffset) to determine conditions associated with changes in f–I gain, self-sustained firing, and adaptation.
Figure 7 shows records from three motoneurons comparing f–I relations, selected AHP trajectories, and membrane potentials for PIC activation and deactivation. The cell shown in Fig. 7A had self-sustained discharge that was maintained after current injection ended. Consistent with this discharge response, it also possessed a prominent NSR that began (Vonset) below the threshold for discharge. Membrane potential trajectories at the start of discharge had minima below Vstart, but depolarized with increasing current and frequency to exceed Vstart near the transition from the subprimary to the primary range (at AHP trajectory 3). Acceleration of discharge in the secondary range was not observed until AHP trajectories exceeded Vonset (at AHP trajectory 6), after which the scoop in the AHP trajectory inverted. With PIC activation the cell continued to depolarize briefly during the current down-ramp, and frequency increased, producing counterclockwise rotation in the f–I relation. During repolarization, membrane potential remained depolarized compared with trajectories at similar injected currents on the up-ramp and they remained positive to Voffset, evidently sustaining the PIC and continued discharge once injected current was withdrawn.
In another motoneuron with a counterclockwise f–I pattern, but with only a slight self-sustained discharge, similar observations were made (Fig. 7B). The subprimary range ended as the AHP trajectory minimum approached Vstart and a brief secondary range started as trajectories exceeded Vonset. However, the effects of PIC activation were less prominent, corresponding to a much smaller difference between Vonset and Voffset than that for the motoneuron just considered. Consequently, Voffset was crossed by trajectories on the down-ramp, approximately at the point where the ascending and descending f–I curves intersected. This evidently was not sufficient to completely remove PIC activation because discharge was sustained at currents less than recruitment current.
These comparisons were made in two other motoneurons with a range of accelerated discharge in the f–I relation, both of which had adapting patterns of discharge. One of these exhibited increased discharge rates, with considerable variability at larger injected currents. In this region trajectory minima achieved potentials within 2 mV of Vonset but did not exceed it. The other motoneuron (represented in Fig. 2C) presented evidence of outward currents at potentials where PICs were activated. Trajectory minima in the range of acceleration reached potentials at which these outward currents were evident, but not potentials in the NSR. Despite the presence of accelerated discharge in both cells, the f–I relations did not exhibit counterclockwise hysteresis associated with substantial PIC activation, unlike the responses shown in Fig. 7, A and B. This comparison indicates that somatic potential trajectories must consistently exceed Vonset for full or substantial PIC activation, although depolarizations that approach Vonset may be sufficient to produce a secondary range in the f–I relation.
Responses of a cell with strong NSR (peak PIC of 6.9 nA) and adapting pattern of discharge are shown in Fig. 7C. Again, the subprimary range ended approximately as trajectory minima exceeded Vstart. In all cells in which responses to current injection and voltage clamp were compared, the trajectory minima matched Vstart on average (mean difference was 0.03 ± 1.67 mV). With increasing injected current, trajectory minima fell well short of Vonset and the secondary range with discharge acceleration was not achieved. Down-ramp trajectories were generally more negative than up-ramp trajectories at similar currents (e.g., 4 vs. 11, 3 vs. 12 and 13), suggesting an increase in net outward current. Interspike trajectories in other motoneurons with NSRs but without secondary ranges were also several millivolts less than Vonset and down-slope trajectories in these adapting cells were often more negative than the up-slope trajectories. Overall, interspike trajectories exceeded potentials at which PICs were initially activated (Vstart) at somatic currents within a few nanoamps of threshold, but much stronger currents were required to depolarize the cells sufficiently to reach the NSR, if present.
DISCUSSION
Our results provide the first direct determination of PICs in rat hindlimb motoneurons, showing that these motoneurons in ketamine–xylazine anesthetized rats are capable of producing strong PICs, often with negative-slope regions (NSRs) of sufficient amplitude to support dendritic plateau potentials and self-sustained discharge. Outward currents are produced as well and these limit the depolarization that can be produced and appear to contribute to the discharge frequency adaptation observed in these cells during somatic current injection. This mixture of inward and outward currents necessitates a caveat regarding estimates of these currents from voltage-clamp recordings, since the I–V plots derived from them represent net inward and outward currents and actual amplitudes of either cannot be distinguished in this mixture. Despite the inherent limitations of the in situ preparation used here, our results show that some features of both inward and outward currents are organized according to motoneuron size, as are discharge properties. In the following text we discuss possible sources for inward and outward currents, their organization, and the relation between discharge patterns and PIC activation.
