Abstract
To fire at high rates, neurons express ionic currents that work together to minimize refractory periods by ensuring that sodium channels are available for activation shortly after each action potential. Vestibular nucleus neurons operate around high baseline firing rates and encode information with bidirectional modulation of firing rates up to several hundred Hz. To determine the mechanisms that enable these neurons to sustain firing at high rates, ionic currents were measured during firing by using the action potential clamp technique in vestibular nucleus neurons acutely dissociated from transgenic mice. Although neurons from the YFP-16 line fire at rates higher than those from the GIN line, both classes of neurons express Kv3 and BK currents as well as both transient and resurgent Na currents. In the fastest firing neurons, Kv3 currents dominated repolarization at all firing rates and minimized Na channel inactivation by rapidly transitioning Na channels from the open to the closed state. In slower firing neurons, BK currents dominated repolarization at the highest firing rates and sodium channel availability was protected by a resurgent blocking mechanism. Quantitative differences in Kv3 current density across neurons and qualitative differences in immunohistochemically detected expression of Kv3 subunits could account for the difference in firing range within and across cell classes. These results demonstrate how divergent firing properties of two neuronal populations arise through the interplay of at least three ionic currents.
INTRODUCTION
Many neurons in vertebrate species operate around high baseline firing rates and encode information via modulations of firing rate, including neurons in cerebellar, vestibular, auditory, and oculomotor circuits as well as fast-firing cortical and basal ganglia neurons. Mammalian vestibular nucleus neurons, for example, fire spontaneously in the awake animal at baseline firing rates of about 30–100 Hz and can increase firing rates up to several hundred Hz (Beraneck and Cullen 2007; Buettner et al. 1978; Cheron et al. 1996; Cullen and McCrea 1993; Fuchs and Kimm 1975; Newlands et al. 2009; Ris et al.1995). To sustain high firing rates, neurons must overcome a critical challenge presented by the biophysical properties of sodium channels: depolarization during the action potential inactivates sodium channels, which then require tens to hundreds of milliseconds to become available for subsequent action potentials. How do neurons maintain the ability to fire at high rates despite sodium channel inactivation?
Two predominant types of ionic currents are thought to be particularly important for conferring the ability to fire fast: sodium currents with resurgent kinetics (Khaliq et al. 2003; Mercer et al. 2007; Raman and Bean 2001) and the Kv3 family of potassium currents (Erisir et al. 1999; Lien and Jonas 2003; McKay and Turner 2004; Wang et al. 1998). In neurons expressing resurgent sodium current, a subset of sodium channels are prevented from inactivation via a blocking particle that competes with the inactivation gate (Grieco et al. 2005). Kv3 currents are activated rapidly at relatively low membrane potentials, making them well suited for mediating rapid repolarization of action potentials (Rudy and McBain 2001).
Although all vestibular nucleus neurons express both resurgent Na current and Kv3 currents (Gittis and du Lac 2007, 2008), the ability to fire fast varies considerably both within and across cell types (Bagnall et al. 2007; Sekirnjak and du Lac 2002) . The fastest vestibular nucleus neurons can sustain firing rates of several hundred Hz, express markers for the neurotransmitters glycine or glutamate, and are labeled in the YFP-16 line of transgenic mice (Bagnall et al. 2007). In contrast, GABAergic neurons labeled in the GIN mouse line fire more slowly in response to maximal depolarization, attaining firing rates that typically saturate between 100 and 200 Hz. Although the ionic currents expressed in YFP-16 and GIN neurons are qualitatively similar (Gittis and du Lac 2007, 2008), quantitative differences in K-channel expression levels raise the possibility that the mechanisms engaged during action potential firing differ across these broad neuronal classes.
The goals of this study were to identify the ionic currents that enable vestibular nucleus neurons to maintain firing at high rates and to determine whether the mechanisms that protect sodium channels from inactivation vary across cell types. To examine currents activated during firing, we used the action potential clamp technique, in which each neuron's own action potentials are used as voltage-clamp stimuli. To ensure adequate voltage-clamp control of identified cell types, recordings were targeted to mature, fluorescently labeled neurons acutely dissociated from GIN and YFP-16 mouse lines. The results indicate that resurgent Na, Kv3, and BK currents contribute differentially to repetitive action potential firing across firing rates and cell types.
METHODS
Animals
Experimental protocols for this study were approved by the Salk Institute Animal Care and Use Committee. Prior to obtaining brain tissue, used for dissociated cell preparations, mice aged 24–40 days (avg = 30 days) were anesthetized deeply with Nembutal interperitoneally and then decapitated.
Cell preparation
Using methods described in Sekirnjak and du Lac (2006), 350 μM coronal slices through the rostral two thirds of the medial vestibular nucleus (MVN) were prepared with a DSK-1500E or Leica VT1000S Vibratome in 4°C carbogenated artificial cerebrospinal fluid (ACSF) containing (in mM): 125 NaCl, 26 NaCHO3, 5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, and 11 glucose. Slices were warmed for 10–30 min at 34°C then maintained at room temperature. Neurons were enzymatically dissociated, as described in Gittis and du Lac (2007), from 24- to 40-day-old mice, either GIN (Oliva Jr et al. 2000) or YFP-16 (Feng et al. 2000) neurons, both in c57bl6 backgrounds. Slices were treated with 40 U/mL papain (Worthington) in 9.4 mg/mL MEM powder (Gibco), 10 mM Hepes, and 0.2 mM cysteine, for 10 min at 30°C. The bilateral vestibular nuclei were removed from a slice, triturated with fire-polished Pasteur pipettes, and dissociated neurons were plated on the glass slide of the recording chamber.
Electrophysiological recording
For the duration of a recording session (2–3 h), neurons were continuously perfused with oxygenated Tyrode's solution (in mM: 150 NaCl, 3.5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, and 10 glucose) and all recordings were done at room temperature. Whole cell recordings were made with borosilicate pipettes (2–4 MΩ), filled with a K-gluconate-based intracellular solution (in mM: 140 K-gluconate, 8 NaCl, 10 Hepes, 0.02 EGTA, 2 Mg-ATP, 0.3 Na2-GTP, and 14 Tris-creatine PO4). The measured liquid junction potential was +15 mV and was corrected off-line.
