Abstract
We describe intracranial local field potentials (LFPs) recorded in the supplementary eye field (SEF) of macaque monkeys performing a saccade countermanding task. The most prominent feature at 90% of the sites was a negative-going polarization evoked by a contralateral visual target. At roughly 50% of sites a negative-going polarization was observed preceding saccades, but in stop signal trials this polarization was not modulated in a manner sufficient to control saccade initiation. When saccades were canceled in stop signal trials, LFP modulation increased with the inferred magnitude of response conflict derived from the coactivation of gaze-shifting and gaze-holding neurons. At 30% of sites, a pronounced negative-going polarization occurred after errors. This negative polarity did not appear in unrewarded correct trials. Variations of response time with trial history were not related to any features of the LFP. The results provide new evidence that error-related and conflict-related but not feedback-related signals are conveyed by the LFP in the macaque SEF and are important for identifying the generator of the error-related negativity.
INTRODUCTION
Human errors in speeded response tasks are signaled by an event-related potential known as the error-related negativity (referred to as ERN or Ne; e.g., Falkenstein et al. 1991; Gehring et al. 1993). The ERN has a frontocentral distribution over the scalp and peaks about 100 ms after the incorrect response in choice-reaction time tasks or the uninhibited response on no-go trials (e.g., Scheffers et al. 1996). Several hypotheses have been proposed to explain ERN occurrence and function (reviewed by Taylor et al. 2007). The first proposes that the ERN reflects a comparison between the representations of the overt error response and the correct response (Falkenstein et al. 1991; Gehring et al. 1993). The second hypothesis proposes that the ERN is related to reward prediction error (e.g., Holroyd and Coles 2002). A third hypothesis proposes that the ERN represents the occurrence of response conflict, i.e., the coactivation of mutually incompatible response processes (e.g., Botvinick et al. 2001; Yeung et al. 2004). However, other hypotheses have been formulated that seek to integrate all of these specific alternatives (Brown and Braver 2007). Resolution among these alternatives should be facilitated by high-resolution neurophysiological data from the source of the ERN. A number of investigations have localized dipole origins for the ERN in the anterior cingulate cortex (ACC) (e.g., Dehaene et al. 1994; Miltner et al. 1997; van Veen and Carter 2002), but contributions cannot be ruled out from more dorsal areas of medial frontal cortex such as the presupplementary and supplementary motor areas (e.g., Miltner et al. 1997).
We have investigated these issues in macaque monkeys performing a saccade stop signal task. The stop signal task requires subjects to inhibit a response at various stages of preparation when a stop signal is presented (Hanes and Schall 1995; Logan and Cowan 1984; reviewed by Verbruggen and Logan 2008). Presaccadic movement and fixation neurons in the frontal eye fields (FEFs) and superior colliculus (SC) exhibit stochastic modulation of activity that is sufficient to control the initiation of saccades (Brown et al. 2008; Hanes et al. 1998; Paré and Hanes 2003). In contrast to the FEFs and the SC, the supplementary eye fields (SEFs) and the ACC do not produce signals with timing sufficient to control saccade initiation (Stuphorn et al. 2010). Instead neurons in SEF signal error, reward, and conflict (Stuphorn et al. 2000), whereas neurons in ACC signal error, reward, and feedback (Ito et al. 2003; see also Amiez et al. 2003, 2005; Matsumoto et al. 2007; Nakamura et al. 2005; Procyk et al. 2000). Thus the apparent colocalization of single-unit activity signaling errors and the inferred dipole of the ERN suggests that these neural signals are the generator of the ERN. The ERN and its presumed hemodynamic correlate have been observed in humans performing the countermanding task (Dimoska et al. 2006; Endrass et al. 2005; Sharp et al. 2010; Stahl and Gibbons 2007) and the antisaccade task (Nieuwenhuis et al. 2001). However, event-related potentials (ERPs) are understood to arise from the summation of synaptic potentials and not neural spikes.
Our laboratory has begun building an empirical bridge between the monkey neurophysiology and human electrophysiology by recording synaptic potentials through local field potentials (LFPs) in monkeys performing tasks for which both single-unit and ERP data have been obtained. The ultimate goal is to directly link intracranial LFP properties to the characteristics of extracranial electrophysiological measures from macaque monkeys (Cohen et al. 2009; Garr et al. 2008; Woodman et al. 2007). The parallels between human electrophysiology and macaque neurophysiological findings suggest that they are different perspectives on a common functional system. However, this inference is uncertain because of the differences between species (the most recent common ancestor of humans and macaques monkeys lived ∼25 million years ago; e.g., Kay et al. 1997; Martin 1993) and differences between measurements (ERPs from the scalp and single-unit recordings in macaques).
Recently we found that LFPs recorded in the dorsal bank of the anterior cingulate sulcus of monkeys performing the saccade stop signal task signaled error and reinforcement in a manner consistent with being the generator of the ERN (Emeric et al. 2008). The specific goal of this study was to determine whether LFPs signaling error, reinforcement, or conflict are observed in the SEF of macaque monkeys, an area that is situated on the dorsal convexity of medial frontal cortex, essentially parallel to the dorsal bank of the cingulate sulcus. The results from this study provide clear evidence that LFPs in the SEF do not contribute to controlling saccade initiation. Furthermore, error-related and conflict-related but not feedback-related LFP modulation occur in the SEF of macaque monkeys. These results provide new and unexpected insights into the cerebral source of the ERN.
METHODS
Data were collected from three male bonnet monkeys (Macaca radiata: 8–10 kg, designated F, M, and U) that were cared for in accordance with the U.S. Department of Agriculture and Public Health Service Policy on the humane care and use of laboratory animals. Each animal was tested for about 4 h/day, 5 days/wk. During testing, water or fruit juice was given as positive reinforcement. Access to water in the home cage was controlled and monitored. Fluids were supplemented as needed. Detailed descriptions of all surgical procedures, electrophysiological techniques, behavioral training, and tasks were previously reported (Hanes and Schall 1995; Hanes et al. 1998).
The experiments were under computer control to present stimuli, record eye movements, and deliver liquid reinforcement. Stimuli were presented on a video monitor (48 × 48°) using computer-controlled raster graphics (512 × 512 pixel resolution or TEMPO Videosync 1,280 × 1,040 pixel resolution). The fixation spot subtended 0.37° of visual angle and the target stimuli subtended from 0.3 to 3° of visual angle, depending on their eccentricity, and had a luminance of 10 or 30 cd/m2 on a 1 cd/m2 background. Eye position was monitored via a scleral search coil or a video-based infrared eye tracker (ASL, Bedford, MA) while the monkeys were head-restrained and seated in an enclosed chair within a magnetic field. Saccades were detected using a computer algorithm that searched for significantly elevated velocity (30°/s). Saccade initiation and termination were defined as the beginning and end of the monotonic change in eye position during the high-velocity gaze shift.