Persistent inward currents in rat hindlimb motoneurons
The responses of rat hindlimb motoneurons to somatic voltage ramps provide clear evidence of substantial PICs, evident as inward deviations from the extrapolated leak current in nearly all motoneurons and as NSRs in many. PICs in rat sacrocaudal motoneurons comprise persistent Na+ and L-type Ca2+ currents (Li and Bennett 2003; Li et al. 2004), with the former activated first with depolarization, about 7 mV below spike threshold. Persistent Na+ current, which is necessary for repetitive discharge (Kuo et al. 2006), is probably responsible for the first inward deviation from leak current in our I–V plots. This deviation occurred about 9 mV below spike threshold and motoneurons incapable of regular repetitive discharge had none or little of this inward current, as indicated by values of gin/gleak.
The ratio of gin to gleak was correlated with input conductance. Care must be taken in ascribing this reduction in slope conductance to the amplitude of the first PIC conductance to be activated with depolarization. The reduction in slope conductance produced with the initial development of inward current depends not only on the magnitude of the conductance but also on its dependence on membrane potential (i.e., ∂g/∂Vm) and the drive potential for this current (presumably Vm − ENa; cf. Koch 1984). Assuming that these two latter factors are uniformly distributed throughout motoneuron pools, this result suggests that persistent Na+ current tends to decrease in relation to motoneuron size. If this is the case, then sustained repetitive discharge may be supported less well in larger motoneurons.
Peak PIC amplitudes and net inward current amplitudes were not correlated with input conductance, consistent with previous observations (Lee and Heckman 1998b). However, these values normalized by recruitment current tended to decrease with input conductance. Since both recruitment current and the current needed to increase discharge frequency (i.e., the inverse of f–I gain) increase with motoneuron size (Turkin et al. 2010), less inward current is generally available to support the development of plateaus and self-sustained discharge in larger motoneurons. This reduced support is consistent with differences in the capacity for self-sustained discharge and discharge acceleration in motoneurons of different size found in the companion article (Turkin et al. 2010). It is also consistent with previously reported differences in discharge patterns associated with PIC activation in cat (Lee and Heckman 1998a) and rat (Button et al. 2006) motoneurons classified according to size or by contraction type, respectively. Considering also the dependence of residual outward current on motoneuron size, our results demonstrate a systematic variation in the distribution of persistent voltage-activated currents and corresponding differences in the ability of motoneurons to produce PIC-facilitated discharge.
Button et al. (2006) concluded that both decerebrate and ketamine–xylazine anesthetized rats expressed PICs based on negative values of ΔI. Our results support this conclusion, although they indicate that PIC amplitude estimates based on ΔI are subject to some uncertainties. Strong PICs were evident in several cells with positive ΔI values and ΔI was best correlated with residual outward current. We used larger currents in this present study to test motoneurons over most of their operating range. This approach favored the development of residual outward currents and adapting patterns of discharge with positive ΔI values, so our ΔI values are not directly comparable with those of Button et al. (2006). However, comparisons of patterns of interspike trajectories and discharge patterns with voltage-clamp-measured PICs showed that PICs required injection of larger currents for full activation and they could not always be readily activated. Thus is it likely that ΔI values observed with smaller injected currents do not reflect peak PICs in NSRs, but rather the amplitudes of PICs activated at less depolarized potentials, without frank activation of dendritic plateau potentials. Perhaps PIC amplitude estimated from ΔI would best correspond to the measure of inward conductance gin/gleak used in this study, but this suggestion remains to be tested.
