Abstract
Speed and accuracy of motor responses to lateralized stimuli are influenced by the spatial overlap between stimulus location and required response. Responses showing high spatial overlap with peripheral cues benefit from a bottom-up driven enhancement of attention to the respective location, whereas low overlap requires top-down modulated reorienting of resources. Here we investigated the interaction between these two processes using a spatial stimulus-response compatibility task. Subjects had to react to lateralized visual stimuli with a button press using either the ipsilateral (congruent condition) or the contralateral (incongruent condition) index finger. Stimulus-driven bottom-up processes were associated with significant contralateral activation in V5, the intraparietal sulcus (IPS) and the premotor cortex (PMC). Incongruent versus congruent responses evoked significant activation in bilateral IPS and PMC, highly overlapping with the activations found for stimulus-driven bottom-up processes, as well as additional activation in bilateral anterior insula and right dorsolateral prefrontal cortex (DLPFC) and temporoparietal junction (TPJ). Moreover, a region anterior to the bottom-up driven activation in the IPS was associated with top-down modulated directionality-specific reorienting of motor attention during incongruent motor responses. Based on these results, we propose that stimulus-driven activation of contralateral IPS and PMC represent key neuronal substrates for the behavioral advantage observed when reacting toward a congruently lateralized stimulus. Additional activation in bilateral insula and right DLPFC and TPJ during incongruent responses should reflect top-down control mechanisms mediating contextual (i.e., task) demands. Furthermore, this study provides evidence for both overlapping and disparate substrates of bottom-up and top-down modulated attentional processes in the IPS.
INTRODUCTION
Spatial stimulus–response compatibility (SRC) refers to the psychological phenomenon that an overlap of spatial properties between stimuli and responses leads to faster and more accurate responses (Fitts and Seeger 1953). A classical example of spatial SRC is the antisaccade task introduced by Hallett (1978). In this task the subject is instructed to fixate a central position and, after presentation of a peripheral target, to perform a saccade away from the target to a mirror symmetrical position. Subjects are usually slower and less accurate when generating these antisaccades, compared with prosaccades, i.e., saccades toward the stimulus (Hallett 1978; Munoz and Everling 2004). Studies investigating SRC using manual responses yielded similar results (Proctor and Reeve 1990). Subjects show faster reaction times (RTs) and higher accuracy when instructed to respond to a lateralized stimulus with the ipsilateral hand (congruent condition), compared with a situation when they have to respond with the contralateral hand (incongruent condition).
In the cognitive literature of SRC, there is general agreement that two response selection processes contribute to performance in a spatial SRC task, depending on the dimensional overlap between stimulus and response (Kornblum et al. 1990). In particular, a direct response selection process is the result of a reflexive attentional orienting toward the location of a stimulus presented outside the current attentional focus. This process then leads to a facilitation of the congruent response (Eimer et al. 1995; Rubichi et al. 1997; Sheliga et al. 1997). In contrast, incongruent responses require an indirect task-dependent response selection process including inhibition of the automatic response facilitated by the reflexive reorientation, endogenous reorienting of attentional properties to the opposite side, and volitional response initiation (Munoz and Everling 2004; Nee et al. 2007; Reuter et al. 2010).
According to the influential model by Corbetta and Shulman (2002) attentional processes are controlled by two interacting networks. A bilateral dorsal frontoparietal network is formed by the dorsal parietal cortex and the dorsal frontal cortex, in particular the frontal eye fields (FEFs) and the dorsal premotor cortex (dPMC). In contrast, the ventral frontoparietal network is composed of the temporoparietal junction (TPJ) and the ventral frontal cortex, including parts of the middle frontal gyrus (MFG), inferior frontal gyrus (IFG), frontal operculum, and anterior insula. Later, Corbetta and collegues (2008) proposed that the dorsal network is responsible for directing attention to sensory stimuli and their locations as a prerequisite for looking or acting toward these. In contrast, the ventral network was hypothesized to be involved in higher-order cognitive processes, such as shifts in task sets, expectations, reward contingencies, and arousal.
Several lines of evidence for differential involvement of the frontoparietal network for stimulus-driven and context-dependent eye movements have been investigated in great detail with human neuroimaging studies as well as electrophysiological recordings in monkeys using the pro-/antisaccade task (e.g., Munoz and Everling 2004; Reuter et al. 2010). In contrast, imaging studies investigating the functional anatomy of the compatibility effect for hand movements have mostly focused on the effect of interference resolution during incongruent responses. These studies have consistently found increased activation in the dPMC and superior parietal cortex extending into the intraparietal sulcus (IPS) when contrasting incongruent with congruent conditions (Dassonville et al. 2001; Iacoboni et al. 1996, 1998; Schumacher and D'Esposito 2002; Schumacher et al. 2003). Thus it has been hypothesized that the parietal cortex is associated with the representation of the stimuli position, whereas the dPMC may use this information to program a context-dependent motor response. Besides activation of this premotor–intraparietal network, some studies also showed increased activation in the right dorsal prefrontal cortex during incongruent responses (Schumacher and D'Esposito 2002; Schumacher et al. 2003). This region has been proposed to exert a mediating or modulatory role on the abovementioned frontoparietal network, controlling that appropriate responses are given in the (incongruent) task context.
Although several neuroimaging studies on manual stimulus–response compatibility have thus focused on the neural correlates of interference resolution during incongruent responses, to the best of our knowledge, there is no functional magnetic resonance imaging (fMRI) study that focused on the differentiation between stimulus-driven reflexive orientation (as the substrate of behavioral facilitation), context-dependent top-down effects independent of direction, and directionally specific reorientation effects. To investigate commonalities and differences between these processes, we performed a spatial SRC experiment in which subjects were instructed to respond to lateralized visual stimuli with the ipsilateral (congruent) or contralateral (incongruent) hand. The focus of our analysis was to characterize the two different aspects of attentional reorienting. On one hand, we investigated which brain areas were associated with reflexive, stimulus-driven orienting toward the target stimulus—i.e., bottom-up driven processes. On the other hand, we aimed at identifying those regions enabling the task-dependent reallocation of attention in the incongruent condition—i.e., top-down modulated reorienting.
METHODS
Subjects
We examined 24 healthy volunteers (age range: 20 to 59 yr, mean age: 29 yr; 11 females) without any record of neurological or psychiatric disorders and normal or corrected-to-normal vision. All subjects gave informed written consent to the study protocol, which had been approved by the local ethics committee of the University of Aachen. Right-hand dominance of the participants was established by means of the Edinburgh handedness inventory (Oldfield 1971), which yielded a mean laterality quotient (LQ) of 88.6 (SD:16.1).
Experimental protocol
Participants were instructed to respond as fast and correctly as possible to briefly presented (200 ms) lateralized (∼11° eccentricity) target stimuli (red dot) by pressing a button on an MRI-compatible response pad (LumiTouch, Burnaby, Canada) according to the task condition:
Congruent condition.
Subjects were instructed to respond with the ipsilateral hand, i.e., pressing with their left index finger to a left-sided stimulus (CL) and with their right index finger to a right-sided stimulus (CR).
Incongruent condition.
