Abstract
Central pain syndrome (CPS) is a debilitating and chronic pain condition that results from a lesion or dysfunction in the CNS. The pathophysiological mechanisms underlying CPS are poorly understood. We recently demonstrated that CPS is associated with suppressed inputs from the inhibitory nucleus zona incerta to the posterior thalamus (PO). As a consequence, activity in PO is abnormally increased in CPS. Because the perception of pain requires activity in the cerebral cortex, CPS must also involve abnormal cortical activity. Here we test the hypothesis that CPS is associated with increased activity in the primary somatosensory cortex (SI), a major projection target of PO that plays an important role in processing sensory-discriminative aspects of pain. We recorded activity of single units in SI in rats with CPS resulting from spinal cord lesions. Consistent with our hypothesis, SI neurons recorded from lesioned rats exhibited significantly higher spontaneous firing rates and greater responses evoked by innocuous and noxious mechanical stimulation of the hindpaw compared with control rats. Neurons from lesioned rats also showed a greater tendency than controls to fire bursts of action potentials in response to noxious stimuli. Thus, the excruciatingly painful symptoms of CPS may result, at least in part, from abnormally increased activity in SI.
INTRODUCTION
Central pain syndrome (CPS) is defined as chronic pain resulting from a lesion or dysfunction in the CNS (Merskey and Bogduk 1994). CPS is a common consequence of spinal cord injury (>50% of patients), multiple sclerosis (30% of patients), and stroke (8% of patients) and therefore afflicts millions of people worldwide (Andersen et al. 1995; Bonica 1991; Osterberg et al. 2005; Siddall et al. 2003). CPS poses a significant clinical problem due to its resistance to treatment. At best, current therapies for CPS can reduce pain for short periods of time but cannot eliminate it completely (Canavero and Bonicalzi 2007; Frese et al. 2006; Hansson 2004; Nicholson 2004). The development of effective long-term treatments is contingent on an understanding of the pathophysiological mechanisms of CPS.
Abnormalities of thalamic function have long been postulated to be involved in CPS (Head and Holmes 1911). In support of this, we recently reported that CPS is associated with suppressed inputs from the inhibitory nucleus zona incerta (ZI) to the posterior thalamic nucleus (PO) in a rodent spinal cord injury model of CPS (Masri et al. 2009); in this model, focal electrolytic lesions are made unilaterally in the ventrolateral quadrant of the spinal cord. As a consequence, we found that CPS was associated with dramatically increased spontaneous and sensory-evoked activity in PO, a thalamic nucleus that receives convergent innocuous and noxious somatosensory inputs by way of the spinothalamic tract (STT), the major ascending pain pathway (Poggio and Mountcastle 1960; Zhang and Giesler 2005). In this model, CPS rats also exhibited abnormally increased activity in vitro in the ventral posterior lateral nucleus (VPL), a thalamic nucleus that also receives somatosensory inputs via the STT (Wang and Thompson 2008). However, our in vivo recordings did not reveal any CPS-related changes in VPL (Masri et al. 2009) although increased VPL activity has been reported by others (Gerke et al. 2003; Hains et al. 2005, 2006; Zhao et al. 2007). Thus activity in both PO and VPL may be abnormally increased in CPS. However, it is unclear how abnormally increased thalamic activity would result in altered pain perception in CPS.
The perception of pain requires activity in the cerebral cortex (Apkarian et al. 2005; Kenshalo and Willis 1991; Neugebauer et al. 2009; Treede et al. 2000). Therefore pain associated with CPS must involve abnormal cortical activity. Whether CPS-related changes in cortical neurophysiology occur is not known. Although several functional neuroimaging studies have reported abnormal cortical responses in CPS (Ducreux et al. 2006; Endo et al. 2008; Hirato et al. 1993; Peyron et al. 1998), to our knowledge there have been no direct recordings from cortical neurons in CPS. Based on our finding that PO activity is abnormally increased in CPS, we expect the cortical targets of PO to show increased activity. Primary somatosensory cortex (SI) is a major projection target of PO (Bureau et al. 2006; Chmielowska et al. 1989; Fabri and Burton 1991; Koralek et al. 1988; Lu and Lin 1993; Nothias et al. 1988) and is involved in processing sensory-discriminative aspects of nociceptive inputs (Apkarian et al. 2005; Bushnell et al. 1999; Coghill et al. 1999; Moulton et al. 2005). In this study, we perform in vivo electrophysiological recordings in CPS and control rats to test the hypothesis that CPS is associated with increased activity in SI neurons.
METHODS
All procedures were approved by the University of Maryland School of Medicine Animal Care and Use Committee. Experiments were conducted according to institutional guidelines, federal regulations, and the guidelines of the International Association for the Study of Pain.
Spinal lesions
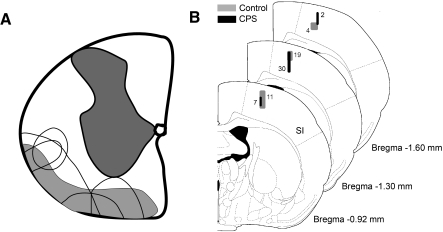
Twelve adult female Sprague-Dawley rats weighing 250–300 g were used in this study. Nine of these rats underwent spinal lesion or sham lesion surgery; the remaining three rats served as naive controls and did not undergo surgical procedures. Surgeries were conducted under strict aseptic conditions. Rats were anesthetized with ketamine/xylazine (100/8 mg/kg ip) and placed on a thermo-regulated heating pad to maintain body temperature. For spinal lesions, a laminectomy was performed to expose the spinal cord between C6 and T2. A quartz-insulated platinum electrode (5 μm tip) was targeted unilaterally to the ventrolateral quadrant of the spinal cord as described previously (Masri et al. 2009; Wang and Thompson 2008). Current (10 μA for 10 s, repeated 4 times) was passed through the electrode to produce an electrolytic lesion (∼0.6 mm3). Figure 1A shows a schematic representation of the lesioned area in five animals. Sham surgery was performed without laminectomy. The analgesic buprenorphine (0.05 mg/kg) was administered every 12 h for 24 h postoperatively.