Data were collected and analyzed using software written in IGOR with a MultiClamp 700B amplifier (Axon Instruments) and an ITC-16 interface (InstruTech). On establishment of the whole cell configuration, neurons exhibited regular, spontaneous action potentials and average membrane potentials of −65.8 ± 3.4 mV in GIN (n = 42) and −66.6 ± 2.2 mV in YFP-16 neurons (n = 47; P = 0.23) . Action potentials were recorded in current-clamp mode, filtered at 10 kHz, and digitized at 40 kHz. To evoke action potentials at different firing rates, DC current was injected at the soma for 1 s, every 5 s with increasing intensities. Data were collected in 3 s epochs, consisting of 1 s of spontaneous firing, 1 s of firing in response to DC current injection, and 1 s of recovery to spontaneous firing rates. The amplitude of injected DC current was typically increased in 40 pA intervals until neurons could not sustain firing over the 1 s step, usually about 200–300 pA.
In voltage-clamp mode, ionic currents were filtered at 8 kHz and digitized at 40 kHz. Whole cell capacitance was compensated through the amplifier circuitry and series resistance was compensated at 70–90%. The average uncompensated series resistance was 2.4 ± 1.3 MΩ in GIN neurons (n = 34) and 2.3 ± 1.2 MΩ in YFP-16 neurons ( n = 26; P = 0.66). The capacitance was measured through the amplifier or by integrating the area of the transient following a step from −65 to −75 mV with whole cell capacitance and series resistance compensation turned off. Average cell capacitance was 6.3 ± 1.6 pF for GIN neurons (n = 34) and 8.6 ± 3.3 pF for YFP-16 neurons (n = 26; P = 0.005).
For action potential clamp, each neuron's own action potentials were measured (10 kHz filter, digitized at 40 kHz) and played back through the amplifier in voltage-clamp mode to measure corresponding spike currents. At spontaneous firing rates, 5 s of action potentials were used as the voltage stimulus. When elevated firing rates were measured, voltage stimuli consisted of a 3 s stimulus of action potentials: 1 s of baseline firing, 1 s of firing at elevated rate, and 1 s return to baseline firing. Action potential currents were pharmacologically isolated as described in the following text. In experiments where hybrid action potential waveforms were used to measure resurgent currents (Figs. 5 and 6), trains of action potentials recorded from each neuron were modified by replacing the falling phase of the action potential (starting at the peak) with a 3 s square pulse whose maximum amplitude matched the peak amplitude of the action potential. For the hybrid spikes used in Fig. 7, voltage stimuli were constructed prior to recordings and the same stimulus was presented to multiple cells.
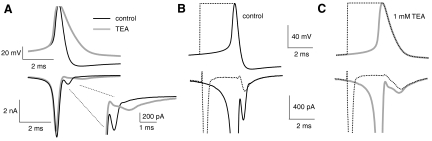
Fig. 5.
Mechanism of Na current protection by Kv3 currents in fast firing, YFP-16 neurons. A, top: average action potential waveforms recorded during 3 s of spontaneous firing in control Tyrode's solution (black) and after application of 1 mM TEA (gray). Bottom: average Na currents evoked by trains of action potentials described above. In voltage clamp, neurons were perfused with a 0 Ca2+ Tyrode's solution containing 20 mM TEA and 5 mM 4-aminopyridine (4-AP). Na currents were measured by subtraction after 1 μM tetrodotoxin (TTX) application and averaged over the last 4 s of the train. Inset: expanded view showing Na currents during action potential repolarization. Note the changes in the amplitude and kinetics of noninactivated Na current after application of 1 mM TEA. B, top: average action potential waveform in Tyrode's solution (black, solid line) and corresponding hybrid spike waveform (dotted line). Bottom: average Na currents evoked by trains of action potentials described above. C, top: average action potential waveform in 1 mM TEA (gray, solid line) and corresponding hybrid spike waveform (dotted line). Bottom: average Na currents evoked by trains of action potentials described above.
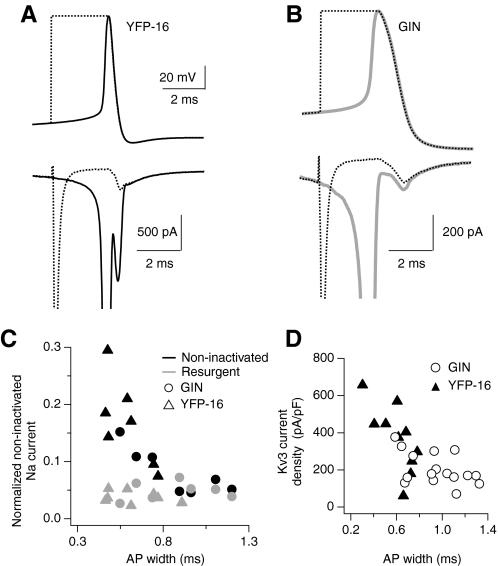
Fig. 6.
Protection of Na currents increases with decreasing action potential width. A, top: average action potential waveform over 3 s of spontaneous firing in a YFP-16 neuron (solid black) and corresponding hybrid spike stimulus (dashed line). Bottom: average Na currents elicited by the voltage stimuli described in A. Note the difference in noninactivated Na current during repolarization of the natural action potential waveform compared with the hybrid spike. Na currents were measured as described in Fig. 5. B: average action potential waveform over 3 s of spontaneous firing in a GIN neuron (solid gray) and corresponding hybrid spike (dashed line). Bottom: average Na currents elicited by the voltage stimuli described in A. Note that the noninactivated Na current is similar for both stimuli. C: noninactivated Na currents measured during repolarization, plotted as a function of action potential width. Noninactivated Na currents were normalized to the peak transient Na current for comparison across cells. Noninactivated Na currents were measured during the repolarization phase of either the natural spike waveform (black symbols) or the hybrid spike stimulus (gray symbols) for GIN (circles, n = 7) and YFP-16 neurons (triangles, n = 7). D: plot of Kv3 current density as a function of action potential width, measured with action potential clamp from spontaneously firing GIN and YFP-16 neurons.
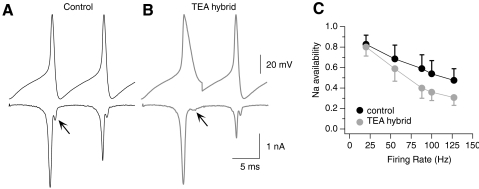
Fig. 7.
Firing rate dependence of Kv3-mediated enhancement of Na currents. A and B, top: voltage-clamp stimuli with 10 ms interspike intervals (100 Hz), created from a pair of action potentials in which Kv3-mediated repolarization is intact for both spikes (A, control Tyrode's) or blocked during the first spike (B, TEA hybrid). Bottom: Na current responses to action potential stimuli, measured by TTX subtraction in 0 Ca2+ Tyrodes + 20 mM TEA and 5 mM 4-AP. Note the change in amplitude and kinetics of noninactivated Na current during action potential repolarization, consistent with a tail current in A but a resurgent current in B. C: Na current availability, measured as the ratio of the Na current amplitude during the second spike compared with the first, at each firing rate tested in n = 9 neurons. Error bars represent SD.