Subjects were instructed to respond with the contralateral hand, i.e., pressing with their left index finger to a right-sided stimulus (ICL) and with their right index finger to a left-sided stimulus (ICR; note that L and R always indicate the respective response hand and not the stimulus side).
Visual stimuli were presented using a presentation software package (Version 11.3) and were displayed on a custom-built, shielded thin-film transistor screen at the rear end of the scanner visible via a mirror mounted on the headcoil (∼12 × 8° viewing angle, 245 mm distance from the subject's eyes). During the experiment, task blocks were periodically alternated with rest periods (“baseline”) lasting 17–23 s (uniformly jittered). Each task block started with an instruction being presented for 500 ms, which informed the subject which of the two experimental conditions (ipsilateral/contralateral response) had to be performed in the upcoming block. Regardless of the condition, between 13 and 16 events per block (randomized 50% left-sided stimulus/50% right-sided stimulus, number of events randomized to avoid anticipation effects) were presented. The interstimulus interval (ISI) was uniformly jittered between 2 and 4.5 s. That is, we used a mixed design with blocked task instructions (congruent/incongruent), but event-related stimulus presentation (left/right). By jittering the ISI within the blocks, events entailing left- and right-sided stimuli could be separated from each other statistically, allowing analysis of side-specific neuronal responses in each experimental condition (CL, CR, ICL, ICR).
In the course of the entire experiment, each of the two conditions (congruent, incongruent) was presented in 18 individual blocks. The order of the ensuing 36 blocks was pseudorandomized and counterbalanced across subjects.
Reaction times <150 or >1600 ms were regarded, respectively, as anticipation errors and missing responses and discarded from the later analysis.
Behavioral data analysis
The behavioral measurements taken during the fMRI experiment were analyzed off-line using MATLAB (The MathWorks, Natick, MA). The effects of experimental factors (condition: congruent/incongruent; stimulus side: left/right) on mean reaction time and percentage of correct responses were compared by a repeated-measures ANOVA (rmANOVA). If the effect of a factor was significant, pairwise comparison was performed by a t-test (P < 0.05, corrected for multiple comparisons using Tukey's procedure).
Functional magnetic resonance imaging
Images were acquired on a Siemens Trio 3T whole-body scanner (Erlangen, Germany) using blood oxygenation level dependent (BOLD) contrast (gradient-echoplanar imaging [EPI] pulse sequence, repetition time [TR] = 2200 ms, in-plane resolution = 3.1 × 3.1 mm, 36 axial slices, 3.1 mm thickness) covering the whole brain. Image acquisition was preceded by four dummy images allowing for magnetic field saturation. These were discharged prior to further processing. Images were analyzed using SPM5 (www.fil.ion.ucl.ac.uk/spm). First, the EPI images were corrected for head movement by affine registration using a two-pass procedure, by which images were initially realigned to the first image and subsequently to the mean of the realigned images. After realignment, the mean EPI image for each subject was spatially normalized to the Montreal Neurological Institute (MNI) single-subject template using the “unified segmentation” approach (Ashburner and Friston 2005). The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move subject data into the space of the MNI tissue probability maps, were then combined with the deformation field transforming between the latter and the MNI single-subject template. The ensuing deformation was subsequently applied to the individual EPI volumes that were hereby transformed into the MNI single-subject space and resampled at 2 × 2 × 2 mm3 voxel size. The normalized images were spatially smoothed using an 8-mm full width at half maximum Gaussian kernel to meet the statistical requirements of the general linear model (GLM) and to compensate for residual macroanatomical variations across subjects.
Statistical analysis
The fMRI data were analyzed using a GLM as implemented in SPM5. Each experimental condition (CL, CR, ICL, ICR) was modeled using a series of stick functions denoting the individual events. These were then convolved with a canonical hemodynamic response function and its first-order temporal derivative. Low-frequency signal drifts were filtered using a cutoff period of 128 s. Parameter estimates were subsequently calculated for each voxel using weighted least squares to provide maximum likelihood estimators based on the temporal autocorrelation of the data (Kiebel et al. 2003). No global scaling was applied. For each subject, simple main effects for each experimental condition were computed by applying appropriate baseline contrasts. These individual first-level contrasts were then fed to a second-level group analysis using an ANOVA (factor: condition; blocking factor: subject) using a random-effects model. In the modeling of variance components, we allowed for violations of sphericity by modeling nonindependence across images from the same subject and allowing unequal variances between conditions and subjects using the standard implementation in SPM5. Simple main effects of each event type (vs. the resting baseline) as well as comparisons between experimental factors were tested by applying appropriate linear contrast to the ANOVA parameter estimates. When testing for comparisons between experimental conditions, we used the minimum conjunction analysis (Nichols et al. 2005) over the differential contrast and the main effect, to ensure that we detected only task-positive effects. With the minimum conjunction analysis, as implemented in SPM5, voxels are declared active in the conjunction analysis only if they pass the statistical threshold in each of the separate contrasts contributing to the conjunction.
For instance, to detect regions specifically associated with increased activation in the incongruent condition, we performed a conjunction analysis over the differential contrast between incongruent versus congruent conditions (ICL + ICR vs. CL + CR) and the composite main effect of the incongruent condition (ICL + ICR). Thereby we ensured that only those voxels were considered active that showed significantly higher activation in the incongruent compared with the congruent condition and, furthermore, were significantly activated (relative to resting baseline) in the incongruent condition. The resulting statistical parametric T-maps [SPM(T)] were then thresholded at P < 0.05 (cluster-level corrected) and anatomically localized using version 1.5 of the SPM Anatomy toolbox (Eickhoff et al. 2005, 2007; www.fz-juelich.de/ime/spm_anatomy_toolbox).
Furthermore, to make sure that both conditions of the composite main effect for incongruency (ICR and ICL) contributed to the observed effect, we inclusively masked with each simple main effect. That is, for the detection of regions associated with the increased activation in the incongruent condition, we inclusively masked the contrast described earlier with the simple main effect of each incongruent condition, that is ICL and ICR, on a P < 0.05 uncorrected level. Hereby voxels driven by only one of the two incongruency conditions (but inactive or even deactivating in the other) were removed from contributing to the significant results.
RESULTS
Behavioral data
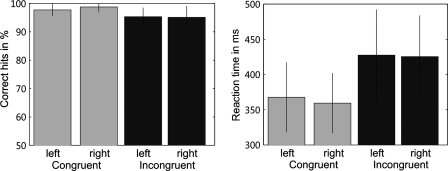
Percentages of correct responses and mean RTs measured during scanning are summarized in Fig. 1. SE values represent between-subjects variability for the respective conditions. In the accuracy of responses and RTs we found a significant main effect of condition (congruent and incongruent). Responses were significantly more accurate [F(1,69) = 40.13, P < 0.01] and RTs significantly faster [F(1,69) = 318.28, P < 0.01] under the compatible compared with the incompatible condition as revealed by rmANOVA.
FIG. 1.
Left panel: the mean percentages of correct responses separately for each response hand in the 2 task conditions (congruent/incongruent). Responses were significantly more accurate under the congruent compared with the incongruent condition. Right panel: the mean reaction times (RTs, ms) for each response hand in the 2 task conditions. The incongruent condition produced significantly longer RTs compared with the congruent condition.