Fig. 1.
A: drawing of coronal section through the cervical spinal cord, showing the location and size of lesions in animals with mechanical hyperalgesia (unfilled areas). Shaded areas represent the location of ascending spinothalamic tract axons, adapted from Fig. 5 in Giesler et al. (1981). B: coronal sections of the rat brain (Paxinos and Watson 1998) showing locations of all recorded neurons in this study. Numbers represent the number of neurons recorded in each shaded area of primary somatosensory (SI) cortex.
Behavioral testing
Rats were habituated to handling for 2 wk before behavioral testing and trained to stand with their forepaws on the experimenter's hand, allowing access to the hindpaws as described by Ren (1999). Animals were not restrained during testing. Behavioral testing consisted of measuring mechanical hindpaw withdrawal thresholds bilaterally using calibrated von Frey filaments (Stoelting, IL). Filaments were applied to the dorsal surface of the hindpaw based on studies demonstrating that threshold changes are more reliably and consistently detected at this site (Ren 1999). Each von Frey filament was applied five times to each hindpaw, and the threshold was defined as the force at which the animal withdrew the paw to three or more of the stimuli (>50% response frequency). Rats underwent behavioral testing on 3 days in the week before surgery to obtain baseline presurgical withdrawal thresholds and on days 7 and 14 postsurgery. The experimenters were not blind to the treatment (sham lesioned versus spinal lesioned).
Extracellular recording and stimulation
Electrophysiological recordings were made from rats ≥1 mo after spinal lesion/sham surgery. The duration between surgery and recording ranged from 1 to 5 mo in each group with the mean duration comparable between groups (2.7 ± 2 mo for sham rats; 3 ± 2 mo for spinal-lesioned rats). Rats were anesthetized with urethane (1.5 g/kg ip), head-fixed in a stereotaxic apparatus, and placed on a thermo-regulated heating pad to maintain body temperature at 37°C. Depth of anesthesia was monitored every 15 min by testing reflexes to pinch and cornea stimulation. Supplemental doses of urethane (0.15 g/kg) were given if necessary. Urethane was selected because it has no, or negligible, effects on glutamatergic and GABAergic transmission and therefore produces only minimal disruption of signal transmission in the neocortex (Sceniak and Maciver 2006).
A craniotomy was performed over SI contralateral to the spinal lesion site. Extracellular recordings of hindpaw-responsive single units were obtained with quartz-insulated platinum electrodes (2–4 MΩ). Spike waveforms were digitized through a Plexon (Dallas, TX) data acquisition system and sampled at 40 kHz. Single units with hindpaw receptive fields were identified by brushing and pinching the hindpaw contralateral to the recording site. Once a well-isolated unit was identified, the following were recorded: 30 s of spontaneous activity and responses to mechanical stimuli applied with a motorized blunt-tipped probe (tip diameter: 1.5 mm; QuickShaft, Faulhaber, Croglio, Switzerland). The motorized probe was used to apply innocuous (5 g) and noxious (200 g) stimuli 20 times each; each probe stimulus was applied for 0.5 s.
At the end of each experiment, electrolytic lesions (5 μA, 20 s) were made to confirm the recording sites (Fig. 1B). Animals were then deeply anesthetized with sodium pentobarbital (60 mg/kg) and perfused transcardially with buffered saline followed by buffered 4% paraformaldehyde. Coronal brain and spinal sections (80 μm thick) were obtained and Nissl-stained to identify recording and lesion sites.
Data analysis
Statistical analyses were performed with SigmaStat (Aspire Software International, Ashburn, VA). Between-group statistical comparisons were assessed with the nonparametric Mann Whitney U test (MWU). Proportional data were analyzed using the χ2 test. The significance level was set at P < 0.05 for all tests.
BEHAVIORAL DATA.
To test whether hindpaw withdrawal thresholds changed over time after surgery, data from spinal-lesioned rats and sham-lesioned rats were analyzed separately with the Friedman test.
ELECTROPHYSIOLOGICAL DATA.
Recorded units were sorted off-line with Plexon's offline Sorter (Plexon, Dallas; TX) using dual thresholds and principal component analyses. Autocorrelograms were generated with NeuroExplorer software (Plexon) to confirm that we obtained recordings from single units. Data from well-isolated single units were further analyzed using the procedures described in the following text.
NeuroExplorer (Plexon) software was used to compute the mean firing rate of each neuron during spontaneous activity periods. For evoked responses, time stamps of well-isolated units and of stimulus triggers were exported to Matlab (MathWorks, Natick, MA) for analyses using custom-written algorithms. Peristimulus time histograms (PSTHs, 20 ms bins) were constructed, and significant stimulus-evoked responses were defined as PSTH bins with response magnitudes significantly exceeding (99% confidence interval) spontaneous activity levels, computed from a 100 ms period preceding the stimuli. Response onset was defined as the first two consecutive bins (poststimulus) that displayed significant responses (above the 99% confidence interval), and response offset was defined as three consecutive bins in which the response duration fell below the 99% confidence interval. The magnitude of the response was defined as the total number of spikes per stimulus occurring between the onset and offset of the significant response. The duration of the significant response was defined as the time (in ms) between response onset and offset.
BURST ANALYSIS.
Bursts of action potentials were identified as clusters of at least three spikes with interspike intervals of <4 ms in which the first spike in the burst has a preceding interspike interval of ≥100 ms (Guido et al. 1995; Lu et al. 1992; Sherman 1996). Three types of bursting activity were evaluated: spontaneous bursting, bursting in response to innocuous mechanical stimuli, and bursting in response to noxious mechanical stimuli.
NEURONAL CLASSIFICATION.
The duration of the extracellularly recorded waveform of each neuron was evaluated using criteria developed by Bruno and Simons (2002) to determine whether it was a fast spiking unit (presumably inhibitory interneuron) or regular spiking unit.