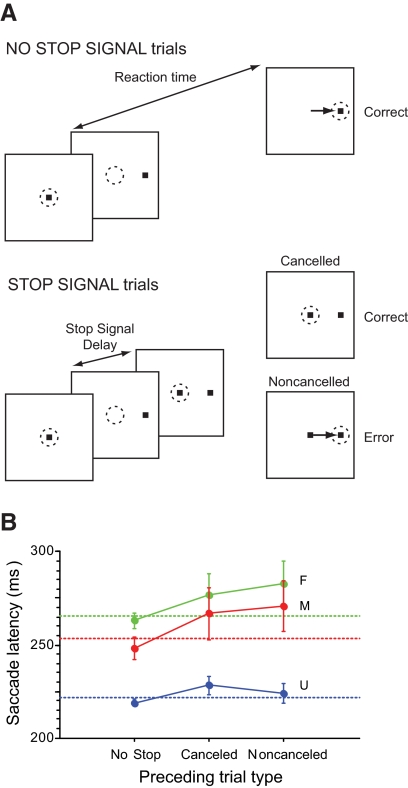
The countermanding task provided the data for this study. All trials began when the monkey shifted gaze to fixate a centrally located stimulus for a variable interval (500–800 ms; Fig. 1). Following this fixation interval, the central stimulus was removed and a peripheral target was simultaneously presented at one of two locations in opposite hemifields, cuing the monkey to make a single saccade to the target. Targets were located along the horizontal axis, typically 10° from the fixation target. For trials with no stop signal, the monkeys were reinforced for making a saccade within 800 ms. In each behavioral session, the delay between fixation of the target and delivery of the reinforcement was constant at 400 ms. On 20–50% of the trials, after a delay (referred to as the stop signal delay [SSD]), the central fixation target reappeared, instructing the monkey to inhibit saccade initiation. Two outcomes were possible on these stop signal trials. First, maintaining fixation on the stop signal for 1,500 ms after the target appeared was reinforced as correct; these trials were referred to as canceled trials. Second, a saccade to the target after the stop signal was presented was considered incorrect; these trials were referred to as noncanceled trials. Noncanceled error trials resulted in no positive reinforcement and a 1,500-ms addition to the intertrial interval.
Fig. 1.
A: saccade countermanding task. Dotted circle indicates focus of gaze at each interval; arrow, the saccade. All trials began with presentation of a central fixation spot. After fixation of this spot for a variable interval, it disappeared simultaneously with the presentation of a target on the left or right. In no stop signal trials, a single saccade to the peripheral target was reinforced as the correct response. In stop signal trials, the fixation spot reappeared after a variable stop signal delay. Maintained fixation was reinforced as the correct response; these are referred to as canceled (or signal-inhibit) trials. If a saccade was produced in spite of the stop signal, no reinforcement was given; these errors are referred to as noncanceled (or signal-respond) trials. B: influence of trial history on response time on no stop signal trials. Columns represent the mean no stop signal reaction time for trials (±SE) with the sequences of preceding trials indicated on the abscissa for each monkey. The horizontal dashed lines are the mean no stop response time for each monkey.
Primary reinforcement in the form of juice and secondary reinforcement in the form of an auditory tone were delivered at the conclusion of correct no stop and canceled trials. Primary but not secondary reinforcement was randomly withheld on 5–15% of the correct trials in each session. In each behavioral session, three to six SSDs of constant value ranging from 25 to 450 ms were used. The values were adjusted across sessions and monkeys to allow for gradual changes in response time so that, on average, the monkeys failed to inhibit approximately half the stop signal trials.
Data acquisition
LFPs were recorded using single tungsten microelectrodes (impedance: 2–5 MΩ at 1 kHz), nonreferenced single ended. The electrode signals were amplified with a high-input impedance head stage (>1 GΩ , ∼2 pF of parallel input capacitance: HST/8m-G1-GR; Plexon, Dallas, TX) and filtered by a multichannel acquisition processor (Plexon). The LFP data were filtered between 0.7 and 170 Hz with two cascaded one-pole low-cut Butterworth filters plus a four-pole high-cut Butterworth filter and sampled at 1 kHz. Both spikes and LFPs were referenced to the guide tube touching the dura.
Data analysis
All recording sites were assessed for the occurrence of excessive noise. Recordings with recurring artifacts during time intervals of interest were excluded from analysis. The mean voltage in the 300 ms preceding target presentation for each valid trial was defined as the baseline and subtracted from the voltage for each trial. SSDs were varied according to the monkeys' performance so that at the shortest SSD, the monkeys generally inhibited the movement in >75% of the stop signal trials and at the longest delay, the monkeys inhibited the movement in <25% of the stop signal trials. No selection was made on the basis of whether the LFP displayed task-related polarization.
To identify intervals of significant LFP modulation across different trial types, single-trial LFPs were time-locked to stimulus presentation or saccade initiation and then time averaged for each trial type. The event-related LFPs were then filtered using a 50th-order low-pass finite impulse response digital filter with a cutoff of 30 Hz. For all comparisons between trial types, a difference wave was produced by subtracting the time-locked LFP in one condition from that in the other (e.g., the difference between noncanceled and latency-matched no stop signal trials). The onset of a significant difference was defined as the instant when the difference wave exceeded ±2SD for ≥50 ms and achieved a difference of ±3SD during that interval. This criterion was used to compare the LFPs on trials with no stop signal to the LFPs on canceled and noncanceled trials. The onset of a significant stimulus-evoked polarization was defined as the instant the stimulus-locked wave exceeded ±2SD of the baseline voltage for ≥25 ms and achieved a difference of ±3SD during that interval.
The rationale and approach for the race model analysis of the countermanding data have been described in detail previously (Hanes et al. 1995, 1998; Logan and Cowan 1984). The data obtained in the countermanding task are the inhibition function (i.e., the probability of noncanceled trials as a function of SSD) and the reaction times in no stop signal trials and in noncanceled stop signal trials. An analysis of these data based on the race model was performed to estimate the stop signal reaction time (SSRT) from the behavioral data collected while recording from each site in the SEF. The SSRT, the length of time that was required to cancel the saccade, was estimated using two methods (Band et al. 2003; Logan and Cowan 1984). The first assumes that SSRT is a random variable, whereas the second method assumes that SSRT is constant. We used the mean of the two values as the estimate of SSRT. Hanes et al. (1998) established the central benefit of the countermanding paradigm as capable of determining whether neurophysiological signals modulate in a manner sufficient to control the initiation of movements. For a neural signal to play a direct role in controlling the initiation of an eye movement, it must be different during trials in which a saccade is initiated compared with trials in which the saccade is inhibited. Moreover, this difference in activity must occur before the SSRT has elapsed—the deadline for the movement to be canceled.
RESULTS
In all three monkeys, the inhibition function was a monotonically increasing function and the mean noncanceled response time (F, 244 ± 67 ms; M, 228 ± 54 ms; U, 208 ± 40 ms) was faster than the mean no stop response time (F, 266 ± 63 ms; M, 253 ± 56 ms; U, 222 ± 38 ms, Fig. 1B). The average ± SD SSRT estimated using both methods described earlier was 85 ± 10 ms (F, 79 ± 10 ms; M, 80 ± 8 ms; U, 95 ± 12 ms).
Emeric et al. (2007) reported the dependence of response time on trial history in the context of countermanding saccades. Specifically, no stop response times increase following canceled stop signal trials but posterror slowing is not observed in this context. Consistent with this finding, significant posterror slowing was not observed in the present data [monkey F, t(7) = −1.06, P = 0.30; monkey M, t(8) = −1.88, t(10), P = 0.07; monkey U, P = 0.62; two-sample t-test; Fig. 1B]. In addition, although no stop response times increased following canceled trials, this postcanceled slowing was not significant [monkey F, t(7) = −0.90, P = 0.38; monkey M, t(8) = −1.5, P = 0.14 ; monkey U, t(10) = −0.99, P = 0.33; Fig. 1B].
Event-related LFP in SEF
We report data from 82 sites in the SEF of three monkeys. Neurophysiological data were recorded serially along acute single penetrations. An individual site consisted of all the behavioral and neurophysiological data recorded from a single location in the cortex.
LFPs recorded from the SEF of monkeys performing the saccade stop signal task exhibited stimulus-related polarization, pronounced presaccadic and postsaccadic polarization, and auditory responses (Fig. 2). Note that in this and all subsequent figures plotting voltage on the ordinate, negative is up according to convention. Also note that only no stop signal trials with saccade latencies of >200 ms were used to produce stimulus- and saccade-evoked potentials to minimize the contribution of any presaccadic LFP polarization to visually evoked components.
Fig. 2.