For a further characterization of the incongruency effect, we correlated the incongruency costs (mean RT toward incongruent stimuli − mean RT toward congruent stimuli) of all subjects, with their individual mean reaction time in the congruent condition. This analysis revealed a significant positive correlation between reaction time and incongruency cost (r = 0.572; P < 0.004). That is, subjects who responded faster in the congruent condition were also less affected by the incongruent condition. To assess whether subjects traded speed for accuracy in the incongruent condition, we further correlated the incongruency costs with the error rate in the incongruent condition. We found that subjects showing smaller incongruency costs also had a higher error rate in the incongruent condition. Thus we indeed found evidence for a trade-off between speed and accuracy.
Imaging data
GENERAL TASK EFFECTS.
In a first step, we wanted to investigate brain regions associated with the general task demands independent of a congruent or incongruent condition. These included detection of the target stimuli and planning as well as initiating of an appropriate motor response. Brain regions associated with the general task demands were delineated by the composite main effect of all experimental conditions [CL + CR + ICL + ICR] (P < 0.05 cluster level corrected). To ensure that all four conditions contributed to the task-positive effect, we additionally inclusively masked this contrast with the simple main effects for each condition on a P < 0.001 uncorrected level. Uncorrected thresholding was used for the masking contrasts because these were intended to constrain only the main analysis to those voxels, where all four individual conditions showed activation higher than baseline. Compared with the remaining contrasts, where we always masked on a P < 0.05 uncorrected level, here we used a more conservative masking threshold. The reason for this was that when looking for the general task effect, we used the composite main effect of all experimental conditions versus the baseline condition. Evidently, however, these baseline contrasts result in much more extended activations than those of the relative contrasts used in the other analyses. Consequently, masking at P < 0.05 would have resulted in vast activations, yielding little to no localizing precision.
The statistical inference, however, was based on the composite main effect and assessed at P < 0.05 after correction for multiple comparisons.
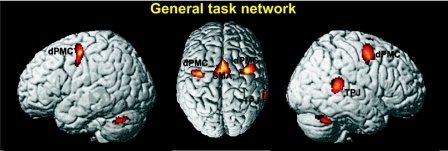
This analysis revealed significant activation in bilateral dPMC (Area 6, 42, −3, 51 and −32, −9, 48), the medial supplementary motor area (SMA) extending into the pre-SMA (−4, 3, 48), the right TPJ (63, −39, 15), as well as activation in the cerebellum (0, −70, −15; 34, −54, −32; −34, −52, −33) and the right caudate nucleus (18, 2, 15) (Fig. 2).
FIG. 2.
The general task network (i.e., regions consistently activated across all conditions). This included bilateral dorsal premotor cortex (dPMC), medial supplementary motor area (SMA), and right temporoparietal junction (TPJ). Moreover, the right nucleus caudatus and the bilateral cerebellum showed significant activation. All activations are shown on a P < 0.05 (cluster-level corrected) level.
BOTTOM-UP PROCESSES.
A main focus of this experiment was the delineation of brain areas associated with stimulus-driven orienting of attention toward the respective stimulus side. To investigate brain regions related to left stimulus-driven bottom-up processes, independent of the subsequent motor response, we used the following approach. We performed a conjunction analysis over the composite main effect for left-sided stimuli (CL + ICR) and the differential contrast between the composite main effect for left-sided stimuli and congruent responses to right-sided stimuli [(CL + ICR) vs. 2 × CR; P < 0.05, cluster level corrected]. The motivation for the first component of this conjunction was that it delineates those voxels that show a higher activity in those trials where left-sided stimuli were shown relative to baseline. That is, all voxels declared active have to show true task-positive activation, whereas, e.g., relative deactivations were not considered. The latter component then tests, where these effects are significantly higher than those related to right stimulus presentation and response, removing, e.g., effects uniformly related to task performance per se. However, we did not include incongruent responses to right-sided stimuli (ICL) in this contrast, even though these evidently also entailed right-sided stimulation. The reason behind this omission was that incongruent left-hand responses required subjects to endogenously reorient spatial attention from the right side to the left side. Consequently, inference on the neural substrates of exogenous reorientation to the left side may be confounded by these top-down mechanisms.
To ensure that both conditions for left-sided stimuli contributed to this effect, we inclusively masked this contrast with the simple main effects for left-sided stimuli, CL and ICR, on a P < 0.05 uncorrected level. Again, thresholding for the masking contrasts was performed at an uncorrected level to constrain the main analysis without losing sensitivity. The statistical inference based on the conjunction analysis, in contrast, was performed at P < 0.05, corrected for multiple comparisons.
In summary, the performed conjunction therefore tests for regions where 1) presentation of left-sided stimuli evoked significant activation, 2) both individual conditions (CL and ICR) showed a positive response, and 3) activation was greater than that for right-sided stimulus presentation.
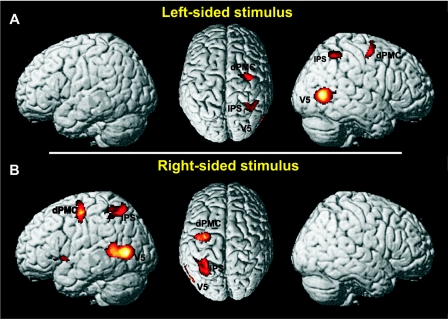
Stimulus-driven bottom-up processes for left lateralized stimuli were associated with contralateral activation in Area V5 (V5, 45, −69, 4), the premotor cortex (PMC) around the dPMC and FEF (Area 6, 36, −12, 52), and the IPS (hIP3, 32, −50, 46) as shown in Fig. 3A. Ipsilateral activation was observed in the cerebellum (−6, −52, −12).
FIG. 3.
A and B: the pattern of activity for left- and right-sided stimulus-driven bottom-up effects. Stimulus-driven bottom-up effects were found in V5, intraparietal sulcus (IPS), and premotor cortex (PMC) contralateral to the stimulus side. Moreover, right-sided stimuli evoked additional activation in anterior insula. All activations are shown on a P < 0.05 (cluster-level corrected) level.
The equivalent contrast for right stimulus-driven bottom-up processes—i.e., {(CR + ICL) ∩ [(CR + ICL) vs. 2 × CL], P < 0.05 cluster level corrected, inclusively masked with CR and ICL on a P < 0.05 uncorrected level}—revealed significant activation in contralateral V5 (V5, −50, −69, 6), the PMC around the dPMC and FEF (Area 6, −42, −14, 51) and the IPS (hIP3, −28, −52, 50) (Fig. 3B). Ipsilateral activation was found in the cerebellum (9, −56, −10). Moreover, we did find significant activation in contralateral anterior insula (−34, 2, −8).
Top-down processes
GENERALIZED TOP-DOWN EFFECTS INDEPENDENT OF DIRECTION.