RESULTS
Behavioral confirmation of CPS in spinal-lesioned animals
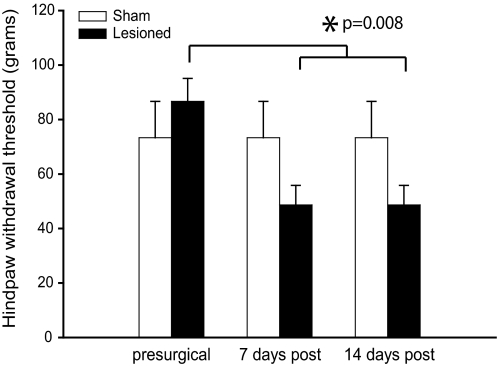
We and others have previously shown that rats with spinal cord lesions develop behavioral signs consistent with CPS, including mechanical and thermal hyperalgesia caudal to the lesion site (Endo et al. 2008; Masri et al. 2009; Mills et al. 2001; Siddall et al. 1995; Wang and Thompson 2008). Consistent with the literature, all spinal-lesioned rats in this study showed a significant decrease in mechanical hindpaw withdrawal thresholds bilaterally within 7 days of the lesion surgery (Fig. 2). Mechanical thresholds decreased from 86.7 ± 21 (SD) g (median 100; range 60–100) to 48.7 ± 18 g (median 60; range 26–60; P = 0.008, Friedman). Sham surgery had no effect on mechanical withdrawal thresholds on either the ipsi- or contralateral hindpaw (Fig. 2). Each animal tested had identical withdrawal thresholds on the ipsilateral and contralateral hindpaw at every time point. As a result, Fig. 2 shows the behavioral data for the contralateral hindpaw; results for the ipsilateral hindpaw were the same and are therefore not shown.
Fig. 2.
Hindpaw mechanical withdrawal thresholds decrease significantly over time after spinal lesions but not after sham surgery. All values represent means ± SE. Thresholds for the right and left hindpaw of each individual animal were identical; therefore only data for the hindpaw contralateral to the lesion are shown.
Properties of SI neurons
Recordings were made from 73 well-isolated single units in SI that responded to hindpaw stimulation (34 from control and 39 from spinal-lesioned animals). Hindpaw responsive neurons were encountered at similar frequencies in both control and spinal lesioned animals. Post hoc histological examination of electrode tracks and lesion sites revealed that all recorded neurons were located in SI (Fig. 1B). Due to the lesion size, the precise laminar location of these neurons could not be defined with confidence. However, in both CPS and control rats, most recorded neurons were located at depths ranging from 0.7 to 1.7 mm below the cortical surface (28/34 neurons in control animals and 35/39 neurons in CPS animals), which we estimate to correspond to layer IV and layer V based on histological examination. A few neurons in each group (6/34 in control animals and 4/39 in CPS animals) were located at depths ranging from 0.4 to 0.6 mm, which we estimate corresponds to layer II/III.
The distribution of receptive fields of recorded SI neurons was similar between the two groups. In control animals, receptive fields included the plantar surface of the hindpaw (22 neurons; 65%), single digits (8 neurons; 23%), multiple digits with a single digit responding preferentially (2 neurons; 6%), and the lateral surface of the hindpaw (2 neurons; 6%). In spinal-lesioned animals, receptive fields included the plantar surface of the hindpaw (27 neurons, 69%), single digits (10 neurons; 26%), and multiple digits with a single digit responding preferentially (2 neurons; 5%). These regions of the hindpaw are included in the hyperalgesic area, that is, the plantar surface of the paw that exhibits profound thermal (heat/cold) hyperalgesia (Masri et al. 2009; Wang and Thompson 2008).
Analysis of the spike waveforms using criteria developed by Bruno and Simons (2002) showed that all recorded neurons were regular-spiking units. The majority of regular-spiking units are thought to be excitatory cells with a small subpopulation composed of GABAergic interneurons (Beaulieu 1993; Bruno and Simons 2002; Kawaguchi and Kubota 1993). Therefore we believe that the majority of neurons recorded in this study consist of excitatory cells.
All recorded neurons responded to light stroking and tapping of the hindpaw as well as to pinching with forceps. The mechanical stimuli we used to evoke responses in the innocuous and noxious range (see methods) cannot be used to reliably distinguish between nociceptive-specific, wide dynamic range, and nonnociceptive SI neurons as was done in previous studies in the rat (Lamour et al. 1982, 1983a,b). This is because it is not possible to selectively activate peripheral nociceptors with these mechanical probes. In addition, our stimulus paradigm does not allow a distinction between wide dynamic range neurons and slowly adapting nonnociceptive neurons. Further, in the previous studies (cited in the preceding text), noxious stimuli (pinch, thermal) were applied only once to each neuron to avoid tissue damage and sensitization associated with repeated stimuli. As a result, the classification of SI neurons in these studies was based on a qualitative comparison between responses to noxious and innocuous stimuli and not on statistical analyses. For these reasons, we made no attempt to classify SI neurons as nociceptive-specific, nonnociceptive, or wide dynamic range neurons.
Neuronal activity is increased in SI of CPS rats
Because CPS is associated with increased activity in PO, we hypothesized that neurons in SI, a major projection target of PO (see introduction) would also show abnormally increased activity. To test this hypothesis, spontaneous activity and stimulus-evoked responses were recorded from SI neurons in spinal-lesioned rats with confirmed mechanical hyperalgesia (n = 6 animals) and from sham-operated (n = 3 animals) and naive (n = 3 animals) controls. There were no significant differences between sham and naive rats for any measure of neuronal activity; therefore the data for these groups were combined (Figs. 1 and 3–5).
Fig. 3.