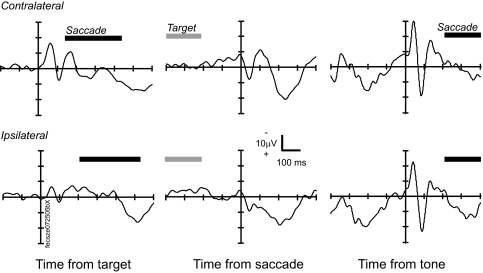
Event-related local field potentials (LFPs) from a representative site in the supplementary eye field (SEF). Left: LFP from no stop signal trials time-locked on the time of target presentation for contralateral (top, 348 trials) and ipsilateral (bottom, 340 trials) targets. Middle: LFP time-locked on the initiation of saccade to contralateral (top) and ipsilateral (bottom) targets. Right: LFP time-locked on the auditory tone used as a secondary reinforcement. The range of target presentation times and saccade initiation times after the target (left) and after the reinforcement (right) is indicated by the bars above the abscissa.
The visual stimulus-evoked polarization of the LFP in SEF was an early negative deflection followed by a positive deflection within 100 ms of stimulus presentation (Fig. 2, left). This response was stronger for stimuli in the contralateral visual field but was not absent for stimuli in the ipsilateral visual field, consistent with the laterality of single-unit responses in SEF (Schall 1991b). The onset of a significant stimulus-evoked polarization was defined as the instant the stimulus-locked wave exceeded ±2SD of the baseline voltage for ≥25 ms and achieved a difference of ±3SD during that interval. Significant stimulus-evoked polarization of the intracranial LFP was observed at the majority of sites in the SEF (10/14 in F, 12/15 in M, 40/53 in U). Significant stimulus-evoked LFP polarization was more common for targets presented contralateral (50/82 sites) than ipsilateral (35/82 sites) to the recording site. The mean ± SD latency of the LFP polarization evoked by contralateral targets was 96 ± 57 ms and that for ipsilateral targets was 96 ± 48 ms. The onset latency was not different for ipsiversive versus contraversive targets (P = 0.72; χ2 = 0.12, Kruskal–Wallis rank-sum test).
A potential similar to the visually evoked potential was also observed following auditory stimuli (Fig. 2, right). The auditory stimulus was a tone delivered as a secondary reinforcer presented 400 ms following a correct trial at the same instant as the primary reinforcer while the target was extinguished. The monkeys would often make a saccade away from the target shortly after reinforcement. Therefore to minimize the contribution of the presaccadic polarization to these sensory components, only no stop signal trials with saccades produced >200 ms after the secondary reinforcer were used to produce stimulus-evoked and saccade-evoked potentials. In potentials time-locked on the auditory tone used as a secondary reinforcer we observed a biphasic potential at 23 of 82 sites across the three monkeys (3/14 in F, 5/15 in M, 15/53 in U). This potential began with a latency of 85 ± 50 ms. This observation is consistent with SEF single-unit auditory responses (Schall 1991b).
A negative-going polarization was observed in the 200 ms preceding the saccade (Fig. 2, middle). However, no presaccadic spike potential or saccade artifact was observed (e.g., Lins et al. 1993), consistent with earlier observations in human patients (e.g., Yamamoto et al. 2004). The presaccadic negativity was stronger for contraversive than that for ipsiversive saccades. Presaccadic polarization was quantified by fitting a regression line to the saccade-evoked potential in the interval from 200 to 15 ms before the saccade. A significant Spearman correlation (α = 0.05) was observed at 87% (71/82) of sites (12/14 in F, 10/15 in M, 18/53 in U). The LFP became significantly more negative prior to contraversive saccades at 54% (44/82 sites) and more positive prior to ipsiversive saccades at 50% (41/82) of the sites in SEF. Overall, the LFP became more negative in the 185 ms prior to contraversive saccades (mean correlation across sites; r = − 0.13) and more positive prior to ipsiversive saccades (mean across sites; r = 0.05). Postsaccadic polarization of the SEF LFPs was commonly observed. We identified LFP polarization in the interval following the saccade at 71/82 of the sites (13/14 in F, 11/15 in M, 41/53 in U). LFP polarization was equally common following contraversive (29/82 sites) and ipsiversive (25/82 sites) saccades. Significant polarization began 234 ± 125 ms after contraversive and 271 ± 120 ms after ipsiversive saccades. The latency was not significantly different for contraversive versus ipsiversive targets (P = 0.30; χ2 = 1.08, Kruskal–Wallis rank-sum test).
Tests of saccade control
The logic of the stop signal task and the measurement of SSRT using the race model suggest particular comparisons between stop signal and no stop signal trials. First, canceled stop signal trials can be compared with those no stop signal trials with latencies long enough that the saccade would have been canceled if a stop signal had occurred. Specifically, the LFP from canceled stop signal trials can be compared with the LFP from no stop signal trials with saccade latencies greater than SSD + SSRT. Second, noncanceled stop signal trials can be compared with those no stop signal trials with latencies short enough that the saccade would not have been canceled if a stop signal had occurred. Specifically, the LFP from noncanceled stop signal trials can be compared with the LFP from no stop signal trials with saccade latencies less than SSD + SSRT. We refer to the subset of no stop signal trials compared with either canceled or noncanceled stop signal trials as latency-matched.
To determine whether LFPs recorded from the SEF were modulated in a manner sufficient to control the production of saccades, we compared the LFP on canceled trials with the LFP on latency-matched no stop signal trials. According to the race model, these are the no stop signal trials in which the GO process was slow enough that the STOP process would have finished before the GO process if the stop signal had occurred. The onset of significant differential polarization was measured for each SSD collected at each site in the SEF. If significant polarization was measured, the time of that polarization was compared with the SSRT estimated from the behavioral data collected during each recording. To determine whether LFP polarization was proportional to response conflict, the average polarity difference between canceled and latency-matched no stop signal trials was measured following the analysis of Stuphorn et al. (2000). To determine whether the LFP signaled error or feedback, we measured polarization following saccade initiation and reward delivery. For each site, the LFP time-locked on saccade initiation on noncanceled trials was compared with the LFP time-locked on saccade initiation on no stop signal trials. Response-locked LFPs were produced for saccades to each target separately and collapsed across targets.
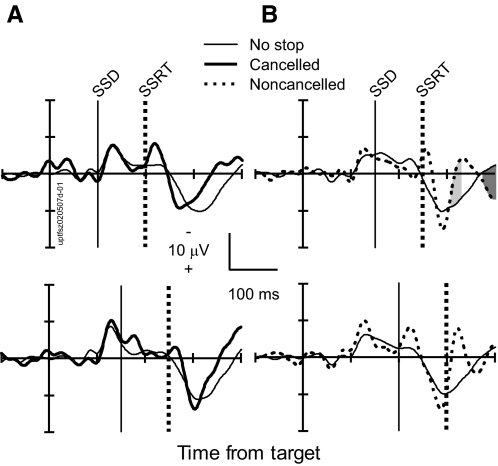
Figure 3 illustrates these comparisons for target-aligned LFPs from a representative site in the SEF. Consider first the comparison between canceled trials and latency-matched no stop signal trials (Fig. 3A). When examined in this manner, movement- and fixation-related but not visual neurons in the FEF and the SC exhibit a pronounced modulation in canceled trials occurring before the SSRT (Brown et al. 2008; Hanes et al. 1998; Paré and Hanes 2003). This modulation occurs in a manner and at a time sufficient to control saccade initiation.
Fig. 3.