Pharmacology
To isolate individual ionic currents during action potentials, neurons were perfused with a series of pharmacological solutions, rapidly delivered using a gravity-driven, VC-6 perfusion valve control system (Warner). Transient Na currents were measured by digital subtraction following the application of 1 μM tetrodotoxin (TTX; Tocris Bioscience). The next solution consisted of either 1 μM paxilline (Tocris Bioscience) to block BK currents or 2 mM MgCl2 in place of 2 mM CaCl2 to block Ca currents and Ca-dependent K currents. Once BK currents were blocked, Kv3 currents were isolated by subtraction following application of 1 mM tetraethylammonium (TEA), which under these conditions was specific for Kv3-containing channels (Gittis and du Lac 2007). In some neurons, two additional voltage-gated K currents were also measured: non-Kv3-containing delayed rectifier K currents (by subtraction after 10 mM TEA) and A-type K currents (by subtraction after 5 mM 4-aminopyridine [4-AP]). Ca2+ currents were measured as the current blocked when CaCl2 was replaced with MgCl2 or when 0.1 mM CdCl2 was added to solutions containing 1 μM TTX, 1 μM paxilline, 20 mM TEA, and 5 mM 4-AP.
In experiments investigating the mechanisms of Na current protection during firing, Ca and K currents were first blocked with 20 mM TEA, 5 mM 4-AP, and 2 mM MgCl2, substituted for 2 mM CaCl2, to minimize the amplitudes of non-Na currents, increasing accuracy of the voltage clamp. Na currents were then isolated by digital subtraction after application of 1 μM TTX.
All stock solutions were prepared in water with the exception of paxilline, which was prepared as a 20,000× stock in DMSO. TTX, TEA, and paxilline were stored at 4°C and 4-AP was stored at −20°C. Unless otherwise noted, drugs were purchased from Sigma.
Data analysis
Action potential parameters were measured using action potential waveforms averaged over 3 s of spontaneous firing or over the last 800 ms of elevated firing rates driven with 1 s of DC current injection. Action potential width was measured half way between action potential threshold and peak. Action potential threshold was defined as the voltage at which the rate of change in membrane potential exceeded 10 V/s. The ionic currents flowing during action potentials were aligned using the peak of the action potential as time 0. Current amplitudes did not vary substantially from action potential to action potential during steady-state firing and were averaged across spikes.
Statistical differences between GIN and YFP-16 neurons were tested with the nonparametric Wilcoxon test for unpaired data. Changes in Na current amplitudes in response to modified action potential waveforms were tested with the Wilcoxon test for paired data. Errors reported in the text are SDs.
Immunohistochemistry
GIN or YFP-16 transgenic mice (2 mo old) were perfused transcardially with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were removed and drop-fixed with the same fixative at 30–60 min at room temperature then sunk in 30% sucrose for 24 h at 4°C. Thin sections (30 μm) containing the MVN were made through the brain stem on a freezing microtome (Microm). For immunocytochemistry of free-floating sections, blocking buffer (2% normal goat serum, 1% bovine serum albumin, and 0.3% Triton X-100 in PBS) was applied for 1 h, followed by primary antibody in working buffer (10-fold dilution of blocking buffer) overnight at 4°C. Sections were washed three times with working buffer and treated with fluoro-conjugated secondary antibody for 1 h at room temperature. After washes in PBS, sections were wet-mounted and coverslipped with 2.5% DABCO (1,4-diazabicyclo-[2.2.2]octane) or Vectashield Hardset (Vector Laboratories). Primary antibodies were mouse anti-Kv3.1b (1:100; NeuroMab), rabbit anti-Kv3.2 (1:100; Chemicon), rabbit anti-Kv3.3 (1:200; Alomone), and mouse anti-Kv3.4 (1:100; NeuroMab). Controls for the specificity of each antibody were performed by the vendor with Western blot analyses of membrane fractions from adult rat brains that were preincubated with purified antigen. An additional control for the Kv3.1 antibody was performed by immunstaining tissue from Kv3.1 null mice (Neuromab). Primary antibodies were detected with Alexa Fluor 594-conjugated goat anti-mouse (1:200; Molecular Probes) or Alexa Fluor 594-conjugated goat anti-rabbit (1:100–200; Molecular Probes).
Confocal images were acquired in 0.1 to 0.5 μm steps on a Leica TCS SP2 AOBS microscope using laser lines of 488 and 561 nm, with ×63 (NA 1.4) objective and ×3 hardware zoom. In most cases, images were collected by sequential scanning to avoid possible fluorophore cross talk. Leica software was used to average sequential z-planes in images (two to six z-planes representing <3 μm total). Images were transferred to Adobe Photoshop (Adobe Systems) for whole-image brightness/contrast adjustment and image overlay.
RESULTS
Vestibular nucleus neurons exhibit different firing ranges
MVN neurons acutely dissociated from brain stem slices fired spontaneously at 5–25 Hz and responded to intracellular depolarization with increases in firing rate (Fig. 1, A and B). Maximum firing rate was defined as the highest average firing rate neurons could attain during 1 s of depolarization without entering depolarization block. Although recordings were made at room temperature, some MVN neurons could sustain firing rates >200 Hz and almost half of the neurons sampled (41/84) could fire faster than 100 Hz.
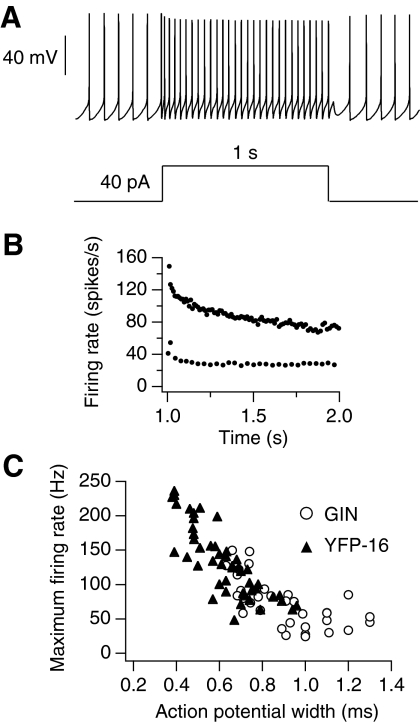
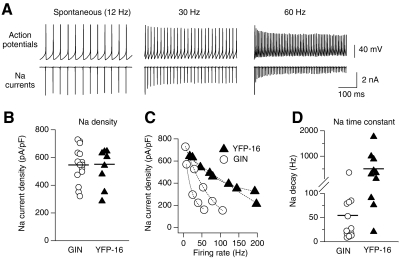
Fig. 1.