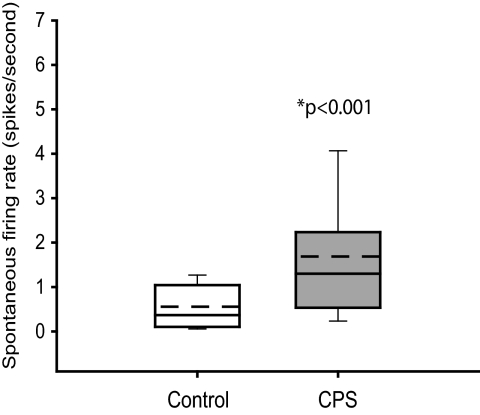
Group data showing that the spontaneous activity is significantly higher in SI neurons from animals with central pain syndrome (CPS, n = 39) than in control rats (n = 34). Boxes represent the 25th to 75th percentile of the distribution; whiskers show the 10th and 90th percentiles. - - -, mean values.
Fig. 4.
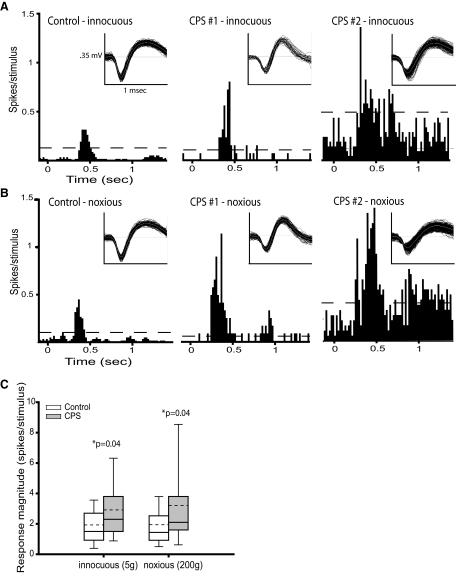
Neuronal activity in SI is enhanced in animals with CPS. A: peristimulus time histograms (PSTHs, 20 ms bins) showing the responses to innocuous mechanical hindpaw stimulation (5 g) of SI neurons from a sham-lesioned rat (left) and a spinal-lesioned rat with behaviorally confirmed CPS (middle and right). Sensory-evoked activity in SI neurons recorded from the CPS rat is markedly higher than in the neuron from the sham rat. Spontaneous activity is also higher in the second SI neuron from the CPS rat (right) than in the neuron from the sham rat. Dashed lines represent, the threshold at which the response significantly exceeded the spontaneous firing rate (99% confidence interval). Inset: representative spike waveforms. B: PSTHs showing the responses to noxious mechanical hindpaw stimulation (200 g) of SI neurons from a sham rat (left) and a CPS rat (middle and right). As in A, the sensory-evoked neuronal activity is higher in the CPS rats. C: group data showing that activity evoked by both innocuous and noxious mechanical stimulation of the hindpaw is significantly higher in SI neurons from animals with CPS (n = 30) than from control rats (n = 28). - - -, mean values.
Fig. 5.
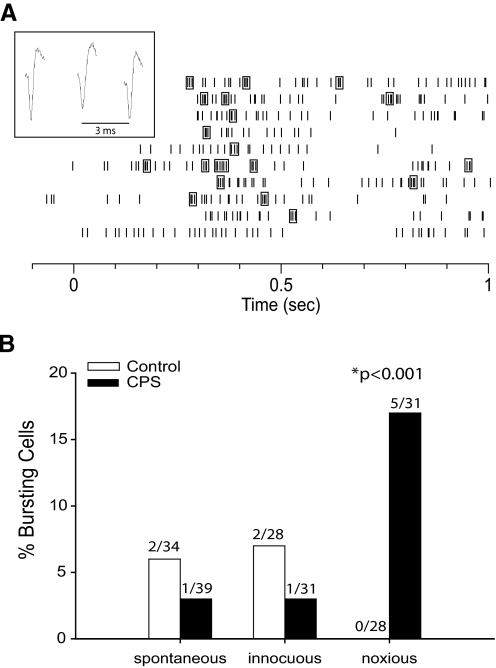
A: raster plot of a bursting neuron's response to 10 applications of a noxious mechanical stimulus to the hindpaw at time = 0 s. Spikes enclosed in boxes represent bursts of action potentials (as defined in methods). Inset: spike waveforms of a burst from this neuron. B: percentage of cells in spinal and sham-lesioned animals that showed bursting activity either spontaneously or in response to innocuous and noxious hindpaw stimuli. The number of cells that showed bursting activity in each category is shown above the bars. The percentage of cells that burst in response to noxious stimuli was significantly higher in CPS rats than control rats (P < 0.001, χ2 test).
Spontaneous firing rates
As a group, SI neurons from CPS rats have significantly higher spontaneous firing rates than controls (median: 1.3 Hz, range: 0.1–6.5 vs. median: 0.37 Hz, range: 0.07-1.9 in controls, P < 0.001, Fig. 3).
Responses evoked by mechanical stimuli
Innocuous (5 g) and noxious (200 g) mechanical stimuli were applied to the hindpaw ipsilateral to the lesion site using a motorized probe. This allowed us to quantitatively analyze PSTHs computed from the responses to these stimuli (see methods). Motorized probe stimuli were applied to the receptive fields of 28 neurons in control rats and 30 neurons in CPS rats. All recorded neurons showed significant responses to both innocuous and noxious stimuli (exceeded the spontaneous firing rate at the 99% confidence interval).
Recordings from representative SI neurons from a sham control animal and an animal with behaviorally confirmed CPS are shown in Fig. 4, A and B. The magnitude of the response evoked by innocuous stimuli (Fig. 4A) is higher in the neurons from the CPS rat (1.9 and 3.8 spike/stimulus) than in the control rat (1.2 spike/stimulus). The spontaneous firing rate of the second neuron from the CPS rat (2.5 Hz) is markedly higher than in the neuron from the control rat (0.8 Hz). Similarly, the responses evoked by noxious stimuli (Fig. 4B) are higher in the CPS rat (3.8 and 9.0 spike/stimulus) than in the control rat (1.4 spike/stimulus).