Absence of saccade control signal at a representative site in SEF. A: comparison of LFPs in correct canceled stop signal trials (thick) and latency-matched no stop signal trials (thin) with stop signal delays (SSDs) of 101 ms (top, 561 no stop trials; 49 canceled trials) and 151 ms (bottom, 348 no stop trials; 59 canceled trials). Intervals in stop signal trials in which polarity was significantly more negative are highlighted by dark gray. Intervals in stop signal trials in which polarity is significantly more positive are highlighted by light gray. B: comparison of LFP in error noncanceled stop signal trials (thick, dotted) and latency-matched no stop signal trials (thin) with SSDs of 151 ms (top, 295 no stop trials; 45 noncanceled trials) and 201 ms (bottom, 478 no stop trials; 43 noncanceled trials).
In contrast to the saccade-related activity in FEF and SC, we observed a significant difference between the LFP recorded on canceled trials and that recorded on latency-matched no stop signal trials in only 5% (20/429) of the SSDs sampled across 82 sites in the SEF (2/75 in F, 3/51 in M, 15/305 in U). In approximately half of these few SSDs (2%, 8/429), the LFP polarity on canceled trials was more negative than that on no stop trials and, in the other half (3%, 12/429), the LFP on canceled trials was more positive than that on no stop trials. However, these rare polarity differences occurred on average 228 ± 205 ms (negative polarity difference) and 297 ± 276 ms (positive polarity difference) after the SSRT. Crucially, no significant polarization difference between canceled trials and no stop signal trials occurred before the SSRT. This result clearly demonstrates that presaccadic LFPs in the SEF do not modulate in a manner sufficient to control the initiation of saccades.
We compared the target-aligned LFP polarization on noncanceled error trials with that on latency-matched no stop signal trials having saccade latencies less than SSD + SSRT (Fig. 3). These are the no stop signal trials in which the GO process was so fast that it would have finished before the STOP process if the stop signal had been presented. On 41% (174/429 SSDs) of the SSDs across 82 sites in the SEF, we observed a significantly enhanced LFP polarization for noncanceled error trials relative to that observed in latency-matched no stop signal trials. Overall, the LFP on 5% of SSDs (25/429) exhibited greater negativity, on 10% (45/429) exhibited greater positivity, and on 24% (104/429 SSDs) exhibited a greater negative polarization followed by a positive polarization on error trials.
On average, the latency of the negative modulation enhancement was 129 ± 166 ms after the SSRT, whereas that of the positive modulation was 190 ± 144 ms. Given the additional time occupied by the SSD, the latency of this LFP modulation is far too long to be attributed to a response to the foveal stop signal.
Tests of conflict signal
The conflict hypothesis posits that activation of mutually incompatible response processes, such as gaze-shifting and gaze-holding, signals the need for control by the executive system (Botvinick et al. 2001; Yeung et al. 2004). This hypothesis can be evaluated using behavioral performance and physiological data from the saccade stop signal task in two ways. The first test involves relating LFP signals in the SEF to the amount of response conflict in different trials.
The amount of conflict produced in the stop signal task can be inferred from an analysis of the race model that states that performance in countermanding tasks can be understood as the outcome of a race between GO and STOP processes (Logan and Cowan 1984). In the saccade stop signal task this race is accomplished through the interaction between gaze-shifting and gaze-holding circuits in the FEF and SC (Hanes et al. 1998; Paré and Hanes 2003). An interactive race model with mutual inhibition between a GO unit and a STOP unit fits performance data as well as the independent race if and only if the timings of modulation of the GO and STOP units correspond to the actual modulation times of movement and fixation neurons (Boucher et al. 2007; Lo et al. 2009). In this framework, the coactivation of movement (GO) and fixation (STOP) units engenders response conflict (Schall and Boucher 2007). Now, canceled trials include a period during which movement (GO) and fixation (STOP) neurons are unusually coactive; this period of coactivation does not occur in noncanceled error trials because the fixation neurons (and the STOP unit in the model) do not turn on before the movement neurons (and the GO unit in the model) reach the threshold of activation to trigger the movement. Furthermore, the magnitude of coactivation of movement (GO) and fixation (STOP) units in canceled trials increases as the probability of a noncanceled saccade increases; this occurs because the activation of the movement (GO) units grows progressively closer to the threshold. Thus a given amount of activation of fixation (STOP) units sufficient to inhibit the growing activation of movement (GO) units multiplied by the magnitude of activation of movement (GO) units will result in higher response conflict. A population of neurons in the SEF of monkeys performing the saccade stop signal task was modulated after SSRT to a degree that was proportional to the probability of a noncanceled saccade and so may signal response conflict (Stuphorn et al. 2000).
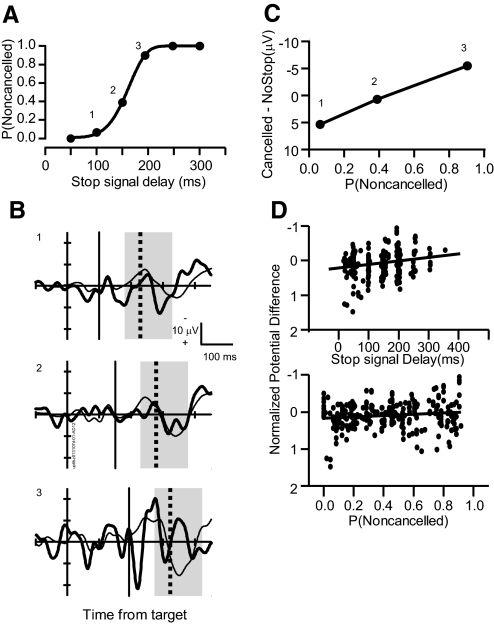
Thus the first test of the conflict-monitoring theory is to determine whether the LFP exhibits polarity differences in canceled compared with latency-matched no stop signal trials that vary systematically with the probability of a noncanceled saccade. To determine whether LFP polarization was proportional to response conflict, the average polarity difference between canceled and latency-matched no stop signal trials was measured following the analysis of Stuphorn et al. (2000). Figure 4 plots the stimulus-evoked LFPs for canceled stop signal trials and for the corresponding latency-matched no stop signal trials at a single site in the dorsal bank of the SEF for the three of six SSDs with sufficient trials (>10) to provide a reliable value. The average difference in LFP polarity between the trial types was measured in the 200-ms interval starting 50 ms before the SSRT. This interval was chosen because it corresponds to the interval in which single-unit modulation related to response conflict was observed in the SEF (Stuphorn et al. 2000). For this representative site in SEF, the LFP polarity difference between canceled trials and latency-matched no stop signal trials increased with SSD and also with increasing probability of producing an errant noncanceled saccade (Fig. 4C). To quantify this relationship, we calculated the regression of the LFP polarity difference between trial types as a function of SSD and as a function of the probability of producing a noncanceled saccade in a given session. The polarity difference in the LFP between canceled and no stop signal trials varied significantly with SSD (slope = −0.0013, r = −0.24, P < 0.01; Fig. 4D, top), as well as with the probability of producing a noncanceled saccade in a stop signal trial (slope = −0.13, r = −0.13, P < 0.01; Fig. 4D, bottom). Thus the polarity difference between canceled and latency-matched no stop signal trials increased with the probability of failing to cancel the saccade.
Fig. 4.
Test for conflict-related LFP polarization. A: inhibition function plots characteristic increasing probability of a noncanceled saccade [P(Noncanceled)] as a function of SSD. B: LFPs from a representative site time-locked on the time of target presentation for canceled trials (thick solid line) at SSDs of 168, 216, and 268 ms (labeled in A) are compared with latency-matched no stop signal trials (thin solid line). Average polarity difference between LFPs in canceled and latency-matched no stop signal trials in the interval from 50 ms before to 100 ms after stop signal reaction time (SSRT, highlighted by gray box) was measured. Vertical thin and thick black lines indicate SSD and SSRT, respectively (B1: 42 no stop trials, 43 canceled trials; B2: 289 no stop trials, 66 canceled trials; B3: 72 no stop trials, 7 canceled trials). C: average polarity difference between canceled and latency-matched no stop signal trials plotted as a function of P(Noncanceled). The increasing trend is significant. D: Z-scored average voltage difference across 436 SSDs plotted as function of SSD (top) and P (Noncanceled) (bottom). The polarity difference became significantly more negative with both increasing SSD and increasing P(Noncanceled).