Firing ranges of acutely dissociated GIN and YFP-16 neurons. A: action potentials from a spontaneously firing YFP-16 neuron, driven to fire at 30 Hz with a 40 pA DC square current injection. B: instantaneous firing rate vs. time for action potentials shown in A and for action potentials elicited in the same neuron by a step of 160 pA (average firing rate for 160 pA = 82 Hz). This was the neuron's maximum firing rate because it could not sustain firing across the step with larger current injections. C: plot of maximum firing rate vs. action potential width (measured at half height) for the population of GIN (n = 36) and YFP-16 neurons (n = 48). There was considerable overlap between the 2 populations, but neurons with the narrowest action potential widths and highest maximum firing rates were YFP-16 and neurons with the broadest action potential widths and lowest maximum firing rates were GIN.
As with recordings from MVN neurons in brain slices (Bagnall et al. 2007), dissociated GIN and YFP-16 neurons differed in their ability to sustain high firing rates. GIN neurons exhibited lower maximum firing rates (24–150 Hz, n = 36) than those of YFP-16 neurons (46–234 Hz, n = 48, P < 0.0001). Although the fastest firing neurons were exclusively YFP-16 neurons and the slowest firing neurons were exclusively GIN neurons, the two populations overlapped at intermediate firing ranges (∼80–150 Hz). Maximum firing rates were well correlated with action potential widths, measured at half-height (Fig. 1C). Action potentials were narrower in YFP-16 neurons (0.62 ± 0.15 ms, n = 48) relative to GIN neurons (0.90 ± 0.19 ms, n = 36, P < 0.0001).
Na current inactivation accumulates more rapidly in GIN than in YFP-16 neurons
The ability of neurons to sustain high firing rates is limited by Na channel inactivation. To measure how Na current availability changes with firing rate, neurons were driven to fire at increasing rates with DC current injections until action potentials could not be sustained across the 1 s step. The recorded trains of action potentials were then used as voltage stimuli in the same neurons to measure Na currents at rates spanning the firing range of each neuron (Fig. 2A). Na currents measured with action potential voltage stimuli were measured by subtraction before and after application of 1 μM TTX. At spontaneous firing rates, Na current densities were similar in both GIN (546 ± 127 pA/pF, n = 15) and YFP-16 neurons (551 ± 141 pA/pF, n = 10, P = 0.76; Fig. 2B). During trains, Na current amplitudes decreased within the first 15 ms to reach steady-state values that varied with firing rate. As neurons approached their maximum firing rates, action potentials became shorter and broader, corresponding to decreased Na currents (Fig. 2, A and C). As shown for four example neurons in Fig. 2C (two GIN and two YFP-16), Na currents decayed more rapidly as a function of firing rate in GIN neurons compared with YFP-16 neurons. Exponential fits to these Na decay curves yielded significantly smaller time constants in GIN (54 ± 88 Hz, n = 15) versus YFP-16 neurons (502 ± 558 Hz, n = 10, P < 0.0001; Fig. 2D). These results suggest that minimizing Na current inactivation at higher firing rates enables YFP-16 neurons to operate over broader dynamic ranges relative to GIN neurons.
Fig. 2.
Na current amplitudes measured across firing ranges of medial vestibular nucleus (MVN) neurons using action potential clamp. A, top: action potentials measured in a GIN neuron during spontaneous firing, at 30 Hz during 60 pA current injection, and at 60 Hz during 120 pA DC current injection. Bottom: Na currents measured in action potential clamp using above action potentials as voltage stimuli. B: Na current densities at spontaneous firing rates, measured with action potential clamp for the population of GIN and YFP-16 neurons. C: Na current densities, measured with action potential clamp, at firing rates spanning the range of two GIN and two YFP-16 neurons. Exponential fits through the data are shown. D: graph showing the time constants of exponential decay of Na currents as a function of firing rate for the population of GIN and YFP-16 neurons. Averages are indicated with horizontal lines.
Ionic currents engaged during action potentials in MVN neurons
To examine why Na current inactivation accumulates more rapidly in GIN neurons, we measured other ionic currents activated during firing. Trains of action potentials at spontaneous firing rates were recorded in each neuron and played back as voltage stimuli. BK currents were measured by subtraction following application of 1 μM paxilline; Kv3 currents after 1 mM TEA (which is specific for Kv3 in MVN neurons once BK currents have been blocked; Gittis et al. 2007); delayed rectifier K currents (IKd) after 10 mM TEA; A-type K currents (IA) after 5 mM 4-AP; and Ca currents after either 0.1 mM CdCl2 or after substitution of 2 mM extracellular CaCl2 with 2 mM MgCl2.
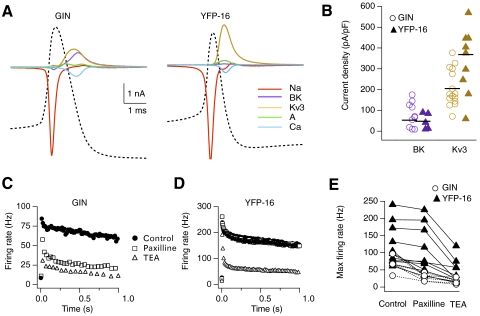
Figure 3A shows activation of the pharmacologically isolated ionic currents during action potentials from representative GIN and YFP-16 neurons. TTX-sensitive Na currents activated during the rising phase of the action potential. Kv3 and BK currents were the predominant K currents activated during action potential repolarization. The remaining outward currents (IKd and IA) contributed <10% of the total repolarizing current. Calcium currents were small and peaked during the falling phase of the action potential. At spontaneous firing rates, BK currents were similarly small in both YFP-16 (48 ± 38 pA/pF, n = 5) and GIN neurons (80 ± 59 pA/pF, n = 10, P = 0.43), but Kv3 current densities were nearly twofold larger in YFP-16 (368 pA/pF, n = 11) versus GIN neurons (205 pA/pF, n = 17, P = 0.01).
Fig. 3.