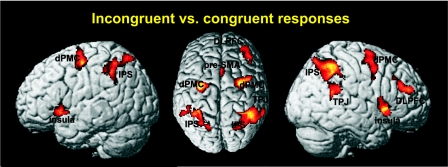
Incongruent responses required the subject to inhibit the reflexive tendency to use the congruent hand, reorient attention to the opposite side, and initiate the incongruent motor response. To investigate areas associated with the computational load required by these processes, we performed a conjunction analysis over the composite main effect of incongruent responses and the differential contrast between incongruent versus congruent responses, i.e., (ICL + ICR) ∩ (ICL + ICR vs. CL + CR), P < 0.05 cluster level corrected. This conjunction was motivated by our aim to identify those regions that were significantly activated by the performance of incongruent responses and, at the same time, were significantly more strongly activated under these conditions than during the performance of congruent responses. To ensure that both incongruent responses with both the left hand and the right hand contributed to this effect, we further masked this contrast inclusively with ICL and ICR on a P < 0.05 uncorrected level—i.e., only those regions where both conditions requiring incongruent responses showed a positive response (activity higher than that at resting baseline) were considered in the main analysis performed, including correction for multiple comparisons. Significantly higher activation for incongruent compared with congruent trials (Fig. 4) was found bilaterally in the anterior IPS (hIP3, 32, −50, 46 and −32, −45, 45), PMC around the dPMC and FEFs (Area 6, 26, −6, 50 and −30, −8, 52), and the anterior insula (52, 18, −4 and −36, 20, −2). Right lateralized activation was found in the dorsolateral prefrontal cortex (DLPFC) (38, 45, 22) and TPJ (Area PFm, 60, −44, 24). Finally, we observed activation in the pre-SMA extending into the cingulate motor cortex (Area 6, 3, 14, 48) and activation in the cerebellum (−4, −78, −32).
FIG. 4.
Incongruent vs. congruent responses evoked significant higher activation in a bilateral network consisting of IPS, PMC, and anterior insula as well as in the medial pre-SMA. Activations in the TPJ and dorsolateral prefrontal cortex (DLPFC) were lateralized to the right side. Congruent vs. incongruent responses did not yield any significant activation. All activations are shown on a P < 0.05 (cluster-level corrected) level.
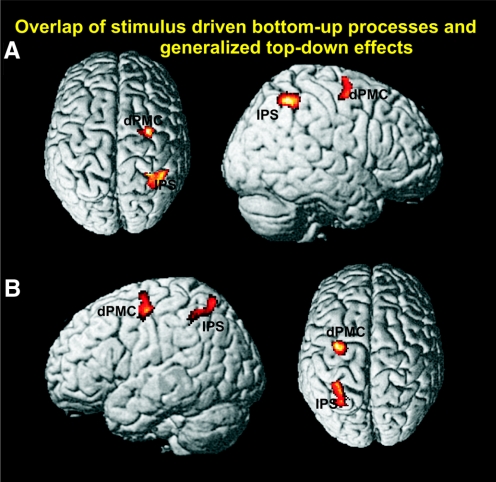
Brain regions associated not only with left stimulus-driven bottom-up effects but also with generalized top-down effects were identified by a conjunction analysis across the respective contrasts, i.e., {(CL + ICR) ∩ [(CL + ICR) vs. 2 × CR]} ∩ [(ICL + ICR) ∩ (ICL + ICR vs. CL + CR)], P < 0.05 cluster level corrected. This analysis revealed common involvement of right IPS (32, −50, 46) and right PMC (28, −4, 64) (Fig. 5A). The equivalent conjunction analysis across right-sided bottom-up effects and generalized top-down effects conversely revealed significant activation of the left IPS (−30, −50, 46) and PMC (−30, −8, 48) (Fig. 5B).
FIG. 5.
Conjunction analysis of left-sided stimulus-driven bottom-up processes and generalized top-down effects revealed common involvement of the right IPS and right dPMC (A). Equivalently, conjunction analysis of right-sided stimulus-driven bottom-up processes and generalized top-down effects showed common activation of the left IPS and dPMC (B). All activations are shown on a P < 0.05 (cluster-level corrected) level.
DIRECTIONALLY SPECIFIC EFFECTS.
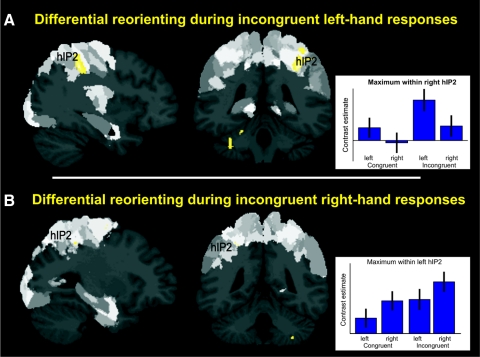
We also investigated which brain regions were associated with the differential (i.e., side-specific) reorienting of attention in the incongruent condition. In the ICL condition, subjects had to endogenously reorient from the right stimulus side to the opposite side to be able to respond incongruently with the left hand. To isolate brain regions that were specifically associated with the reorienting of attention from the right side to the left side, we used the following approach. We performed a conjunction analysis over incongruent left-hand responses and the contrasts between incongruent left-hand responses and each other experimental condition [ICL ∩ (ICL vs. CL) ∩ (ICL vs. CR) ∩ (ICL vs. ICR)], P < 0,05 cluster level corrected. That is, we aimed at identifying brain regions not only significantly activated (relative to baseline) by endogenous reorientation to the left side but also significantly stronger in that condition as in any other. Because the reorienting from the right side to the left side was the only thing that was specific for the ICL condition compared with the three other conditions, this rather conservative contrast thus allows isolation of directionally specific top-down processes.
As shown in Fig. 6A this analysis revealed significant activation in the junction between right postcentral sulcus and IPS (hIP2, 36, −40, 42) and in the left cerebellum (−15, −46, −16).
FIG. 6.
A: the most anterior part of the right IPS (hIP2) was associated with differential reorienting from right to left side during incongruent left-hand responses. Activations are shown on a P < 0.05 (cluster-level corrected) level. Hypothesized activation in corresponding left anterior IPS for differential reorienting from left to right side was revealed by a small volume correction. For better visualization of activation in left anterior IPS, B shows activation on an uncorrected level (P < 0.005). Regions of the brain that are dark gray represent regions that, until now, have not been mapped using observer-independent cytoarchitectonic analysis (Schleicher et al. 2005).
We also performed the equivalent contrast for differential reorienting of attention from the left to the right side during incongruent right hand responses (Fig. 6B). This contrast revealed the hypothesized activation in the corresponding left IPS (hIP2, −28, −45, 48). This activation was significant when correcting for multiple comparisons in a spherical VOI (8 mm radius) based on the IPS coordinates reported in Vogt et al. (2007).