The second test involves determining whether LFP signals in the SEF relate to adjustments of performance; specifically, the magnitude of the response time adjustment on a given trial should increase more after trials in which more conflict occurred on the previous trial (e.g., Kerns et al. 2004). Consistent with this, in other data we have found a tendency for saccade latency to be elevated following canceled stop signal trials (Emeric et al. 2007); however, this elevation seems to arise from slow fluctuations of response time across trials (MJ Nelson, L Boucher, GD Logan, TJ Palmeri, and JD Schall, unpublished data). As reported earlier, unfortunately, in this particular data set the trend for longer saccade latency after canceled stop signal trials was not significant, so this test could not be performed. However, a more detailed test probes whether saccade latency is elevated following canceled trials that happen to occur on trials with a longer stop signal delay such that the race between the GO and STOP processes is more advanced, thereby engendering more presumed conflict. In this data set we found no significant correlation (within or across sessions) between saccade latency on trial n + 1 and SSD on canceled stop signal trial n (mean across sessions, r = 0.04, P = 0.41). It should be recognized, though, that performance varies stochastically across SSDs, so a better estimate of the amount of putative conflict occurring on trial n + 1 is the session-wise probability of failing to cancel at that SSD. Still, we found no significant correlation within or across sessions between saccade latency on trial n + 1 and the SSD of the noncancelled saccade on trial n (mean across sessions, r = 0.03, P = 0.47).
These analyses used an estimate of putative conflict inferred from the state of the response processes responsible for performing the task (Boucher et al. 2007; Schall and Boucher 2007). Another approach is to use a brain signal that may signal conflict. Therefore we measured the trial-by-trial correlation between the magnitude of LFP polarization in the interval around SSRT in trial n and the response time adjustment in trial n + 1 (Fig. 5). For each trial, the maximum negative-going deflection in the 200-ms interval starting 50 ms before SSRT was plotted against the change in reaction time on the subsequent no stop trial. Although a significant correlation was observed at some sites, across all the sites examined, response time changes were not correlated with the magnitude of the LFP negativity on canceled trials (r = 0.003, P = 0.897). It is possible that simple voltage magnitude is not the most sensitive measure and thus we explored other measures. No significant correlation was observed between response time changes across trials and the area under the local minima (r = 0.013, P = 0.693), the latency of the local minima relative to SSRT (r = 0.005, P = 0.761), or the mean voltage in the 200-ms interval starting 50 ms before SSRT (r = 0.013, P = 0.420).
Fig. 5.
Test for conflict related LFP polarization. A: LFP aligned on the estimate of SSRT for the subset of 35 canceled stop signal trials that were followed by no stop signal trials from a single session. Red circles mark peak negative polarity in the interval from 50 ms before to 150 ms after SSRT. B: peak negative polarity plotted as a function of the response time adjustment on the subsequent no stop trial. No trend was evident. C: distribution of correlations between peak negativity in canceled trials and response time adjustment in the next trial. No relationship was found across the 82 sites examined.
To rule out the possibility that the single trial event-related LFPs and response time adjustments were simply too noisy to extract a meaningful correlation, we performed a separate analysis in which the measure of response time adjustment was the difference between the trial n + 1 response time and the mean response time in each session. For each session, we constructed two separate event-related LFPs aligned on SSRT for canceled trials: 1) canceled trials followed by no stop trials with response times greater than the median no stop response time and 2) canceled trials followed by no stop trials with response times less than the median no stop response time. Only 4 of the 82 sites demonstrated significant polarizations when comparing the event-related LFP on canceled trials preceding no stop trials with slower than average response times versus those preceding no stop trials with faster than average response times.
To summarize, at many sites in SEF the magnitude of negative polarization of the LFP around SSRT scaled with the variation of performance in a manner consistent with a measure of putative response conflict. However, no measure of the LFP related to changes in response time. Thus the data are consistent with one aspect of the conflict hypothesis but are inconsistent with another aspect.
Tests of error signal
Modulation of the intracranial SEF LFP following saccade production was common for both no stop signal trials and noncanceled trials. Figure 6 plots comparisons of the response-locked LFPs from the SEF on error noncanceled trials and all no stop signal trials. A response-locked frontocentral negativity, known as the correct response negativity (CRN), is associated with the execution of correct responses (e.g., Vidal et al. 2000). A small CRN was observed following saccades on no stop signal trials, but following error saccades a more pronounced negative polarization was observed. This intracranial error-related potential was defined as the onset of the first significant negative-going potential following the saccade. Overall, an intracranial error-related potential was identified in 54% (44/82) of the sites when the LFP was combined across targets (8/14 in F, 11/15 in M, 28/53 in U). We calculated a grand-average LFP from the response-locked potential recorded across all 82 sites. The clear polarization observed at the individual sites is evident in the grand-average LFP. In this grand average, a statistically significant negativity began 33 ms after the saccade and peaked 110 ms after the saccade. Measured across individual sites, this potential began 93 ± 44 ms after saccade initiation. This LFP modulation was observed after both contraversive and ipsiversive saccades but was lateralized at some sites, being somewhat less common following contraversive (23/82 sites) than ipsiversive (35/82 sites) saccades. Measured site by site, the latency of this modulation following contraversive saccades was 105 ± 47 ms and that following ipsiversive saccades was 123 ± 58 ms; these distributions were not significantly different (P = 0.53; χ2 = 0.53, Kruskal–Wallis rank-sum test). The earlier onset for the grand average and combined data compared with the site-by-site values is a simple result of improving signal-to-noise through averaging.
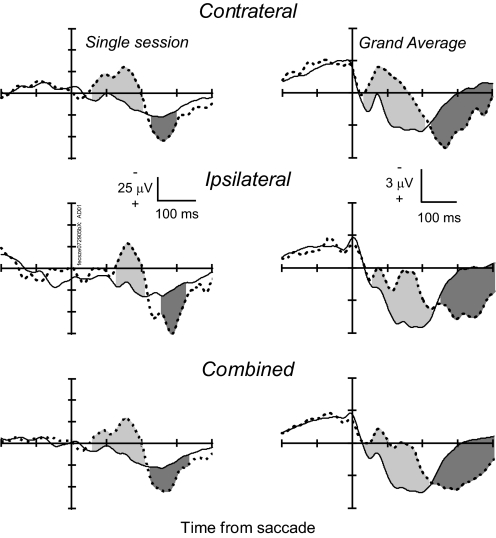
Fig. 6.
Error-related LFP. Left: LFP from a representative site aligned on saccade initiation for error noncanceled stop signal trials (thick dashed) and correct no stop signal trials (solid) for contraversive (top, 88 no stop trials; 34 noncanceled trials), ipsiversive (middle, 38 no stop trials; 13 noncanceled trials), and both combined (bottom) saccades. Right: grand average LFP from 82 sites in the SEF aligned on saccade initiation for contraversive (top), ipsiversive (middle), and combined (bottom) error noncanceled and correct no stop signal trials. Intervals in which the polarity of the LFP in noncanceled error trials was significantly more negative than that in no stop signal trials is indicated by the light gray fill. Intervals in which polarity of the LFP in noncanceled error trials was significantly more positive than that in no stop signal trials is indicated by dark gray fill.