Residual outward current
Rat hindlimb motoneurons possess an outward current that is activated with depolarization and appears to deactivate slowly, persisting on the return of somatic voltage toward resting potential. The distribution of this residual outward current depends on input conductance and its strong correlation with ΔI suggests that it plays a primary role in the discharge adaptation observed in most motoneurons during current ramps. Several candidates for this current should be considered. Li and Bennett (2007) previously showed that a dendritic potassium SK current is activated by a Ca2+ L-type current. However, apamin does not alter the I–V relation in rat sacrocaudal motoneurons near resting potential (see Fig. 6 in Li and Bennett 2007). Na+-activated K+ current is activated by persistent Na+ current and has suitable properties (Budelli et al. 2009); it has been shown to be present in neonatal motoneurons (Safronov and Vogel 1996). Residual outward current was present in cells with negligible inward currents and we did not find any correlation between inward current amplitude and residual outward current, suggesting that neither of these two candidates is responsible. However, inward currents could have been masked by outward currents (see Fig. 2C), so this observation is inconclusive. Another possibility is the M-current, an important regulator of neuron excitability mediated by KCNQ channels (Jentsch 2000). This current is activated near resting potential but increases with depolarization with a half-activation potential of about −40 mV (Brown and Adams 1980). M-currents have been found in turtle motoneurons (Alaburda et al. 2002) and rat neonatal motoneurons (Rivera-Arconada and Lopez-Garcia 2005); mouse motoneurons also have muscarine-sensitive K+ currents (Miles et al. 2007). KCNQ channels are present in the initial segment of mouse motoneurons (Pan et al. 2006) and other spinal neurons (Devaux et al. 2004).
The size dependence of residual outward current and its correlation with ΔI suggest that it contributes to discharge adaptation in rat hindlimb motoneurons and to the greater extent of adaptation found in larger motoneurons (Turkin et al. 2010). However, interpretation of Fig. 5D and conclusions concerning the role of residual outward current in discharge adaptation are complicated by differences in driving potential and the extent of residual outward current activation during voltage-clamp determinations and current-evoked discharge. A rough estimate can be made of these differences. The peak Vm during voltage-clamp determinations of residual outward current was −35 mV, whereas the mean Vm during interspike intervals at the peak of current injection in the nonaccommodating cells shown in Fig. 5D was −47 mV. Inspection of Fig. 6B suggests that residual outward currents would be about 2.5- to 3-fold stronger with depolarizations to −35 mV compared with levels expected during discharge. This would be offset somewhat by differences in driving potential. We assume that residual current has a reversal potential of −80 mV and has its greatest effect on discharge at potentials positive to the mean interspike trajectory potential, at about −45 mV. Drive potentials for the residual outward current would be 35 mV during discharge and 25 mV during voltage-clamp determinations at −55 mV, a factor of 1.4. This would make the residual outward current measured by voltage clamp 1.8- to 2.1-fold as large as that expected during discharge. These calcuations assume no deactivation of residual outward current during repolarization to −55 mV, so these estimates are likely somewhat high.
Slow inactivation of fast Na+ channels is sufficient to explain discharge adaptation of mouse motoneurons, which is scarcely affected by blocking any of several outward currents, including M-currents, or by reducing persistent sodium current (Miles et al. 2005). Although the preceding calculations suggest that other factors such as Na+ inactivation contribute to discharge adaptation (see also Turkin et al. 2010), the level of residual outward current appears sufficient to contribute substantially. This discrepancy may represent a difference between species or could indicate that the residual outward current in rat motoneurons is merely correlated with the susceptibility of fast Na+ channels to slow inactivation, but is not causally related to the adaptation. Alternatively, the difference between test conditions (1-s pulses vs. longer ramps) may have produced different forms of adaptation. We excluded cells with clear evidence of fast-Na+ inactivation sufficient to produce accommodation from the comparison of ΔI and residual outward current; thus different forms of adaptation may have been observed in this study and that of Miles et al. (2005). Pending identification of residual outward current and direct demonstration of its contribution to discharge adaptation, the present results suggest that this outward current reduces discharge frequencies to an extent dependent on the level of depolarization and that this mechanism for reducing discharge frequency is best developed in larger motoneurons.
Persistent inward currents and patterns of motoneuron discharge
Comparisons of persistent currents obtained by somatic voltage clamp and discharge patterns elicited by ramp current injection are consistent with contributions of both outward and inward currents to patterns of discharge and differences in recruitment − derecruitment current differences. PICs support repetitive discharge and their activation with stronger depolarizing currents produces dendritic plateau potentials, counterclockwise f–I hysteresis, and self-sustained discharge. The residual outward currents demonstrated in this study are associated with adapting patterns of discharge. These results are consistent in many respects with previous comparisons of discharge and PICs in decerebrate cat hindlimb motoneurons (Lee and Heckman 1998a,b) and sacrocaudal motoneurons of chronic spinal rats (Bennett et al. 2001; Li and Bennett 2003; Li et al. 2004), although differences between these studies indicate that the composition and magnitudes of persistent currents vary by preparation with corresponding variation in discharge characteristics.