As a group, neurons from CPS rats show higher noxious stimulus-evoked responses than controls (median: 2.1 spike/stimulus, range: 0.4–15.7 vs. median: 1.4 spike/stimulus, range: 0.2–6.7 in controls, P = 0.04, Fig. 4C). Neurons from CPS rats also show higher responses to innocuous stimuli than controls (median: 2.3 spike/stimulus, range 0.4-11.7 vs. median: 1.5 spike/stimulus, range: 0.3–5.8 in controls, P = 0.04, Fig. 4C). There was no significant difference between CPS and control rats in the duration of either the innocuous-evoked responses (P = 0.7) or the noxious-evoked responses (P = 0.1). There was also no significant difference between CPS and control rats in the onset latency of the response to either innocuous (P = 0.8) or noxious (P = 0.3) mechanical stimulation.
Bursting activity is increased in SI of CPS rats
It has been suggested previously that CPS, in both humans and animal models, is associated with abnormally high incidence of bursting activity in thalamic nuclei (Lee et al. 2005; Lenz et al. 1989; Vierck et al. 1990; Wang and Thompson 2008; Weng et al. 2003; but see Dostrovsky 2007). Therefore, we were interested in examining whether SI neurons also showed increased bursting in CPS. (See methods for definition and classification of bursts.)
In both CPS and control animals, spontaneous bursts of action potentials and bursts in response to innocuous mechanical stimuli were rare (1 or 2 cells in each group, Fig. 5). Burst frequencies were comparable between groups both during spontaneous firing (0.01 Hz in CPS rats vs. 0.009 Hz in controls) and in response to innocuous stimuli (0.009 Hz in CPS rats vs. 0.01 Hz in controls). However, in response to noxious mechanical stimuli, 17% of cells from CPS animals showed bursting activity (5/31 cells), whereas no cells from control animals showed bursting activity (P < 0.001, χ2 test).
DISCUSSION
Our overarching hypothesis is that suppression of inhibitory inputs from ZI to PO contributes to CPS, with the consequent abnormally increased activity in PO transmitted up to its cortical targets. Here we tested the hypothesis that CPS is associated with increased spontaneous and evoked activity in SI, a major cortical target of PO (see introduction). Consistent with this hypothesis, we found that spontaneous activity was significantly increased in SI neurons of CPS rats compared with controls. We also found that CPS was associated with significant increases in responses evoked in SI neurons by both innocuous and noxious mechanical stimuli and that the tendency for SI neurons to burst in response to noxious stimuli was significantly greater in CPS animals. Overall, our data support the hypothesis that neuronal activity is abnormally increased in SI of CPS animals.
In our study, SI neurons showed responses that significantly exceeded the spontaneous firing rate to both innocuous and noxious mechanical stimuli. However, the magnitude of the responses was somewhat lower than previously reported by Lamour et al. (1982, 1983a,b). This discrepancy might reflect differences in anesthetics used (urethane vs. halothane). More likely these differences are due to the different type of stimuli used, especially to evoke noxious stimuli. We used discrete mechanical stimuli of different forces, a procedure that allowed for repeated application of noxious stimuli to compute the statistical significance of the responses. Lamour and collaborators (1982, 1983a,b) used strong pinch or thermal stimuli that were applied only once to prevent tissue damage and sensitization.
It is also possible that our spinal lesions interrupted the transmission of information from the periphery to the cortex, although this is unlikely based on our behavioral data that clearly demonstrate that the animals exhibit pronounced hyperalgesia. Despite these differences between our and Lamour's studies, we found that following spinal lesions, spontaneous neuronal firing in SI increased by 350% and evoked neuronal activity increased by upto 220%, indicating that SI responses are grossly abnormal in CPS.
SI pathophysiology in CPS
Our findings are consistent with evidence from the few published functional neuroimaging studies of CPS patients. A recent study of patients with CPS resulting from spinal cord injury found significant reorganization of SI that correlated with levels of ongoing pain (Wrigley et al. 2009). A positron emission tomography study of patients with poststroke CPS found that allodynia produced by rubbing a cool stimulus on the skin was associated with hyperperfusion of the contralateral thalamus, SI, second somatosensory cortex (SII), and anterior insula, compared with applying the stimulus to a nonallodynic body site (Peyron et al. 1998). Hirato et al. (1993) reported a positive correlation between the severity of spontaneous CPS pain and the level of glucose metabolism in the area around the central sulcus (SI and possibly SII) in patients with poststroke CPS. A functional MRI study of cold and mechanical allodynia in syringomyelia patients (characterized by lesions of the spinothalamic tract) reported activation of thalamus, SI, SII, and anterior insula (Ducreux et al. 2006). However, because quantitative comparisons with nonallodynic stimulation were not performed, it is unclear whether activity in these areas is increased in CPS. Along with these studies of human patients, a recent functional MRI study of rats with spinal cord injury-induced CPS found increased sensory-evoked SI responses (Endo et al. 2008). Taken together with our findings that SI neuronal activity is abnormally increased in rats with focal lesions of the spinal cord, these studies suggest that regardless of the type of original insult to the CNS, SI abnormalities are involved in CPS.
Additional CPS mechanisms
Other cortical areas might also be involved in CPS (Apkarian et al. 2005; Neugebauer et al. 2009), notably SII and the anterior insula, which both receive projections from PO (Alloway et al. 2003; Carvell and Simons 1986, 1987; Gauriau and Bernard 2004b; Spreafico et al. 1987). SII likely plays an important role in the sensory-discriminative aspects of pain (Apkarian et al. 2005). The anterior insula appears to be involved in interoceptive processing and contributes to the affective dimension of pain (Craig 2002, 2003b). Future electrophysiological studies of SII and anterior insula in our rat model of CPS are planned and will be key to elucidating the role of these cortical areas in the pathophysiology of the disease.