We also observed a later, positive-going potential following errors. This was defined as the onset of the first significant positive-going potential following the saccade. Overall an intracranial error-related positive potential was identified in 66% (54/82) of the sites when the LFP was combined across targets (12/14 in F, 10/15 in M, 32/53 in U). The error-related positivity in the grand average began 233 ms and peaked 461 ms after the onset of the error saccade. Measured across sites, this potential began 257 ± 89 ms after saccade initiation. The positivity was equally common following contraversive (36/82 sites) and ipsiversive (39/82 sites) saccades. Its latency following contraversive saccades (253 ± 102 ms) was not significantly different from that following ipsiversive saccades (271 ± 95 ms; P = 0.13; χ2 = 2.25, Kruskal–Wallis rank-sum test).
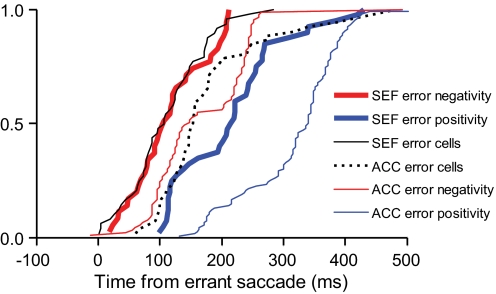
We compared the latency of these negative- and positive-going error-related potentials to the onset of error-related spike rate modulation in the SEF (Stuphorn et al. 2000) and the ACC (Ito et al. 2003) (Fig. 7). Error-related unit modulation occurs earlier in the SEF than that in the ACC (Ito et al. 2003) and the negative-going error-related potential in ACC is coincident with the error-related unit modulation in ACC (Emeric et al. 2008). The negative-going potential in the SEF was coincident with the SEF error cell modulation (P = 0.20; χ2 = 1.65, Kruskal–Wallis rank-sum test) and occurred significantly earlier than the ACC negative-going error potential (P < 0.01; χ2 = 28.37, Kruskal–Wallis rank-sum test). The positive-going error potential in the SEF occurred significantly later than the SEF error cell modulation (P < 0.01; χ2 = 58.38, Kruskal–Wallis rank-sum test) and also later than the ACC error cell modulation and the ACC negative-going error potential (P < 0.01; χ2 = 55.91, Kruskal–Wallis rank-sum test). The positive-going error-related potential in the SEF occurred earlier than the ACC positive-going error-related potential (P < 0.01; χ2 = 14.17, Kruskal–Wallis rank-sum test).
Fig. 7.
Cumulative distributions of onset time of LFP error-related negative polarity (red) and LFP error-related positive polarity (blue) in SEF (thick) and anterior cingulate cortex (ACC, thin). Also plotted are the latencies of error-related single-unit modulation in SEF (black solid) and ACC (black dotted).
Several studies have examined the relationship between the ERN and posterror adjustments (e.g., Debener et al. 2005). We examined the trial-by-trial covariation of the error-related LFP and the response time adjustment on the n + 1 trial (Fig. 8). For each noncanceled trial that was followed by a no stop signal trial, the maximum negative-going deflection in the 250-ms interval starting at initiation of the noncanceled error saccade and the maximum positive-going deflection in the 300-ms interval starting 200 ms after saccade initiation were plotted against the difference in saccade latency on the subsequent no stop trial. Significant correlations were observed at some recording sites and across all the sites examined response time changes were correlated with the magnitude of the peak LFP negativity (t = 2.45, P < 0.05), with the mean voltage in this interval (t = 2.480, P < 0.05) but not with the peak positivity (t = 0.34, P = 0.74) (Fig. 8B). However the sign of the correlation means that response times became longer as the negative-going polarization in SEF decreased in magnitude.
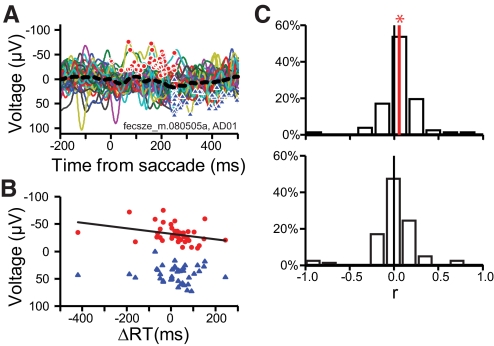
Fig. 8.
Error-related LFP and the response time (RT) adjustment. A: response-locked LFP for noncanceled stop signal trials that were followed by no stop signal trials (top). ●, peak negative value in the 250-ms interval following the response on each of the 32 individual trials. ▲, peak positive value in 250- to 500-ms interval following the response on each individual trial. Peak negative and positive polarization plotted against the response time adjustment on the subsequent no stop trial (bottom). B: distribution of the correlation coefficients for peak negativity (top) and peak positivity (bottom) as a function of RT adjustment. Each site in SEF contributed one data point. The red vertical line indicates that the distribution is significantly different from 0 (black vertical line).
We performed a separate analysis in which the measure of response time adjustment was the difference between the trial n + 1 response time and the mean response time in each session. For each session we constructed two separate event-related LFPs aligned on saccade initiation: 1) for noncanceled trials followed by no stop signal trials with response times greater than the median no stop response time on trials with no stop signal and 2) for noncanceled trials followed by no stop trials with response times less than the median no stop response time on trials with no stop signal. At 15 of the 82 sites significant differences were observed when comparing the event-related LFP on canceled trials preceding no stop trials with slower than average response times versus those preceding no stop trials with faster than average response times. There was no significant difference in the grand average collapsed across sessions.
Tests of reinforcement-feedback signal
To determine whether LFPs in the SEF were modulated by feedback about reinforcement, neural signals can be time-locked to the reinforcement when it was delivered and when it was occasionally withheld in correct no stop signal trials. This could be done because the delay between the end of the saccade to the target and delivery of reinforcement was fixed at 400 ms and therefore entirely predictable. Emeric et al. (2008) observed a significant negative-going potential in the LFP recorded from ACC after the time when reinforcement would have been delivered compared with trials when reinforcement was delivered. Although we observed clear modulation relative to the tone (Fig. 2C) we did not observe a significant potential at any of the 82 sites examined when reinforcement was withheld, unexpectedly creating a reinforcement prediction error. Thus the LFPs in macaque SEF do not signal reinforcement feedback.
Location of recording sites
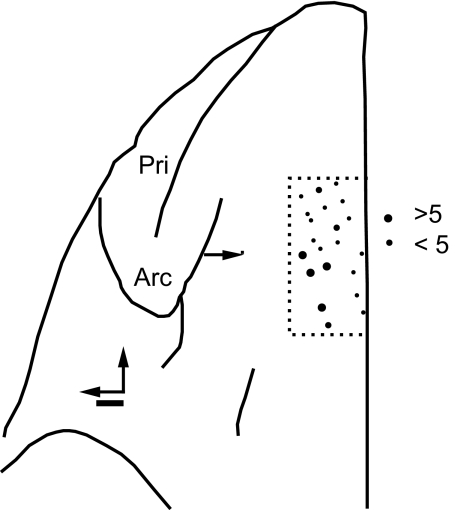
Nearly all of the intracranial error-related potentials were recorded from the dorsal convexity in area F7, as judged by the task-related activity of the neurons encountered in SEF (Stuphorn et al. 2000, 2010), electrically evoked saccades (Stuphorn and Schall 2006), and anatomical landmarks or histological features (Matelli et al. 1991). The sites with intracranial error-related potentials were sampled from cylindrical wells centered 29 mm anterior to the interaural line and either on the midline (monkeys M and F) or 5 mm lateral to the midline (monkey U). The sites with intracranial error-related potentials were distributed in a strip extending 7 mm laterally from the midline (Fig. 9). Stuphorn et al. (2000) reported no consistent colocalization of conflict and error neurons in SEF. This lack of colocalization was likewise evident in the present data.
Fig. 9.
Location of sites with error LFP signals. Top view of the left frontal lobe of monkey U. Neural activity was sampled within the region bounded by the thin dashed line. The number of error-related LFPs recorded is indicated by the size of the circles. The arcuate (Arc) and principal (Pri) sulci are labeled. The horizontal arrow marks 29 mm anterior to the interaural line. Scale bar: 1 mm.