Ionic currents activated during firing in MVN neurons. A: pharmacologically identified ionic currents contributing to action potentials in representative GIN and YFP-16 neurons. Currents were measured using voltage stimuli consisting of a train of action potentials recorded in each neuron at spontaneous firing rates. Currents and action potential waveforms were averaged over the last 4 s of a 5-s recording. B: current densities of 1 μM paxilline-sensitive BK (purple) and 1 mM tetraethylammonium (TEA)-sensitive Kv3 currents (yellow) activated during action potentials at spontaneous firing rates in GIN and YFP-16 neurons. Averages are indicated with horizontal lines. C and D: instantaneous firing rates vs. time measured in a GIN neuron (C) and a YFP-16 neuron (D), firing at each neuron's maximum firing rate under control conditions, after application of 1 μM paxilline, and again after 1 mM TEA. E: summary of effects of 1 μM paxilline and 1 mM TEA on maximum firing rate for the population of GIN and YFP-16 neurons.
To determine the role of Kv3 and BK currents in setting neuronal firing range, changes in maximum firing rates were measured after pharmacologically blocking first BK currents with 1 μM paxilline, then Kv3 currents with 1 mM TEA. The effect of blocking these currents was markedly different in GIN and YFP-16 neurons. Figure 3C shows instantaneous firing rate versus time measured in a GIN neuron with a maximum firing rate of 66 Hz (average rate during 1 s step). Blocking BK currents reduced the maximum firing rate to 25 Hz. Subsequent blockade of Kv3 currents produced a small additional reduction in maximum firing rate to 15 Hz. Similar effects were observed across the population of GIN neurons, where BK blockade reduced maximum firing rates to an average of 41% of control values (from 66 ± 23 to 27 ± 11 Hz; n = 5), and Kv3 current blockade further reduced maximum firing rates to 25% of control values (17 ± 8 Hz; Fig. 3E). In contrast, blocking BK currents in YFP-16 neurons (Fig. 3D) had little effect on maximum firing rates (83% of control values; 118 ± 59 to 98 ± 68 Hz; n = 11), but subsequent blockade of Kv3 currents reduced maximum firing rates to 37% of control levels (44 ± 34 Hz; Fig. 3E). These results indicate that the firing ranges of GIN and YFP-16 neurons are differentially influenced by Kv3 and BK currents.
Contribution of BK and Kv3 to action potential repolarization depends on firing rate
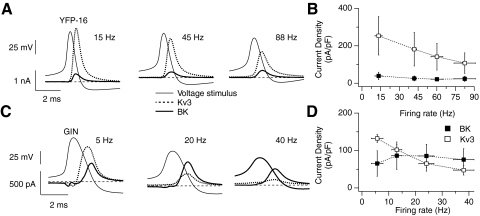
The contribution of repolarizing potassium currents at different firing rates was probed by driving neurons to fire with 1 s DC current injections of increasing intensities and using the resulting action potentials as voltage command stimuli in the same neuron. In both types of neurons, Kv3 current amplitudes decreased as firing rates increased, but BK currents remained relatively constant. In YFP-16 neurons ( n = 10), Kv3 currents dominated action potential repolarization at all firing rates (Fig. 4, A and B). This was also observed in the fastest firing GIN neurons (n = 2; data not shown) but in the majority of GIN neurons (with maximum firing rates <80 Hz, n = 7), BK currents became the dominant repolarizing current at firing rates >20 Hz (Fig. 4, C and D). These results demonstrate that the contributions of BK and Kv3 currents to action potential repolarization at high firing rates differ between YFP-16 neurons and the slower firing GIN neurons.
Fig. 4.
Changes in BK and Kv3 currents across the firing ranges of GIN and YFP-16 neurons. A and C: BK and Kv3 currents recorded in a YFP-16 neuron (A) or GIN neuron (C) using action potential voltage stimuli. BK and Kv3 currents were measured with voltage stimuli measured at different firing rates in each neuron: 15, 45, and 88 Hz in the YFP-16 neuron; 5, 20, and 40 Hz in the GIN neuron. Firing rates indicated on the x-axis are within ±5 Hz of actual firing rate. The currents are aligned to the average action potential waveform (voltage stimulus). Zero current is indicated with a horizontal, dashed line. B and D: average current densities of BK and Kv3 currents recorded in YFP-16 (B, n = 10) and GIN (D, n = 7) neurons using action potential stimuli spanning the firing ranges of each neuron.
Fast Kv3-mediated repolarization enhances Na current availability
To understand why Na current inactivation accumulates more slowly in neurons with large Kv3 currents, we analyzed Na currents in YFP-16 neurons with narrow action potentials (0.53 ± 0.13 ms, n = 7), where the contribution from BK currents was minimal (Fig. 5). In these neurons, spontaneous action potentials (10–20 Hz for 5 s) were recorded before and after blocking Kv3 currents with 1 mM TEA, resulting in a doubling of action potential width (Fig. 5A, top). These trains of “control” and “TEA” action potentials were then used as voltage stimuli in the same neurons to measure Na currents. Resulting Na currents consisted of large initial transient components, followed by smaller second components observed during action potential repolarization (Fig. 5A). The second component represents current flowing through Na channels that were not inactivated (Raman and Bean 1997, 1999). Blocking Kv3 currents significantly reduced the peak amplitude of non-inactivated Na currents from 1250 ± 680 to 460 ± 240 pA (n = 7, P = 0.02).
A fraction of Na channels in fast-firing neurons are protected from inactivation by an endogenous blocking particle whose release from the pore produces a “resurgent” Na current (Raman and Bean 1997). To measure resurgent Na current during action potential repolarization, a modified voltage protocol was used in which the rising phase of each action potential was replaced with a 3 ms depolarizing square pulse (Do and Bean 2003; Enomoto et al. 2006) (Fig. 5, B and C). Surprisingly, the peak amplitude of resurgent Na currents measured with hybrid spike stimuli was significantly smaller (by 74 ± 13%, n = 7, P = 0.004) than that of noninactivated Na currents measured with “control” action potential stimuli (Fig. 5B). This suggests that noninactivated Na currents observed during natural action potential stimuli were mainly not resurgent currents. This was not the case when TEA was applied to block fast action potential repolarization. The peak amplitude of noninactivated Na currents measured with “TEA” action potential stimuli was not significantly different from the peak amplitude of resurgent Na currents measured with “TEA hybrid” stimuli (difference of 33 ± 20%, n = 7, P = 0.097; Fig. 5C).
These results indicate that Na channels can be protected from inactivation during firing both by a resurgent mechanism and by Kv3 currents. Noninactivated Na currents measured with “control” action potential stimuli represent tail currents through Na channels that have not yet entered inactivated or blocked states. Therefore Kv3-mediated action potential repolarization protects Na channels from inactivation by hyperpolarizing the membrane sufficiently fast that some Na channels transitioned directly from the open to closed state, bypassing both inactivated and blocked states.