Although our data are consistent with the notion that abnormal increases in PO activity in CPS lead to increases in SI activity, this evidence is correlative and does not definitively demonstrate that PO causes the increases in SI activity in CPS rats. For example, VPL and the ventral posterior medial nucleus (VPM) also relay nociceptive information to SI (Gingold et al. 1991; Landry and Deschenes 1981; Shi and Apkarian 1995; Shi et al. 1993) and could potentially be involved. While we have previously demonstrated through in vivo electrophysiological recordings that there are no changes in VPL or VPM activity in rats with CPS induced by our spinal lesions (Masri et al. 2009), conflicting evidence has been reported in the literature. In our spinal lesion model of CPS, abnormally increased VPL activity has been reported in vitro (Wang and Thompson 2008). Furthermore, recordings made in vivo in the spinal contusion injury model of CPS have revealed that VPL neurons show greater spontaneous and evoked activity and enlarged receptive fields (Gerke et al. 2003; Hains et al. 2005, 2006; Zhao et al. 2007). One explanation for these disparate findings is that our CPS model involves injury to a relatively small region of the spinal cord, whereas the extent of injury elicited by spinal contusion is much greater and may potentially involve more suprapsinal structures. Thus the relative roles of PO, VPL, and VPM in the increased SI activity we see in CPS rats remain to be elucidated.
Both SI and the thalamic reticular nucleus (TRN) could potentially contribute to the abnormally increased activity of PO neurons observed in CPS rats. Corticothalamic projections from SI target PO (Alloway et al. 2003; Bourassa et al. 1995), and therefore increased SI activity in CPS may drive increased PO responses. TRN is inhibited by noxious mechanical stimulation (Peschanski et al. 1980), potentially resulting in disinhibition of PO. However, we consider these possible mechanisms unlikely, as we do not find changes in VPL (Masri et al. 2009), a major target of both corticothalamic projections from SI as well as TRN (Alloway et al. 2003; Bourassa et al. 1995; Yen and Jones 1983). Furthermore, the major source of excitatory input to TRN is from SI (Liu and Jones 1999). Thus, we would predict that animals with CPS would have increased excitatory drive to TRN, resulting in increased thalamic inhibition, which is not consistent with our findings.
Several ascending pathways relay nociceptive signals from the periphery to suprapsinal structures. While we have focused on the STT and the projections of its thalamic targets to the cortex, it is possible that the spinobulbar pathway could play a role in cortical changes in CPS through indirect projections from the parabrachial nucleus (Alden et al. 1994; Bernard et al. 1994; Bourgeais et al. 2001). Furthermore, the STT itself is complex and differs across species. In the primate, the STT terminates in numerous thalamic nuclei, including VPL and VPM, which project to SI and SII; the posterior portion of the ventral medial nucleus (VMpo), which relays nociceptive signals to SI and the insular cortex; and the medial dorsal nucleus (MD), which projects to the anterior cingulate cortex (Craig 2003a, 2004; Craig and Zhang 2006; Dostrovsky and Craig 2005; Gingold et al. 1991; Ray and Price 1993; Shi and Apkarian 1995). In the rat, the STT terminates in similar nuclei (including the VPL, VPM, and MD) as well as PO and the nucleus submedius (Gauriau and Bernard 2004a; Poggio and Mountcastle 1960; Zhang and Giesler 2005); however, the VMpo is absent in the rat (Dostrovsky and Craig 2005), and thus its possible involvement in CPS mechanisms cannot be tested in this species. Despite this limitation, the literature on CPS in humans and the findings of our study are consistent in that they demonstrate a role of SI abnormalities in CPS regardless of potential interspecies differences in the underlying mechanisms.
SI and the processing of pain
Although the role of SI in nociceptive processing is controversial, several lines of evidence support the notion that SI is a key component of the cortical network that is responsible for pain perception (Apkarian et al. 2005; Kenshalo and Willis 1991; Peyron et al. 2000). The controversy stems from early reports that SI lesions in humans did not produce analgesia (Head and Holmes 1911) and from functional neuroimaging studies in humans that showed inconsistent activation of SI in response to painful stimuli (Bushnell et al. 1999). However, Head and Holmes' data (1911) as well as subsequent studies in monkeys (Kenshalo et al. 1989; Peele 1944) showed that lesions in SI impaired the ability to localize or discriminate intensity of noxious stimuli. Evaluation of the functional neuroimaging data suggested that SI activation might be difficult to detect due to limitations of imaging technology and statistical methodology (Apkarian et al. 2005; Bushnell et al. 1999). Several other lines of evidence support a role for SI in pain processing. Anatomical studies in rats and nonhuman primates have shown that SI is innervated by thalamic nuclei (including PO, VPL, and VPM) that contain nociceptive neurons (Bureau et al. 2006; Dostrovsky and Craig 2005; Guilbaud et al. 1980; Kenshalo et al. 1980; Poggio and Mountcastle 1960). Electrophysiological investigations (including ours) have found SI single units that respond to noxious stimuli in rats (Lamour et al. 1982) and non-human primates (Kenshalo and Isensee 1983; Kenshalo et al. 1988). With regard for the specific role SI may play in pain processing, electrophysiological studies in animals and human functional neuroimaging studies have found that SI encodes stimulus intensity (Coghill et al. 1999; Kenshalo et al. 1988; Lamour et al. 1982; Moulton et al. 2005). Consistent with this notion, hypnotic suggestions that altered perceived pain intensity produced correlative changes in SI activity (Hofbauer et al. 2001). In addition, nociceptive processing in SI is somatotopically organized (Andersson et al. 1997; DaSilva et al. 2002; Kenshalo and Isensee 1983; Kenshalo and Perkins 1984). Taken together, the literature suggests a role for SI in the sensory-discriminative aspects of pain processing. Thus, abnormal increases in SI activity may in part underlie the excruciatingly painful symptoms associated with CPS.