DISCUSSION
We obtained clear evidence in macaque monkeys performing a saccade stop signal task that LFPs in SEF are modulated by visual and auditory stimuli and before saccades. Among sites with presaccadic potentials, none exhibited modulation sufficient to control the initiation of saccades when analyzed in the framework of the race model of countermanding performance (Hanes et al. 1998; Logan and Cowan 1984). The LFP also exhibited signals that could be used for performance monitoring. SEF LFP signaled errors and conflict but not reward prediction error. The relationship of these signals to changes of response time across trials was ambiguous or absent in this data set. These data provide useful new insights into the role of SEF in controlling saccade production and into the origin of extracranial event-related potentials.
Event-related potentials during the stop signal task
Several reports have described ERPs from human subjects performing stop signal tasks (Bekker et al. 2005; De Jong et al. 1990, 1995; Dimoska et al. 2006; Endrass et al. 2007; Kok et al. 2004; Naito and Matsumura 1994; Pliszka et al. 2000; Ramautar et al. 2004, 2006a,b; Stahl and Gibbons 2007; van Boxtel et al. 2001). This literature indicates the following general observations. First, larger N2 and P3 components are observed in stop signal trials compared with no stop signal trials and these components differ when comparing canceled and noncanceled stop signal trials. Similarly, in macaque SEF, we observed a trend toward greater LFP polarization on canceled stop signal trials (Fig. 4). Second, ERP components have not been consistently identified that modulate before SSRT on canceled trials to provide signals sufficient to control the initiation of movements. In macaque SEF, this was replicated (Fig. 3). These results can contribute to resolving whether the N2 observed on canceled trials is an index of the efficacy of stopping through inhibition or conflict (e.g., Dimoska et al. 2006; Kok et al. 2004). Third, the ERN has been observed on noncanceled trials in the saccade stop signal task. In macaque SEF, this was replicated (Fig. 6). However, the interpretation of the negativity after errors may be somewhat ambiguous because it may be the enhanced stop-signal aligned N2 on noncanceled stop signal trials. Thus in general, intracranial LFPs recorded in macaque SEF as well as in ACC (Emeric et al. 2008) appear to correspond to ERPs recorded from human participants. Confirmation of this identification, however, requires further investigation coordinated across species, task conditions, and effectors.
Stimulus-related modulation
The LFPs in SEF were consistently polarized in the interval following visual and auditory stimuli. The visual-evoked LFP modulation was observed consistently and at short latency relative to the stimulus. This is consistent with reports of SEF single units with visual activity (Amador et al. 2004; Pouget et al. 2005; Schall 1991a). In contrast, the LFPs in the ACC were only weakly polarized in the interval following stimulus presentation (Emeric et al. 2008). This difference has an anatomical explanation: SEF receives many more afferents from visual cortical areas than does ACC. Specifically, the SEF receives visual afferents from the medial superior temporal visual area, the superior temporal polysensory area, the lateral intraparietal area, and FEF (Huerta and Kaas 1990; Schall et al. 1993). In contrast, the ACC receives weaker visual afferents from the parietooccipital area, area 7A, and the inferotemporal area TG (Van Hoesen et al. 1993) as well as SEF (Huerta and Kaas 1990; Luppino et al. 1990) and FEF (Huerta et al. 1987; Stanton et al. 1993; Wang et al. 2004).
The auditory stimulus-evoked LFP modulation was observed less frequently than visually evoked LFP modulation but still at short latency relative to the stimulus. This is consistent with reports from single-unit studies observing activity in SEF elicited by auditory stimuli (Schall 1991a).
Presaccadic modulation and control of saccade initiation
The LFPs in the SEF were consistently polarized in the presaccadic interval. This is consistent with SEF single-unit studies that observed increased activity relative to saccade onset (Hanes et al. 1995; Schall 1991b; Schlag and Schlag-Rey 1987) and may be a source of the readiness potential observed for visually guided saccades (Evdokimidis et al. 1991; Everling et al. 1996).
Microstimulation, anatomical data, single-unit, and lesion studies in macaque monkeys have led to a subtle understanding of the role of SEF in contributing to the control of saccade initiation (reviewed by Olson 2003; Schall and Boucher 2007; Tehovnik et al. 2000). Although eye movements can be evoked from SEF using low currents (Schlag and Schlag-Rey 1985), lesions of SEF cause only modest and relatively high level impairments of gaze control (Husain et al. 2003; Parton et al. 2007; Pierrot-Deseilligny et al. 2002; Schiller and Chou 1998, 2000). In humans performing a saccade stop signal task, the pattern of blood oxygenation level dependent (BOLD) activation in SEF is different from that in FEF and has been interpreted as less directly involved in saccadic control (Curtis et al. 2005).
The countermanding paradigm provides a clear criterion for determining whether neurons generate signals sufficient to control the production of movements (Hanes et al. 1998). The key test is whether the activity of neurons is different between trials with a movement (no stop signal or noncanceled trials) and trials with no movement (canceled trials) and, critically, whether such a difference occurs before SSRT. If some neural modulation occurs after SSRT, then according to the race model that identifies SSRT with the time of inhibition of the movement, then the modulation is too late to contribute to controlling response initiation (Boucher et al. 2007; Logan and Cowan 1984). Specifically, if a neural signal is to be sufficient to control movement then a significant difference in the activity on canceled trials versus the activity on no stop trials, it must occur before SSRT. Presaccadic movement and fixation neurons but not visual neurons in the FEF and SC satisfy this criterion (Hanes et al. 1998; Paré and Hanes 2003). However, although many SEF neurons are active during the preparation and execution of saccades, in the saccade stop signal task, these neurons with apparent movement-related activity fail to produce signals sufficient to control gaze (Stuphorn et al. 2010). Consistent with this pattern of single-unit modulation, the present analysis of field potentials in SEF revealed very few sites with modulation sufficient to control gaze. At sites with a significant LFP modulation on canceled trials, that modulation occurred well after SSRT (Fig. 3). This is further evidence against SEF having a direct role in the control of gaze shifts.
Although SEF appears to have no direct control of saccade initiation, other evidence demonstrates its capacity to influence saccade production more subtly. First, the activity of numerous SEF neurons was correlated with response time and varied with sequential adjustments in saccade latency. Moreover, trials in which the monkeys canceled or produced a saccade in a stop signal trial were distinguished by a modest difference in the discharge rate of these SEF neurons before presentation of the stop signal or target (Stuphorn et al. 2010). Finally, previous work has reported that subthreshold microstimulation of the SEF improves stop signal task performance in monkeys by delaying saccade initiation (Stuphorn and Schall 2006). These results provide a useful perspective on a recent hypothesis that identifies the stopping process with a circuit between the presupplementary motor area, the inferior frontal gyrus, and the subthalamic nucleus (Aron et al. 2007; Mars et al. 2009).
Performance monitoring
ERROR PROCESSING.
At many sites in SEF we observed negative-going potentials followed by positive-going potentials following noncanceled error saccades. This LFP modulation was not observed in correct canceled stop signal trials (data not shown). Therefore the LFP modulation was not evoked by the stop signal. The LFP modulation occurred after both contra- and ipsiversive error saccades. Therefore it is unlikely that this modulation is due to a strictly movement-evoked potential. We thus interpret this LFP modulation as signaling the occurrence of an error. Intracranial error-related potentials have been previously observed in the ACC of macaque monkeys (Emeric et al. 2008; Gemba et al. 1986). In addition, intracranial error-related potentials have been observed in humans (Brázdil et al. 2002, 2005). This evidence leads us to the hypothesis that the error-related potentials observed in this study are intracranial analogs of the ERN and the Pe.