Dissociation of bottom-up and top-down processes in IPS
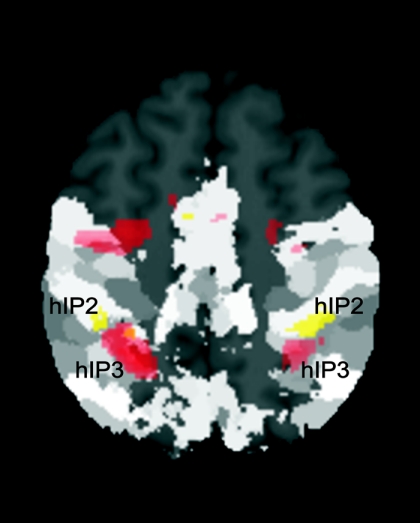
To further dissociate stimulus-driven bottom-up processes for left- and right-sided stimuli and top-down modulated directionality-specific reorienting of attention during incongruent responses, we overlayed these contrasts and compared them with the histological organization of the IPS (Choi et al. 2006; Scheperjans et al. 2008a,b). As shown in Fig. 7 this analysis revealed a functional and anatomical separation of bottom-up and top-down modulated reorienting in the IPS. Whereas bottom-up effects evoked activation in the middle part of the contralateral IPS (hIP3), top-down-driven reorienting of attention during incongruent responses was associated with activation in a more anterior part of the IPS (hIP2).
fig. 7.
Overlay of stimulus-driven bottom-up processes for left- and right-sided stimuli (red) and top-down modulated differential reorienting for left and right incongruent responses (yellow) with the Anatomy toolbox revealed an anatomical dissociation of these processes. Stimulus-driven orienting toward stimulus side was associated with contralateral activation in medial posterior parts of the IPS (hIP3). In contrast, top-down modulated differential reorienting during incongruent left- and right-hand responses revealed activation in the most anterior part of the IPS (hIP2). Activations are shown on a P < 0.05 (cluster-level corrected) level. However, for better visualization of activation in left anterior IPS, differential reorienting during incongruent right-hand responses is shown on an uncorrected level (P < 0.005). Regions of the brain that are dark gray represent regions that, until now, have not been mapped using observer-independent cytoarchitectonic analysis (Schleicher et al. 2005).
DISCUSSION
We used fMRI to investigate bottom-up driven and top-down modulated attentional reorienting in a spatial SRC paradigm. Both processes evoked activation in a dorsal frontoparietal network, especially the dPMC and the IPS. In the latter region, overlapping as well as (functionally and anatomically) dissociated effects were observed. Incongruent responses were additionally associated with significantly higher activation in a more ventral network consisting of bilateral anterior insula, right TPJ and DLPFC as well as increased activation in the pre-SMA.
Behavioral data
The observed reaction-time difference between congruent and incongruent responses is in accordance with previous data on similar paradigms. Most studies reported an increase in RTs of 40 to 80 ms when subjects had to react incongruently to a lateralized visual stimulus (Iacoboni et al. 1996; Matsumoto et al. 2004; Proctor and Reeve 1990). These differences are deemed to reflect the extra computational load associated with the inhibition of the reflexive answer and the generation of the appropriate incongruent response.
Further assessment of individual differences in reaction times revealed that congruent and incongruent RTs were significantly correlated. Moreover, subjects who responded faster in the congruent condition also showed significantly smaller incongruency costs. These results highlight the influence that subject-specific factors—i.e., personal traits such as the overall visuomotor processing speed—have on the performance of the SCR task in addition to the omnipresent incongruency effect.
Imaging data
GENERAL TASK NETWORK.
The general task network refers to areas consistently activated throughout all conditions, i.e., the bilateral dPMC, the medial SMA, the right TPJ, as well as the cerebellum and the right nucleus caudatus (Fig. 2). These regions thus seemed to be associated with the general cognitive demands of this task, i.e., not only target detection but also planning and initiating of a context-dependent motor response with the respective hand.
This general task network highly parallels activations found in another study of our group in which subjects were instructed to respond to arrows with the corresponding hand while the direction and timing of the arrows were varied independently (Jakobs et al. 2009). The dPMC is known to play a crucial role in linking spatial encoding of targets with movement plans and implementation of stimulus response mapping in the context of reactive behavior (Chouinard and Paus 2006; Chouinard et al. 2005; Halsband and Passingham 1985). In a comparison of the present findings with previous reports on pro-/antisaccade paradigms, it appears that the dPMC activations observed in our experiment might include parts of what is usually referred to as the FEFs. These regions are considered to be key structures for voluntary control of saccadic eye movements (Connolly et al. 2002; Cornelissen et al. 2002) in tasks similar to the manual paradigm used here. Moreover, they have also been shown to be activated by visually guided pointing to the extent that an experiment trying to separate activations for saccade- and hand-specific antimovements in healthy subjects showed high overlap in prefrontal and parietal regions (Connolly et al. 2000). The question thus arises how specific the systems in the posterior part of the superior frontal sulcus and adjacent precentral gyrus are for the control of hand versus eye movements or whether they may be part of a generalized action-based spatial orientation system.
The SMA, on the other hand, has been known to be involved in the initiation of internally as well as externally triggered movements (Nachev et al. 2008), exerting context-specific influence on the motor cortices (Grefkes et al. 2008). Finally, the nucleus caudatus and more generally the basal ganglia are constituents of cortical–subcortical loops for movement preparation and action selection (Jueptner and Krukenberg 2001; Middleton and Strick 2000).
We therefore propose that these regions are involved in the planning and preparation of the accurate motor response. This interpretation is well in line with a study trying to separate the volitional generation component from inhibitory processes in the antisaccade task (Reuter et al. 2010). These authors found the FEFs and supplementary eye field (SEF) to be specifically involved in the preparation component of volitional saccades generation, suggesting that the neural components for generation and inhibition of saccades are independent to a large extent.
In contrast to these action-related regions, the temporoparietal junction has been proposed to be part of a right-lateralized ventral attention network (Corbetta and Shulman 2002; Corbetta et al. 2008) specialized toward the detection of behaviorally relevant stimuli (Himmelbach et al. 2006; Jakobs et al. 2009; Karnath and Dieterich 2006; Macaluso et al. 2002). Increased activity in the right TPJ has been observed, independently of the sensory modality, when subjects have to break their current attentional set to reorient to task-relevant stimuli (Astafiev et al. 2006; Corbetta and Shulman 2002; Corbetta et al. 2000, 2005; Kincade et al. 2005). The TPJ may thus be regarded as a computational hub integrating both sensory bottom-up information and top-down-mediated task demands (Husain and Nachev 2007; Jakobs et al. 2009; Kilner et al. 2007).
When looking for regions associated with the actual motor output—that is, side-specific motor responses—we found increased activation in regions related to movement execution, such as SMA, M1, and the basal ganglia contralateral to the respective respond hand (Supplemental Fig. S1).1 Moreover, ipsilateral activation was found in the cerebellum.
BOTTOM-UP PROCESSES.
Stimulus-driven bottom-up processing of the peripheral cue evoked activation in the visual cortex (including Area V5), the intraparietal sulcus (IPS), and the premotor cortex (PMC) around the dPMC and FEF (dPMC/FEF) contralateral to the stimulus side.
Area V5 is a motion-sensitive region (Corbetta et al. 1991; Malikovic et al. 2007; Zeki et al. 1991) known to respond to fast changes in the visual field such as the briefly presented stimuli used here. Activation in this region should thus reflect sensory processing and, in particular, spatial identification of the visual cue.