GRANTS
This project was supported by National Institute of Neurological Disorders and Stroke Fellowship F32NS-064775 to R. L. Quiton and Grants 051799 to A. Keller and 055896 to S. M. Thompson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol 341: 289– 314, 1994 [DOI] [PubMed] [Google Scholar]
- Alloway KD, Hoffer ZS, Hoover JE. Quantitative comparisons of corticothalamic topography within the ventrobasal complex and the posterior nucleus of the rodent thalamus. Brain Res 968: 54– 68, 2003 [DOI] [PubMed] [Google Scholar]
- Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain 61: 187– 193, 1995 [DOI] [PubMed] [Google Scholar]
- Andersson JL, Lilja A, Hartvig P, Langstrom B, Gordh T, Handwerker H, Torebjork E. Somatotopic organization along the central sulcus, for pain localization in humans as revealed by positron emission tomography. Exp Brain Res 117: 192– 199, 1997 [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463– 484, 2005 [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Numerical data on neocortical neurons in adult rat with special reference to the GABA population. Brain Res 609: 284– 292, 1993 [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. The parabrachial area: electrophysiological evidence for an involvement in visceral nociceptive processes. J Neurophysiol 71: 1646– 1660, 1994 [DOI] [PubMed] [Google Scholar]
- Bonica JJ. History of pain concepts and pain therapy. Mt Sinai J Med 58: 191– 202, 1991 [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschenes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fiber study using biocytin as an anterograde tracer. Eur J Neurosci 7: 19– 30, 1995 [DOI] [PubMed] [Google Scholar]
- Bourgeais L, Monconduit L, Villanueva L, Bernard JF. Parabrachial internal lateral neurons convey nociceptive messages from the deep laminas of the dorsal horn to the intralaminar thalamus. J Neurosci 21: 2159– 2165, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966– 10975, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol 4: e382, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96: 7705– 7709, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V. Central Pain Syndrome: Pathophysiology, Diagnosis and Management. New York: Cambridge Univ Press, 2007 [Google Scholar]
- Carvell GE, Simons DJ. Somatotopic organization of the second somatosensory area (SII) in the cerebral cortex of the mouse. Somatosens Res 3: 213– 237, 1986 [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Thalamic and corticocortical connections of the second somatic sensory area of the mouse. J Comp Neurol 265: 409– 427, 1987 [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Carvell GE, Simons DJ. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285: 325– 338, 1989 [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 82: 1934– 1943, 1999 [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655– 666, 2002 [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26: 1– 30, 2003a [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500– 505, 2003b [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477: 119– 148, 2004 [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol 499: 953– 964, 2006 [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22: 8183– 8192, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO. The thalamus and human pain. In: Central Neuropathic Pain: Focus on Poststroke Pain, edited by Henry JL, Panju A, Yashpal K. Seattle, WA: IASP Press, 2007, p. 101–112 [Google Scholar]
- Dostrovsky JO, Craig AD. Ascending projection systems. In: Wall and Melzack's Textbook of Pain, edited by McMahon S, Koltzenburg M. Oxford: Churchill Livingstone, 2005, p. 187–204 [Google Scholar]
- Ducreux D, Attal N, Parker F, Bouhassira D. Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain 129: 963– 976, 2006 [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain 138: 292– 300, 2008 [DOI] [PubMed] [Google Scholar]
- Fabri M, Burton H. Topography of connections between primary somatosensory cortex and posterior complex in rat: a multiple fluorescent tracer study. Brain Res 538: 351– 357, 1991 [DOI] [PubMed] [Google Scholar]
- Frese A, Husstedt IW, Ringelstein EB, Evers S. Pharmacologic treatment of central post-stroke pain. Clin J Pain 22: 252– 260, 2006 [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468: 24– 56, 2004a [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 24: 752– 761, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience 117: 715– 722, 2003 [DOI] [PubMed] [Google Scholar]
- Giesler GJJ, Spiel HR, Willis WD. Organization of spinothalamic tract axons within the rat spinal cord. J Comp Neurol 195: 243– 252, 1981 [DOI] [PubMed] [Google Scholar]
- Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol 308: 467– 490, 1991 [DOI] [PubMed] [Google Scholar]
- Guido W, Lu SM, Vaughan JW, Godwin DW, Sherman SM. Receiver operating characteristic (ROC) analysis of neurons in the cat's lateral geniculate nucleus during tonic and burst response mode. Vis Neurosci 12: 723– 741, 1995 [DOI] [PubMed] [Google Scholar]
- Guilbaud G, Peschanski M, Gautron M, Binder D. Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8: 303– 318, 1980 [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain 128: 2359– 2371, 2005 [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol 95: 3343– 3352, 2006 [DOI] [PubMed] [Google Scholar]
- Hansson P. Post-stroke pain case study: clinical characteristics, therapeutic options and long-term follow-up. Eur J Neurol 11, Suppl 1: 22– 30, 2004 [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 34: 102– 254, 1911 [Google Scholar]
- Hirato M, Horikoshi S, Kawashima Y, Satake K, Shibasaki T, Ohye C. The possible role of the cerebral cortex adjacent to the central sulcus for the genesis of central (thalamic) pain–a metabolic study. Acta Neurochir Suppl 58: 141– 144, 1993 [DOI] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol 86: 402– 411, 2001 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70: 387– 396, 1993 [DOI] [PubMed] [Google Scholar]
- Kenshalo DRJ, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res 454: 378– 382, 1988 [DOI] [PubMed] [Google Scholar]
- Kenshalo DRJ, Giesler GJJ, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 43: 1594– 1614, 1980 [DOI] [PubMed] [Google Scholar]
- Kenshalo DRJ, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol 50: 1479– 1496, 1983 [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Perkins WC. Organizations of primate SI cortical nociceptive neurons. Pain Suppl 2: S312, 1984. [Google Scholar]