We found that strong PICs with dendritic plateaus are not activated at currents near spike threshold and no linear type 3 f–I relations were observed in which PICs activated before recruitment (Turkin et al. 2010). In contrast, type 3 responses are often found in chronic spinal cord transected rats; potentials required for activation of Ca2+ L-currents are lower relative to spike threshold than those in motoneurons with counterclockwise type 4 f–I relations and late PIC activation (Li et al. 2004). The latter group of motoneurons consists of small cells with lower values of rheobase and motoneurons with smaller values of input conductance and rheobase also compose fully bistable cells in decerebrate cats (Lee and Heckman 1998a,b) and rat hindlimb motoneurons with f–I patterns consistent with PIC activation. However, larger motoneurons in decerebrate cats tend to be partially bistable with late PIC activation, whereas larger rat hindlimb motoneurons tend to have adapting patterns of discharge. The differences in PIC activation and discharge characteristics between larger motoneurons in these different preparations indicate that the relative amplitudes of inward and outward currents and their effective threshold of activation are labile. They also stress the importance of neuromodulatory factors on PIC expression and plasticity in the modulation and expression of PICs following spinal injury and disuse (Harvey et al. 2006a,b; Lee and Heckman 2000; Li et al. 2007).
The striking alterations that can be produced in I–V relations by pharmacological reduction of outward currents (e.g., Lee and Heckman 1999; Powers and Binder 2003) demonstrate the potential for control of discharge characteristics through neuromodulation of outward currents. Although K+ SK currents activated by dendritic Ca2+ currents do not appear to change following chronic spinal transection (Li and Bennett 2007), the lability and modulation of other outward currents that influence discharge, such as the residual outward current described here, remain to be determined. To our knowledge, residual outward currents have not been previously described in mammalian motoneurons. Considering the differences between preparations just described and the ostensible influence of residual outward currents on discharge, these currents merit investigation as a target of neuromodulation and a source of lability following injury. Regardless of the lability of these and other intrinsic currents, our results emphasize the importance of considering both inward and outward currents and their interactions when evaluating mechanisms that govern discharge and the changes that may occur following injury and neurological disease.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-054282 to R. Jung, a Barrow Neurological Foundation grant to T. M. Hamm, and a University of Arizona Undergraduate Biology Research Program stipend to N. K. Bandekar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. C. J. Heckman for a helpful discussion on the in situ application of somatic voltage-clamp recordings.
REFERENCES
- Alaburda A, Perrier JF, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol 540: 875–881, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001 [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283: 673–676, 1980 [DOI] [PubMed] [Google Scholar]
- Budelli G, Hage TA, Wei A, Rojas P, Jong YJ, O'Malley K, Salkoff L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci 12: 745–750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Gardiner K, Marqueste T, Gardiner PF. Frequency–current relationships of rat hindlimb alpha-motoneurones. J Physiol 573: 663–677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Cahill F, Gardiner PF. Spike frequency adaptation of rat hindlimb motoneurons. J Appl Physiol 102: 1041–1050, 2007 [DOI] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci 24: 1236–1244, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- Koch C. Cable theory in neurons with active, linearized membranes. Biol Cybern 50: 15–33, 1984 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 3–34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998a [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998b [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol 82: 2518–2527, 1999 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol 566: 2–32, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol 98: 1042–1047, 2007 [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26: 2599–2613, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol 89: 615–624, 2003 [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Lopez-Garcia JA. Effects of M-current modulators on the excitability of immature rat spinal sensory and motor neurones. Eur J Neurosci 22: 3091–3098, 2005 [DOI] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Properties and functions of Na(+)-activated K+ channels in the soma of rat motoneurones. J Physiol 497: 727–734, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982 [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol 78: 1740–1742, 1997 [DOI] [PubMed] [Google Scholar]
- Turkin VV, O'Neill D, Jung R, Hamm TM. Comparison of frequency-current relations and persistent inward currents in rat motoneurons measured in situ. Soc Neurosci Abstr 35: 860.12, 2009 [Google Scholar]
- Turkin VV, O'Neill D, Yarkov A, Jung R, Hamm TM. Characteristics and organization of discharge properties in rat hindlimb motoneurons. J Neurophysiol 104: 1549–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]