Differential contribution of Kv3 currents across MVN neurons
To determine the extent to which Kv3-mediated protection of Na channels is engaged across the population of MVN neurons, we measured noninactivated Na currents during action potential repolarization in GIN and YFP-16 neurons. Figure 6A shows the average action potential waveform recorded from a spontaneously firing YFP-16 neuron with a narrow action potential (width: 0.46 ms). The Na current measured in action potential clamp had a large noninactivated component during action potential repolarization whose peak was 63% larger {100 × [(1 − peak resurgent current/peak noninactivated current)]} than the resurgent current alone, measured with a hybrid spike. In contrast, in a GIN neuron with a much broader action potential (width: 1.2 ms), the noninactivated Na current observed during action potential repolarization was similar in amplitude (25% larger) and kinetics to the resurgent Na current measured with a hybrid spike (Fig. 6B).
Across the population, neurons with the widest action potentials (>0.8 ms) exhibited noninactivated Na currents whose amplitude and kinetics could not be distinguished from those of resurgent Na currents (peak amplitude of noninactivated currents was 13 ± 49% larger; n = 6, P = 0.33; Fig. 6C). In contrast, neurons with action potentials narrower than 0.8 ms had noninactivated Na currents that were larger than expected through a purely resurgent mechanism (70 ± 0.16%, n = 11, P = 0.001) and increased in amplitude as action potential widths decreased. As shown in Fig. 6D, action potential widths were well correlated with Kv3 current amplitudes.
Kv3-mediated protection enhances Na currents at high firing rates
To examine the firing range over which Kv3 currents contribute most to sodium channel availability, we measured Na currents evoked by voltage stimuli constructed from two pairs of action potentials with and without pharmacological block of Kv3 channels. Figure 7A shows a pair of control action potentials, separated by an interspike interval of 10 ms (100 Hz) and the corresponding Na currents measured with action potential clamp. The first action potential elicited a large transient Na current and the second action potential elicited a smaller transient Na current (ratio = 0.68), indicating a reduction in Na channel availability. Figure 7B shows the same pair of action potentials but the repolarizing phase of the first action potential was replaced by that measured after blocking Kv3 currents with 1 mM TEA. In response to “TEA hybrid” stimuli, the first action potential elicited a similar transient Na current but a much smaller transient Na current during the second action potential (ratio = 0.43). Blocking Kv3-mediated action potential repolarization changed the amplitude and kinetics of the noninactivated Na current (arrows in Fig. 7, A and B), indicative of more resurgent current and less tail current (see Fig. 5).
The effect of Kv3-mediated action potential repolarization on Na channel availability was measured across a range of firing rates (25, 55, 88, 100, and 127 Hz; Fig. 7C). As interspike intervals became shorter, Na channel availability decreased during both “control” and “TEA hybrid” stimuli. However, Na channel availability was significantly greater at all firing rates when Kv3-mediated repolarization was intact (n = 9, P < 0.0001). The presence of Kv3 repolarizing currents influenced sodium currents during the second action potential only modestly at low firing rates (by 4 and 14% at 25 and 55 Hz, respectively) but increased sodium channel availability by 33–35% at higher firing rates (Fig. 7C). These results indicate that Kv3 currents extend sodium channel availability predominantly at interspike intervals <20 ms.
Differential expression of Kv3 channel subunits in YFP-16 and GIN neurons
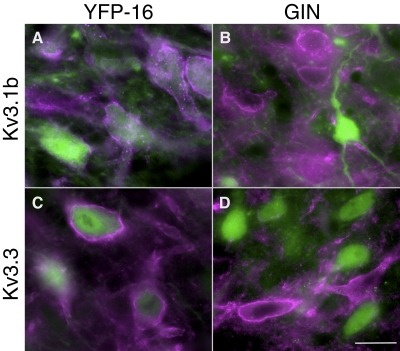
The results presented earlier, together with published data on whole cell potassium currents (Gittis and du Lac 2007), demonstrate that Kv3 currents are larger in YFP-16 than those in GIN neurons and that graded expression of Kv3 currents underlies the continuous distribution of firing ranges in MVN neurons. To examine the molecular basis for these observations, we analyzed expression of Kv3.1b, Kv3.2, Kv3.3, and Kv3.4 subunits via immunohistochemistry in fixed sections prepared in tandem from YFP-16 and GIN mice. Immunostaining with antibodies generated against the Kv3.1b, Kv3.3, and Kv3.4 subunits resulted in immunofluorescence primarily along neuronal membrane surfaces (Fig. 8). In contrast, antibodies to the Kv3.2 subunit resulted in a diffuse pattern of immunofluorescence within neuronal cell bodies with only occasional staining of membrane surfaces (not shown). The percentages of neurons in YFP-16 and GIN lines that were immunostained by antibodies to each Kv3 subunit are shown in Table 1. Most YFP-16 neurons (91%) were immunopositive for Kv3.1b (Fig. 8A); in contrast, relatively few GIN neurons (23%) showed surface staining with Kv3.1b antibodies. Kv3.2 immunostaining was also present in some GIN neurons and in the majority of YFP-16 neurons (Table 1). Immunostaining against Kv3.3 showed the most striking differential expression (Fig. 8, C and D): almost all YFP-16 neurons were immunopositive for Kv3.3, whereas no GIN neurons were. Kv3.4 was expressed in the majority of neurons from both lines (Table 1). These results indicate that the differences in fast repolarizing currents in YFP-16 and GIN neurons result from differential expression of Kv3 channel subunits.
Fig. 8.
Kv3 subunit expression varied across GIN and YFP-16 neurons. A and B: Kv3.1b immunostain in YFP-16 (left) and GIN-16 (right) transgenic mice. Green fluorescent protein (GFP) and yellow fluorescent protein (YFP) fluorescence are green and Kv3.3 staining is purple. Images are z stacks (average of 6 planes in 0.5 μm steps) taken with a ×63 objective and ×3 digital zoom. Kv3.1b staining was more frequently found in YFP-16 relative to GIN neurons. C and D: same as A and B but for Kv3.3 subunits. Scale bar is 10 μm.
Table 1.
Expression of Kv3 channel subunits to GIN and YFP-16 neurons
| Neuron | Kv3.1b | Kv3.2 | Kv3.3 | Kv3.4 |
|---|---|---|---|---|
| GIN | 18/79 (23%) | 38/125 (30%) | 0/119 (0%) | 77/89 (87%) |
| YFP-16 | 128/140 (91%) | 96/110 (87%) | 86/92 (94%) | 110/121 (91%) |
Cell counts were in three 30-μm sections from each of two GIN transgenic mice and two YFP-16 transgenic mice. The table lists the ratio of neurons that were immunopositive for each Kv3 channel subunit to the number of fluorescently labeled neurons examined in each mouse line. The percentages of GIN and YFP-16 neurons expressing each subunit are shown in bold.