The IPS and the dPMC/FEF have consistently been associated with overt and covert attentional reorienting (Nobre 2001) and conceptualized to form a dorsal frontoparietal attention network (Corbetta and Shulman 2002; Corbetta et al. 2008). Among these, the dPMC/FEF is considered to reflect motor attention, i.e., the automatic preparation of motor responses to an attended location in space (Cisek and Kalaska 2005; Jackson and Husain 1996; Rosen et al. 1999). In contrast to the more action-related role of the PMC, the posterior parietal cortex and, in particular, the IPS is involved in coding space and in directing spatial attention (Bremmer et al. 2001; Colby and Goldberg 1999; Corbetta and Shulman 2002; Halligan et al. 2003; Nobre 2001). Here, we found the significant contralateral IPS activation for stimulus-driven bottom-up processes to be located in area hIP3, the human homologue of the monkey medial intraparietal area (MIP), which receives input from extrastriate visual areas (Cavada 2001; Felleman and Van Essen 1991) and is also directly connected with the dPMC (Colby and Duhamel 1991; Galletti et al. 1996; Rizzolatti et al. 1998). Because this region thus lies in a unique position for linking sensory input with motor responses, it has been proposed that MIP contributes to the construction of spatial target representations that can be used by the dPMC/FEF for computing a respective movement vector (Cohen and Andersen 2002).
We therefore propose the following mechanism for the behavioral advantage when reacting to a congruently lateralized visual stimulus. Spatial properties (such as location) of the cue are first captured by activation of the contralateral visual cortex, in particular V5. This information is then forwarded in a bottom-up driven fashion to the IPS (hIP3) on the same hemisphere, resulting in an automated reorientation of spatial attention toward the side of the cue. Based on the connections of the IPS (hIP3) with the PMC, the latter region is also activated in a feedforward manner. Hereby preparation and initiation of motor response to the side of the cue should be facilitated, resulting in shorter reaction times.
A somehow unexpected finding might be the extreme lateralization of the bottom-up effects observed in our experiment. Despite the well-established contralateral bias in the voluminous nonhuman primate literature most neuroimaging studies in humans reported only weak lateralization effects for saccades as well as visually guided pointing (Connolly et al. 2005, 2007; Curtis and Connolly 2008). Although it is difficult to explain why other fMRI studies did not observe this well-documented effect in the nonhuman primate literature, our experimental design might have had a few advantages that allowed us to find lateralized bottom-up effects with fMRI. First, we used a very simple paradigm consisting of only four conditions with a long scanning time, allowing us to present 126 individual events for each condition. Since we additionally used a relatively high number of subjects (24) compared with previous fMRI studies, we would speculate that potentially the statistical power was higher in the present experiment. Moreover, when looking for left-side-specific bottom-up processes, we contrasted neural responses to left-sided stimuli independent of the motor response with congruent responses for right-sided stimuli (and vice versa). Thereby, weak activation in ipsilateral dPMC may have been eliminated due to subtracting with congruent responses for the other side.
TOP-DOWN PROCESSES.
Generalized top-down effects independent of direction. The incongruent condition requires inhibition of the automatic tendency to react toward the target stimulus (based on the bottom-up-driven processes outlined earlier) as well as a top-down-modulated spatial reorienting toward the opposite side and an initiation of the response with the contralateral hand. These processes were associated with increased activity bilaterally in IPS, dPMC/FEF and anterior insula, the medial pre-SMA, and right-lateralized activations in the temporoparietal junction (TPJ) and dorsolateral prefrontal cortex (DLPFC). We would thus conclude that these regions form key nodes of the response inhibition and reorientation network. Our experimental design, however, does not allow a clear differentiation between these different subprocesses that take place in the incongruent condition. Thus we will take the indirect approach of comparing our activations with those reported in previous experiments focusing on the neural substrates of these individual processes. Hereby, we will cautiously give some possible suggestions about the functional roles of each region and potential interactions among them.
Our results are in good agreement with comparable studies investigating spatial response selection for hand movements. In most of these studies, stimulus–response compatibility was varied across blocks by requiring participants to make either a compatible or incompatible keypress to the cued location (e.g., Iacoboni et al. 1996). Moreover, some of them varied the difficulty of response selection by manipulating the number of possible stimulus locations (Dassonville et al. 2001; Schumacher et al. 2003, 2007). These studies have shown consistent activation in the frontoparietal network when contrasting incongruent with congruent conditions. In particular, the parietal cortex (superior parietal cortex extending into the IPS) has been proposed to be associated with the coordination of the visual information, whereas the dPMC has been proposed to take part in the response-selection process of the accurate motor response (Nee et al. 2007; Schumacher et al. 2003).
In our experiment, we found substantial overlap between activations in the dorsal frontoparietal network associated with stimulus-evoked activity (bottom-up effects) and incongruent responding to stimuli independently of the cue localization (generalized top-down effects) (Fig. 5). This indicates that within the IPS and the dPMC the same neuronal populations that are driven by automated bottom-up processes also increase their activation when the demand on spatial processing (as in the incongruent condition) increases. Further evidence for this interpretation comes from visual search tasks in monkeys showing that neurons in the lateral intraparietal area and FEF do not only code for sensory bottom-up processes but are also modulated by top-down signals that are related to visual expectancy or goals (Colby et al. 1996; Thompson et al. 1997; Wolfe 1994). Our study thus provides further support that the IPS and dPMC are fundamentally involved in processing spatial features of stimuli and responses in SRC tasks (Beurze et al. 2007; Schumacher et al. 2003, 2007). Depending on the task condition and the modulating influence of, e.g., the TPJ or DLPFC, the IPS and the dPMC might map visual stimuli with an accurate motor response in either an automatic (congruent) or top-down-modulated (incongruent) fashion, as already suggested by Corbetta et al. (2008).
Beside activation in this attention network, bilateral anterior insula, right DLPFC, and medial pre-SMA were specifically associated with the increased cognitive control required in the incongruent condition.
A key component in performing externally structured tasks—like those presented in virtually every neuroimaging experiment—is the ability to adopt what is termed “task sets,” containing sensory processing pathways, cognitive categorizations, stimulus–response associations, and motor outputs, i.e., the knowledge of “what to do” (Meiran 1996). The anterior insula has recently been proposed to be a central part of the human brain network responsible for the initiation and maintenance of such goal-directed task sets (Dosenbach et al. 2006). This view also aligns well with the hypothesis of Corbetta and collegues (2008) that the anterior insula may represent the source of top-down signals restricting activation of, e.g., the TPJ to behaviorally (in the light of the current task set) relevant stimuli. We would therefore propose that observed activation of the anterior insula reflects the more complex instruction, and thus task set, to be maintained in the incongruent-response task. The insula may then act as a modulator to other regions like the DLPFC and the TPJ sustaining specific processes in the execution of this task set.
The (right) dorsolateral prefrontal cortex on the other hand has repeatedly been proposed to play an important role in monitoring and voluntary controlling of the motor system (Shallice 2004; Vogt et al. 2007). Supporting this view, activation of the dorsolateral prefrontal cortex has also been consistently reported in SRC-related paradigms, in particular during antisaccade generation (Desouza et al. 2003; Ettinger et al. 2005, 2008; Ford et al. 2005; McDowell et al. 2002). In this context, the DLPFC has been proposed to be associated with the successful suppression of the stimulus-driven automatic reaction to shift attention and fixation in the opposite direction. The involvement of the right DLPFC in action inhibition, however, is not limited to tasks requiring a spatial reorientation. In a recent study investigating a stop-signal and a go/no-go task within the same subjects, the right DLPFC was demonstrated to be the only region commonly activated in both tasks and, furthermore, showed a correlation with behavioral performance (Zheng et al. 2008). We would thus propose that the observed activation of the DLPFC reflects the inhibition of the stimulus-driven tendency to react with the ipsilateral hand in favor of the required, voluntarily executed, contralateral response.