- Kenshalo DR, Thomas DA, Dubner R. Somatosensory cortex lesions change the monkey's reaction to noxious stimulation. J Dent Res Abstr 68: 897, 1989 [Google Scholar]
- Kenshalo DR, Willis WD. The role of the cerebral cortex in pain sensation. In: Normal and Altered States of Function, edited by Peters A, Jones EG. New York: Plenum, 1991, vol. 9, p. 153–212 [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res 463: 346–351, 1988 [DOI] [PubMed] [Google Scholar]
- Lamour Y, Guilbaud G, Willer JC. Rat somatosensory (SmI) cortex: II. Laminar and columnar organization of noxious and non-noxious inputs. Exp Brain Res 49: 46– 54, 1983a [DOI] [PubMed] [Google Scholar]
- Lamour Y, Willer JC, Guilbaud G. Neuronal responses to noxious stimulation in rat somatosensory cortex. Neurosci Lett 29: 35– 40, 1982 [DOI] [PubMed] [Google Scholar]
- Lamour Y, Willer JC, Guilbaud G. Rat somatosensory (SmI) cortex. I. Characteristics of neuronal responses to noxious stimulation and comparison with responses to non-noxious stimulation. Exp Brain Res 49: 35– 45, 1983b [DOI] [PubMed] [Google Scholar]
- Landry P, Deschenes M. Intracortical arborizations and receptive fields of identified ventrobasal thalamocortical afferents to the primary somatic sensory cortex in the cat. J Comp Neurol 199: 345– 371, 1981 [DOI] [PubMed] [Google Scholar]
- Lee JI, Ohara S, Dougherty PM, Lenz FA. Pain and temperature encoding in the human thalamic somatic sensory nucleus (ventral caudal): inhibition-related bursting evoked by somatic stimuli. J Neurophysiol 94: 1676– 1687, 2005 [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496: 357– 360, 1989 [DOI] [PubMed] [Google Scholar]
- Liu XB, Jones EG. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J Comp Neurol 414: 67– 79, 1999 [PubMed] [Google Scholar]
- Lu SM, Guido W, Sherman SM. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J Neurophysiol 68: 2185– 2198, 1992 [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res 10: 1– 16, 1993 [DOI] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: A role in central pain. J Neurophysiol 102: 181– 191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. Classification of Chronic Pain. Seattle, WA: IASP, 1994 [Google Scholar]
- Mills CD, Grady JJ, Hulsebosch CE. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J Neurotrauma 18: 1091– 1105, 2001 [DOI] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol 93: 2183– 2193, 2005 [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev 60: 226– 242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BD. Evaluation and treatment of central pain syndromes. Neurology 62: S30– 6, 2004 [DOI] [PubMed] [Google Scholar]
- Nothias F, Peschanski M, Besson JM. Somatotopic reciprocal connections between the somatosensory cortex and the thalamic Po nucleus in the rat. Brain Res 447: 169– 174, 1988 [DOI] [PubMed] [Google Scholar]
- Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis–prevalence and clinical characteristics. Eur J Pain 9: 531– 542, 2005 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- Peele TL. Acute and chronic parietal lobe ablations in monkeys. J Neurophysiol 7: 269– 286, 1944 [Google Scholar]
- Peschanski M, Guilbaud G, Gautron M. Neuronal responses to cutaneous electrical and noxious mechanical stimuli in the nucleus reticularis thalami of the rat. Neurosci Lett 20: 165– 170, 1980 [DOI] [PubMed] [Google Scholar]
- Peyron R, Garcia-Larrea L, Gregoire MC, Convers P, Lavenne F, Veyre L, Froment JC, Mauguiere F, Michel D, Laurent B. Allodynia after lateral-medullary (Wallenberg) infarct. A PET study. Brain 121: 345– 356, 1998 [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30: 263– 288, 2000 [DOI] [PubMed] [Google Scholar]
- Poggio GF, Mountcastle VB. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Central nervous mechanisms in pain. Bull Johns Hopkins Hosp 106: 266– 316, 1960 [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 337: 1– 31, 1993 [DOI] [PubMed] [Google Scholar]
- Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav 67: 711– 716, 1999 [DOI] [PubMed] [Google Scholar]
- Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol 95: 3865– 3874, 2006 [DOI] [PubMed] [Google Scholar]
- Sherman SM. Dual response modes in lateral geniculate neurons: mechanisms and functions. Vis Neurosci 13: 205– 213, 1996 [DOI] [PubMed] [Google Scholar]
- Shi T, Apkarian AV. Morphology of thalamocortical neurons projecting to the primary somatosensory cortex and their relationship to spinothalamic terminals in the squirrel monkey. J Comp Neurol 361: 1– 24, 1995 [DOI] [PubMed] [Google Scholar]
- Shi T, Stevens RT, Tessier J, Apkarian AV. Spinothalamocortical inputs nonpreferentially innervate the superficial and deep cortical layers of SI. Neurosci Lett 160: 209– 213, 1993 [DOI] [PubMed] [Google Scholar]
- Siddall P, Xu CL, Cousins M. Allodynia following traumatic spinal cord injury in the rat. Neuroreport 6: 1241– 1244, 1995 [DOI] [PubMed] [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103: 249– 257, 2003 [DOI] [PubMed] [Google Scholar]
- Spreafico R, Barbaresi P, Weinberg RJ, Rustioni A. SII-projecting neurons in the rat thalamus: A single- and double-retrograde-tracing study. Somatosens Res 4: 359– 375, 1987 [DOI] [PubMed] [Google Scholar]
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain 87: 113– 119, 2000 [DOI] [PubMed] [Google Scholar]
- Vierck CJJ, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci 10: 2077– 2095, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci 28: 11959– 11969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HR, Lenz FA, Vierck C, Dougherty PM. Physiological changes in primate somatosensory thalamus induced by deafferentation are dependent on the spinal funiculi that are sectioned and time following injury. Neuroscience 116: 1149– 1160, 2003 [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141: 52– 59, 2009 [DOI] [PubMed] [Google Scholar]
- Yen CT, Jones EG. Intracellular staining of physiologically identified neurons and axons in the somatosensory thalamus of the cat. Brain Res 280: 148– 154, 1983 [DOI] [PubMed] [Google Scholar]
- Zhang X, Giesler GJJ. Response characterstics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol 93: 2552– 2564, 2005 [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27: 8893– 8902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]