DISCUSSION
This study demonstrates that vestibular nucleus neurons use several mechanistic strategies to ensure adequate sodium channel availability in the face of maintained high firing rates. First, these neurons express high levels of sodium channels with biophysical properties that enable slow entry into and rapid recovery from the inactivated state (Gittis and du Lac 2008). Second, the presence of resurgent sodium current indicates that some sodium channels escape inactivation by being blocked during the depolarizing phase of the action potential. Third, high expression of rapidly activated Kv3 currents protects sodium currents from inactivation by transitioning channels directly from the open to the closed state during action potential repolarization. Finally, in GIN neurons that express relatively low levels of Kv3 currents, expression of BK currents extends the firing range in depolarized firing regimes that compromise the availability of voltage-dependent Kv3 currents.
Although previous studies using conventional voltage-step stimuli demonstrated the presence of Kv3 and resurgent Na currents in MVN neurons (Gittis and du Lac 2007, 2008), assessing the contributions of these currents across neuronal firing ranges required the use of the action potential clamp method (Bean 2007). Our measurements of Na tail currents evoked during action potential repolarization indicate that some Na channels are protected from inactivation during firing. By comparing the time course and magnitude of the Na tail currents evoked during natural action potentials with those evoked by hybrid action potentials designed to assess the contributions of resurgent Na currents, we infer that the resurgent mechanism protects a fixed fraction of Na channels from inactivation across all firing rates and that rapid Kv3-mediated repolarization is particularly important at high firing rates. Computer models suggest that Kv3 currents affect Na channel availability by changing the kinetics of the blocked state that gives rise to resurgent Na current (Akemann and Knopfel 2006; Zagha et al. 2008). Our results, along with a recent study of fast-firing Purkinje and cortical neurons (Carter and Bean 2009) indicate that Kv3 currents enhance Na channel availability simply by repolarizing the membrane so rapidly that some channels are precluded from entering either inactivated or blocked states.
MVN neurons comprise a heterogeneous population capable of firing at high sustained rates but varying in firing range (Bagnall et al. 2007). The results from the action potential clamp studies presented here together with conventional voltage-clamp studies of Na and K currents (Gittis and du Lac 2007, 2008) indicate that the firing range of vestibular nucleus neurons is set primarily by Kv3 currents. The fastest firing neurons, from the YFP-16 line, express Kv3 currents that are larger and have more rapid kinetics than those from slower firing GIN neurons. In contrast, the kinetics of Na channel activation and inactivation are equivalent in YFP-16 and GIN neurons (Gittis and du Lac 2008), implying that differences in Na channel availability are determined by expression levels of Kv3 currents. Immunostaining against Kv3 channel subunits suggests that qualitative differences in channel isoform expression, primarily in Kv3.3 subunits, contribute to variations in Kv3 currents across neurons.
It is well established that the kinetics and voltage-dependent properties of Kv3 channels are tuned to promote fast firing. They exhibit rapid activation kinetics that minimizes action potential duration and rapid deactivation kinetics that reduces refractory periods (Erisir et al. 1999; Lien and Jonas 2003; Rudy and McBain 2001). Although both Kv3.3 and Kv3.4 subunits have particularly fast kinetics, the differential expression of Kv3.3 but not Kv3.4 subunits in MVN neurons (Table 1), together with the existing literature (Brooke et al. 2010), suggest that Kv3.3 subunits play particularly important roles in determining neuronal firing properties. Kv3.3 subunits influence both the tonic firing and complex spike activity of Purkinje cells (Akemann and Knopfel 2006; Zagha et al. 2008). Mutations or deletions of Kv3.3 subunits result in movement disorders and altered olivocerebellar functioning (Joho et al. 2006; McMahon et al. 2004; Waters et al. 2006). In Kv3.3 or Kv3.1/3.3 knockout mice, motor deficits are partially rescued by selective reexpression of Kv3.3 in Purkinje cells (Hurlock et al. 2008). Remaining deficits could reflect altered firing of deep cerebellar nucleus neurons (Hurlock et al. 2009) and/or altered signaling through vestibular nucleus neurons.
Although Kv3 currents are particularly critical for action potential repolarization at high firing rates in YFP-16 neurons, as has been shown in electrosensory pyramidal cells (Fernandez et al. 2005), BK currents dominate repolarization at the highest firing rates in GIN neurons (Fig. 4). These data indicate that the contributions of ion channels to neuronal output can change as a function of neuronal firing rate, as first illustrated in studies of Aplysia (Ma and Koester 1995, 1996). A role for BK channels in enabling fast action potential firing has also been observed during burst firing in Purkinje cells (McKay and Turner 2004), but contrasts with the situation in hippocampal pyramidal neurons, in which accumulating inactivation of BK currents promotes action potential broadening (Gu et al. 2007; Shao et al. 1999). The observation that spike generation in YFP-16 and GIN neurons is linear, although repolarizing currents can differ between the two populations, adds to observations in other cell types that neurons with different underlying currents can produce similar firing outputs (McAnelly and Zakon 2000; Schulz et al. 2006; Swensen and Bean 2003).
The vestibular system is remarkable for its speed and accuracy, maintained by experience-driven plasticity (Brandt and Dieterich 1999; Gittis and du Lac 2006; Straka et al. 2005). Information processing in the vestibular system is thought to occur along parallel processing streams, some of which are tuned for fast signal transmission, whereas others play a more integrative role (Beraneck et al. 2007; Biesdorf et al. 2008; Lisberger et al. 1994; Ramachandran and Lisberger 2006). Based on transmitter content and anatomical location of YFP-16 and GIN neurons in the vestibular nuclei (Bagnall et al. 2007), YFP-16 neurons likely represent premotor neurons in fast pathways, whereas GIN neurons correspond to local inhibitory neurons in slower, integrative pathways (Beraneck et al. 2007; Biesdorf et al. 2008; Buttner-Ennever 1992; Epema et al. 1988; Sekirnjak and du Lac 2006).
Both Kv3 and BK currents can be regulated by experience and neuronal activity. In MVN neurons, synaptic inhibition triggers rapid and long-lasting reductions in BK currents that result in increases in intrinsic excitability (Nelson et al. 2003) via reductions in CaMKII activity (Nelson et al. 2005). Our results indicate that such activity-dependent reductions of BK currents would have little effect on the fastest firing projection neurons but would reduce the firing range of slower firing inhibitory interneurons. Kv3 channels can also be modified by phosphorylation in an experience-dependent fashion (Song et al. 2005). Graded expression of Kv3 currents in MVN neurons in fast and slow pathways appears to develop during the first few weeks postnatally, as inferred from developmental decreases in action potential width (Murphy and du Lac 2001). This raises the possibility that postnatal maturation of vestibular nerve activity (Hurley et al. 2006; Rusch et al. 1998; Wooltorton et al. 2007) contributes to the tuning of intrinsic firing of central vestibular neurons.