Likewise, the pre-SMA has also been proposed to play an important role in the executive control of motor behavior (Cunnington et al. 2002, 2005). This includes inhibiting responses, switching between task rules, or linking stimuli to changing responses (Cunnington et al. 2003; Garavan et al. 2003; Nachev et al. 2007, 2008; Paus 2001; Rushworth et al. 2002; Simmonds et al. 2008; Swainson et al. 2003; Wager et al. 2004). In line with this view, recent electropysiological recordings in nonhuman primates showed selectivity of pre-SMA activity when the animal had to switch from an automatic to a controlled response (Isoda and Hikosaka 2007). For interpretation of the current data, we would follow this view rendering the pre-SMA the effector of the inhibitory control established by the DPLFC.
A remarkable concordance may be noted in the activation patterns related to SRC and antisaccade paradigms, which underlines the close resemblance of the neurophysiological processes underlying the bottom-up and top-down control of hand and eye movements (Astafiev et al. 2003; Connolly et al. 2000). In particular, there is a large body of human neuroimaging literature and electrophysiological recordings in nonhuman primates demonstrating the engagement of a dorsal frontoparietal attention network (IPS and FEF/dPMC) in the initiation of antisaccadic incongruent actions (Munoz and Everling 2004; Pierrot-Deseilligny et al. 2001). In this context, the IPS, especially the lateral intraparietal area (LIP), has been proposed to form the interface between sensory and motor processing, whereas the FEFs have a crucial role in the performing of voluntary saccades (Dias and Segraves 1999; Gaymard et al. 1999; Rivaud et al. 1994). Given the current knowledge on the neuronal correlates of voluntary hand actions (Chouinard and Paus 2003; Wise et al. 1997), we would interpret the observed activations in the IPS and the dPMC in the same fashion. Furthermore, the DLPFC has been assumed to support the suppression of automatic, reflexive responses, whereas the SEF may play a key role in implementing control when there is conflict between several competing saccadic responses (Coe et al. 2002 Stuphorn et al. 2000). As detailed earlier, the DLPFC may hold a similar role in the control of lateralized hand movements, whereas the pre-SMA should be equivalent in function to the SEF.
Directionally specific effects.
Directionality-specific reorientation of attention in the incongruent condition evoked lateralized activation in the most anterior part of the IPS (hIP2). Comparison with activation for stimulus-driven bottom-up effects and cytoarchitectonic probability maps then revealed an anatomic dissociation between both processes (Fig. 7). This distinction is in line with the current view of the primate IPS, where its posterior parts receive projections from several visual areas and are associated with visuospatial information processing (Bisley and Goldberg 2003; Blatt et al. 1990). They are therefore postulated to sustain overt and covert shifts of visuospatial attention (Nobre 2001). In contrast, its more anterior parts project mainly to premotor areas and are associated with sensorimotor processing (Matelli et al. 1986; Murata et al. 2000; Rizzolatti et al. 1998). Consequently, more anterior regions within the IPS are conceptualized to be more closely linked to action planning and are therefore involved in the reorienting of motor attention (Rushworth et al. 2003).
Our observation of bottom-up-driven activation of the posterior IPS, i.e., area hIP3, and top-down modulation of the more anterior area hIP2 thus corresponds to the distinction between visuospatial and motor-related dominance of, respectively, the posterior and anterior IPS.
Conclusions
We used fMRI to investgate stimulus-driven bottom-up and task-dependent top-down modulation of attentional reorienting in a spatial SRC task.
We propose that stimulus-driven activation of contralateral IPS (hIP3) and, consecutively, the dPMC should represent key neuronal substrates for the behavioral advantage observed when subjects had to react toward a congruently lateralized stimulus. In contrast, incongruent responses involved inhibition of this stimulus-associated priming and executing the appropriate (contralateral) motor response. These processes are sustained by the anterior insula (task-set maintenance), the right DPLFC (inhibition), the TPJ (integration of task-set and sensory input), and the pre-SMA (volitional initiation of movement). Overlap of activation associated with stimulus-evoked activity independent of the task set and incongruent responding to stimuli independently of the cue localization in the IPS and the dPMC highlight the role of these two regions in integrating information about spatial stimuli with an appropriate motor response.
Additionally, in line with the current view of a functional differentiation of the IPS, bottom-up and top-down directionality-specific reorienting could be anatomically and functionally dissociated in the IPS. Whereas stimulus-driven orienting of visuospatial attention evoked activation in area hIP3 of the IPS, task-dependent differential reorienting of motor attention resulted in activation of the most anterior part of the IPS (hIP2).
GRANTS
This work was supported by a grant from the Human Brain Project/Neuroinformatics Research (National Institute of Biomedical Imaging and Bioengineering, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health [NIMH]) to K. Zilles; Human Brain Project/NIMH Grant R01-MH-074457-01A1 to S. B. Eickhoff; and a grant from the Helmholtz Initiative on Systems Biology to K. Zilles, S. B. Eickhoff, and E. C. Cieslik.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage 26: 839–851, 2005. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. Eur J Neurosci 23: 591–596, 2003. [DOI] [PubMed] [Google Scholar]
- Beurze SM, de Lange FP, Toni I, Medendorp WP. Integration of target and effector information in the human brain during reach planning. J Neurophysiol 97: 188–199, 2007. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science 299: 81–86, 2003. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol 299: 421–445, 1990. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Duhamel JR, Graf W, Fink GR. Space coding in primate posterior parietal cortex. NeuroImage 14: S46–S51, 2001. [DOI] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia 39: 742–756, 2001. [DOI] [PubMed] [Google Scholar]
- Cavada C. The visual parietal areas in the macaque monkey: current structural knowledge and ignorance. NeuroImage 14: S21–S26, 2001. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495: 53–69, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci 25: 2277–2284, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist 12: 143–152, 2003. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron 45: 801–814, 2005. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci 22: 5081–5090, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci 3: 553–562, 2002. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia 29: 517–537, 1991. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol 76: 2841–2852, 1996. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349, 1999. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Cant JS, Munoz DP. Effector-specific fields for motor preparation in the human frontal cortex. NeuroImage 34: 1209–1219, 2007. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, DeSouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol 84: 1645–1655, 2000. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol 94: 605–611, 2005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297, 2000. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. Selective attention modulates extrastriate visual regions in humans during visual feature discrimination and recognition. Ciba Found Symp 163: 165–175, 1991. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215, 2002. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Tansy AP, Stanley CM, Astafiev SV, Snyder AZ, Shulman GL. A functional MRI study of preparatory signals for spatial location and objects. Neuropsychologia 43: 2041–2056, 2005. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Kimmig H, Schira M, Rutschmann RM, Maguire RP, Broerse A, den Boer JA, Greenlee MW. Event-related fMRI responses in the human frontal eye fields in a randomized pro- and antisaccade task. Exp Brain Res 145: 270–274, 2002. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage 15: 373–385, 2002. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. NeuroImage 20: 404–412, 2003. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E. Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum Mov Sci 24: 644–656, 2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol 99: 133–145, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. NeuroImage 13: 1–14, 2001. [DOI] [PubMed] [Google Scholar]
- Desouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related fMRI. J Neurophysiol 89: 1016–1023, 2003. [DOI] [PubMed] [Google Scholar]
- Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory guided saccades. J Neurophysiol 81: 2191–2214, 1999. [DOI] [PubMed] [Google Scholar]
- Dickman SJ, Meyer DE. Impulsivity and speed–accuracy tradeoffs in information processing. J Pers Soc Psychol 54: 274–290, 1988. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron 50: 799–812, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36: 511–521, 2007. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25: 1325–1335, 2005. [DOI] [PubMed] [Google Scholar]
- Eimer E, Hommel B, Prinz W. S-R compatibility and response selection. Acta Psychologica 90: 301–313, 1995. [Google Scholar]
- Ettinger U, Antonova E, Crawford TJ, Mitterschiffthaler MT, Goswani S, Sharma T, Kumari V. Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. NeuroImage 24: 487–494, 2005. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related fMRI. Cereb Cortex 18: 1148–1159, 2008. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Seeger CM. S-R compatibility: spatial characteristics of stimulus and response codes. J Exp Psychol 46: 199–210, 1953. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J Neurophysiol 94: 429–440, 2005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage 25: 661–667, 2005. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Battaglini PP, Shipp S, Zeki S. Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the macaque monkey. Eur J Neurosci 8: 30–52, 1996. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage 20: 1132–1139, 2003. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res 129: 288–301, 1999. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage 41: 1382–1394, 2008. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res 18: 1279–1296, 1978. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Fink GR, Marshall JC, Vallar G. Spatial cognition: evidence from visual neglect. Trends Cogn Sci 7: 125–133, 2003. [DOI] [PubMed] [Google Scholar]
- Halsband U, Passingham RE. Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis). Behav Brain Res 18: 269–277, 1985. [DOI] [PubMed] [Google Scholar]
- Himmelbach M, Erb M, Karnath HO. Exploring the visual world: the neural substrate of spatial orienting. NeuroImage 32: 1747–1759, 2006. [DOI] [PubMed] [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci 11: 30–36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Brain–behavior relationships: evidence from practice effects in spatial stimulus–response compatibility. J Neurophysiol 76: 321–331, 1996. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Bimodal (auditory and visual) left frontoparietal circuitry for sensorimotor integration and sensorimotor learning. Brain 121: 2135–2143, 1998. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10: 240–248, 2007. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Husain M. Visuomotor functions of the lateral pre-motor cortex. Curr Opin Neurobiol 6: 788–795, 1996. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. NeuroImage 47: 667–677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Krukenberg M. Motor system: cortex, basal ganglia, and cerebellum. Neuroimaging Clin N Am 11: 203–219, 2001. [PubMed] [Google Scholar]
- Karnath HO, Dieterich M. Spatial neglect: a vestibular disorder? Brain 129: 293–305, 2006. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Glaser DE, Friston KJ. A heuristic for the degrees of freedom of statistics based on multiple variance parameters. NeuroImage 20: 591–600, 2003. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cogn Process 8: 159–166, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci 25: 4593–4604, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus–response compatibility: a model and taxonomy. Psychol Rev 97: 253–270, 1990. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J. Supramodal effects of covert spatial orienting triggered by visual or tactile events. J Cogn Neurosci 14: 389–401, 2002. [DOI] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex 17: 562–574, 2007. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol 251: 281–298, 1986. [DOI] [PubMed] [Google Scholar]
- Matsumoto E, Misaki M, Miyauchi S. Neural mechanisms of spatial stimulus-response compatibility: the effect of crossed-hand position. Exp Brain Res 158: 9–17, 2004. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, Braff DL. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry 51: 216–223, 2002. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. J Exp Psychol Hum Learn 22: 1423–1442, 1996. [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 31: 236–250, 2000. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228, 2004. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601, 2000. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage 36, Suppl. 2: T155–T163, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci 7: 1–17, 2007. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JP. Valid conjunction inference with the minimum statistic. NeuroImage 25: 653–660, 2005. [DOI] [PubMed] [Google Scholar]
- Nobre AC. The attentive homunculus: now you see it, now you don't. Neurosci Biobehav Rev 25: 477–496, 2001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424, 2001. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain 114: 1473–1485, 1991. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Reeve T. Stimulus– Response Compatibility: An Integrated Perspective Amsterdam: Elsevier, 1990. [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry 59: 313–320, 2002. [DOI] [PubMed] [Google Scholar]
- Reuter B, Kaufmann C, Bender J, Pinkpank T. Kathmann N Distinct neural correlates for volitional generation and inhibition of saccades. J Cogn Neurosci 22: 728–738, 2010. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res 102: 110–120, 1994. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296, 1998. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci 11: 135–152, 1999. [DOI] [PubMed] [Google Scholar]
- Rubichi S, Nicoletti R, Iani C, Umiltà C. The Simon effect occurs relative to the direction of an attention shift. J Exp Psychol Hum Percept Perform 23: 1353–1364, 1997. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592, 2002. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. NeuroImage 20, Suppl. 1: S89–S100, 2003. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18: 2141–2157, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18: 846–867, 2008b. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Cole MW, D'Esposito M. Selection and maintenance of stimulus-response rules during preparation and performance of a spatial choice-reaction task. Brain Res 1136: 77–87, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, D'Esposito M. Neural implementation of response selection in humans as revealed by localized effects of stimulus-response compatibility on brain activation. Hum Brain Mapp 17: 193–201, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M. Neural evidence for representation-specific response selection. J Cogn Neurosci 15: 1111–1121, 2003. [DOI] [PubMed] [Google Scholar]
- Shallice T. The fractionation of supervisory control. In: The Cognitive Neurosciences (3rd ed.), edited by Gazzaniga MS. Cambridge, MA: MIT Press, 2004, p. 943–956 [Google Scholar]
- Sheliga BM, Craighero L, Riggio L, Rizzolatti G. Effects of spatial attention on directional manual and ocular responses. Exp Brain Res 114: 339–351, 1997. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46: 224–232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2002. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci 15: 785–799, 2003. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol 77: 1046–1050, 1997. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund HJ, Rizzolatti G, Fink GR. Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. NeuroImage 37: 1371–1383, 2007. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage 22: 1679–1693, 2004. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premoter and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. [DOI] [PubMed] [Google Scholar]
- Wolfe J. Guided search 2.0. A revised model of visual search. Psychon Bull Rev 1: 202–228, 1994. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci 11: 641–649, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Oka T, Bokura H, Yamaguchi S. The key locus of common response inhibition network for no-go and stop signals. J Cogn Neurosci 20: 1434–1442, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.