GRANTS
This work was supported by National Eye Institute Grant EY-11027 and the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank B. Bean, I. Raman, and T. Kodama for helpful discussion and M. Grivich for IGOR program development.
REFERENCES
- Akemann W, Knopfel T. Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons. J Neurosci 26: 4602–4612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, Stevens RJ, du Lac S. Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations. J Neurosci 27: 2318–2330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol 98: 1549–1565, 2007 [DOI] [PubMed] [Google Scholar]
- Beraneck M, Pfanzelt S, Vassias I, Rohregger M, Vibert N, Vidal PP, Moore LE, Straka H. Differential intrinsic response dynamics determine synaptic signal processing in frog vestibular neurons. J Neurosci 27: 4283–4296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesdorf S, Malinvaud D, Reichenberger I, Pfanzelt S, Straka H. Differential inhibitory control of semicircular canal nerve afferent-evoked inputs in second-order vestibular neurons by glycinergic and GABAergic circuits. J Neurophysiol 99: 1758–1769, 2008 [DOI] [PubMed] [Google Scholar]
- Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann NY Acad Sci 871: 293–312, 1999 [DOI] [PubMed] [Google Scholar]
- Brooke RE, Corns L, Edwards IJ, Deuchars J. Kv3.3 immunoreactivity in the vestibular nuclear complex of the rate with focus on the medial vestibular nucleus: targeting of Kv3.3 neurones by terminals positive for vesicular glutamate transporter 1. Brain Res 1345: 45–58, 2010 [DOI] [PubMed] [Google Scholar]
- Buettner UW, Buttner U, Henn V. Transfer characteristics of neurons in vestibular nuclei of the alert monkey. J Neurophysiol 41: 1614–1628, 1978 [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA. Patterns of connectivity in the vestibular nuclei. Ann NY Acad Sci 656: 363–378, 1992 [DOI] [PubMed] [Google Scholar]
- Carter BC, Bean BP. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast spiking neurons. Neuron 64: 898–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Escudero M, Godaux E. Discharge properties of brain stem neurons projecting to the flocculus in the alert cat. I. Medial vestibular nucleus. J Neurphysiol 76: 1759–1774, 1996 [DOI] [PubMed] [Google Scholar]
- Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol 70: 828–842, 1993 [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci 26: 3412–3422, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epema AH, Gerrits NM, Voogd J. Commissural and intrinsic connections of the vestibular nuclei in the rabbit: a retrograde labeling study. Exp Brain Res 71: 129–146, 1988 [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999 [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51, 2000 [DOI] [PubMed] [Google Scholar]
- Fernandez FR, Mehaffey WH, Molineux ML, Turner RW. High-threshold K+ current increases gain by offsetting a frequency-dependent increase in low-threshold K+ current. J Neurosci 25: 363–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol 38: 1140–1161, 1975 [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol 16: 385–390, 2006 [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Firing properties of GABAergic versus non-GABAergic vestibular nucleus neurons conferred by a differential balance of potassium currents. J Neurophysiol 97: 3986–3996, 2007 [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Similar properties of transient, persistent, and resurgent Na currents in GABAergic and non-GABAergic MVN neurons. J Neurophysiol 99: 2060–2065, 2008 [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron 45: 233–244, 2005 [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26: 10253–10269, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlock EC, Bose M, Pierce G, Joho RH. Rescue of motor coordination by Purkinje cell-targeted restoration of Kv3.3 channels in Kcnc3-null mice requires Kcnc1. J Neurosci 29: 15735–15744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci 28: 4640–4648, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho RH, Street C, Matsushita S, Knopfel T. Behavioral motor dysfunction in Kv3-type potassium channel-deficient mice. Genes Brain Behav 5: 472–482, 2006 [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23: 4899–4912, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci 23: 2058–2068, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol 72: 928–953, 1994 [DOI] [PubMed] [Google Scholar]
- Ma M, Koester J. Consequences and mechanisms of spike broadening of R20 cells in Aplysia californica. J Neurosci 15: 6720–6734, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16: 4089–4101, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnelly ML, Zakon HH. Coregulation of voltage-dependent kinetics of Na(+) and K(+) currents in electric organ. J Neurosci 20: 3408–3414, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, Turner RW. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci 20: 729–739, 2004 [DOI] [PubMed] [Google Scholar]
- McMahon A, Fowler SC, Perney TM, Akemann W, Knopfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci 19: 3317–3327, 2004 [DOI] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci 27: 13552–13566, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, du Lac S. Postnatal development of spike generation in rat medial vestibular nucleus neurons. J Neurophysiol 85: 1899–1906, 2001 [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 46: 623–631, 2005 [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron 40: 609–620, 2003 [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51, 2000 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Lin N, Wei M. Response linearity of alert monkey non-eye movement vestibular nucleus neurons during sinusoidal yaw rotation. J Neurophysiol 102: 1388–1397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20: 3354–3368, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Transformation of vestibular signals into motor commands in the vestibuloocular reflex pathways of monkeys. J Neurophysiol 96: 1061–1074, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 19: 1663–1674, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J 80: 729–737, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris L, de Waele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol 74: 2087–2099, 1995 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24: 517–526, 2001 [DOI] [PubMed] [Google Scholar]
- Rusch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 9: 356–362, 2006 [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Intrinsic firing dynamics of vestibular nucleus neurons. J Neurosci 22: 2083–2095, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Physiological and anatomical properties of mouse medial vestibular nucleus neurons projecting to the oculomotor nucleus. J Neurophysiol 95: 3012–3023, 2006 [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Strom JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, Bhattacharjee A, Hamann M, Forsythe ID, Oliver DL, Kaczmarek LK. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nat Neurosci 8: 1335–1342, 2005 [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76: 349–392, 2005 [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci 23: 9650–9663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol 509: 183–194, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet 38: 447–451, 2006 [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, Lysakowski A, Eatock RA. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol 97: 1684–1704, 2007 [DOI] [PubMed] [Google Scholar]
- Zagha E, Lang EJ, Rudy B. Kv3.3 channels at the Purkinje cell soma are necessary for generation of the classical complex spike waveform. J Neurosci 28: 1291–1300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]