The presence of an error-related LFP in SEF is most likely derived from the presence of single units that exhibit increased activity after error responses (Nakamura et al. 2005; Stuphorn et al. 2000). The correspondence in latency of the LFP error negativity and the single-unit discharge rate error modulation (Fig. 7) is consistent with this association. The error-related LFP negativity in ACC also occurs concomitantly with the error-related single-unit discharge rate modulation within ACC (Emeric et al. 2008); of note, though, the error-related spike and LFP modulation in ACC are significantly later than those in SEF. Both the temporal coincidence of LFP and spike modulation in SEF and ACC associated following errors are interesting to contrast with other research showing that a modulation of LFP polarization associated with saccade target selection occurs significantly later than the target selection spike rate modulation (Cohen et al. 2009; Monosov et al. 2008). The basis of this difference between areas and functional signals is uncertain at this time.
Evidence supporting the hypothesis that the LFP in SEF contributes to the ERN recorded extracranially is found in the timing of the intracranial field potential relative to the human ERN. In humans performing manual stop signal tasks, an ERN is recorded that exhibits a peak negative deflection 80 ms after the error response (e.g., Kok et al. 2004; Ramautar et al. 2004, 2006a,b). Similarly, the ERN measured during an antisaccade task peaked 80 ms after error saccades (Nieuwenhuis et al. 2001). We found that across individual sites, the error-related LFP occurred on average 93 ms after the errant saccade. Given task differences, measurement error, and conduction time differences between the larger human and smaller macaque brains, these time values seem comfortably comparable.
More evidence supporting the hypothesis that the LFP in SEF contributes to the ERN recorded extracranially is obtained in the specific form of the potential. Similar to the grand-average error-related LFP in SEF and in ACC (Emeric et al. 2008), the ERN waveform in humans after saccades appears double-peaked (Nieuwenhuis et al. 2001; Van't Ent and Apkarian 1999). However, the response-locked ERN may overlap with the stop signal-locked N2; therefore the negative-going potentials observed following noncanceled errors may reflect both stop signal and error-related processing (e.g., Dimoska et al. 2006; Ramautar et al. 2004, 2006a,b).
The ERN was originally interpreted as an error detection signal resulting from a mismatch between the response and the outcome of response selection (Falkenstein et al. 1990, 1991; Gehring et al. 1993). This error-detection hypothesis originally included the premise that ERN magnitude relates to response time adjustments (Coles et al. 1995; Gehring et al. 1993). Several studies have examined this relationship with diverse results using ERPs (Debener et al. 2005; Gehring and Fencsik 2001; Gehring et al. 1993; Scheffers et al. 1996) and functional magnetic resonance imaging (fMRI) (Debener et al. 2005; Garavan et al. 2003). We found that the variations in response time adjustment covaried with the magnitude of negative-going, but not the positive-going, error-related field potential modulation on the preceding noncanceled trial, similar to other recent studies with human participants (Gehring and Fencsik 2001; Nieuwenhuis et al. 2001). However, the magnitude of the negative-going error-related potential decreased with increasing response time, which is inconsistent with a signal that should lead to longer response times. In previous work in the ACC, we found no significant correlation between the single-trial amplitude and response times on the subsequent trial across the sample of sites (Emeric et al. 2008). Consistent with this observation, patients with damage to the medial frontal lobe, including ACC and the supplementary motor area, do not consistently exhibit deficits in posterror adjustments (Fellows and Farah 2005; Modirrousta and Fellows 2008). Thus the error-related LFP recorded in the SEF of monkeys performing a saccade stop signal task may monitor but does not appear to contribute appreciably to the executive control of saccade production.
REINFORCEMENT LEARNING.
When errors were committed in this task, positive reinforcement is not received. Other research with human participants has described a frontocentral negativity that is elicited by events signaling error, loss of reinforcement, or punishment (e.g., Gehring and Willoughby 2002a,b; Miltner et al. 1997). This event-related potential has been referred to as the feedback-related negativity. More specifically, it has been hypothesized that the mesencephalic dopamine system conveys a reward prediction error signal to the frontal cortex when errors are committed that is manifest through ACC activity producing the ERN (Holroyd et al. 2002, 2005). Consistent with this, single units in ACC that discharge after errors are also active when earned reinforcement is withheld (Ito et al. 2003; see also Niki and Watanabe 1979). Also in monkeys performing the saccade stop signal task, other neurons in ACC modulate in a manner directly paralleling that of dopamine neurons (Ito et al. 2003). Emeric et al. (2008) observed error-related and reinforcement-feedback potentials in the dorsal bank of the ACC in macaque monkeys performing a saccade stop signal task. Specifically, a negative-going LFP was observed in ACC on correct no stop signal trials when reinforcement was withheld. In addition, Vizoli and Procyk (2009) observed feedback ERPs in monkeys that were attenuated by administration of the D2-like receptor antagonist haloperidol (but see Godlove 2010).
When tested by withholding reward on successful trials, we did not observe such feedback-related modulation in the SEF LFPs. This is somewhat unexpected because some single units in SEF have been found to be active during both anticipation and receipt of juice rewards (Amador et al. 2000; Stuphorn et al. 2000). However, in these single-unit studies of SEF, the effect of withholding reward on successful trials was not tested. In summary, the current LFP data cannot support the conclusion that the SEF contributes to the feedback-related negativity, but it remains possible that SEF contributes otherwise to the sensitivity to reinforcement.
RESPONSE CONFLICT.
Another hypothesis states that the ERN signals response conflict that is supposed to arise when mutually incompatible response processes are activated concurrently (e.g., Botvinick et al. 2001, 2004). The modulation of the N2 event-related potential during high-conflict trials has been emphasized as evidence for this conflict hypothesis (Yeung et al. 2004). According to this interpretation, the N2 and the ERN originate from the same neural process but are just observed at different times; response conflict on correct trials is supposed to precede the response and is manifested as the N2, whereas response conflict on error trials follows the response and is manifested as the ERN. A frontal N2 component is observed when movements are inhibited in the stop signal task (e.g., Dimoska et al. 2006; Ramautar et al. 2004; Schmajuk et al. 2005; van Boxtel et al. 2001) or in go/no-go tasks (e.g., Bokura et al. 2001; Pfefferbaum et al. 1985). In the SEF LFP, we observed a negative polarization coinciding with the N2 that had greater magnitude on canceled trials (Fig. 3). Additional evidence for a conflict representation has been provided in fMRI studies reporting BOLD activation in SEF that varies with inferred magnitude of conflict (Curtis et al. 2005). BOLD activation in the supplementary motor area has been observed under conditions of response conflict as well (Garavan et al. 2003).
Earlier research from this laboratory described single-unit activity in the SEF that could be identified with signaling the amount of conflict engendered by the unusual state of coactivation of gaze-holding and gaze-shifting when saccade preparation was canceled in stop signal trials (Stuphorn et al. 2000). The key evidence was that the magnitude of the elevated discharge rate in canceled trials increased with the probability that the saccade would not be canceled at the given SSD across the session. The probability of producing a saccade increases with the magnitude of accumulation of presaccadic movement-related activity toward the threshold to trigger the saccade (Brown et al. 2008; Hanes and Schall 1996; Ray et al. 2009). The closer the gaze-shifting activity is to the threshold before it is inhibited by gaze-holding activity, the greater the conflict. These interaction processes can be modeled through mutual inhibition between GO and STOP units (Boucher et al. 2007; Lo et al. 2009; Wong-Lin et al. 2010) and preliminary results indicate that the coactivation of the GO and STOP units can be used to optimize performance on subsequent trials (Schall and Boucher 2007). In this study we found that the magnitude of SEF LFP modulation increased with the probability of noncanceled saccades (Fig. 4). Thus the polarization of the LFP in SEF parallels the modulation of discharge rate of the particular neurons in a manner that is consistent with signaling the magnitude of conflict.
The general conflict hypothesis also posits that the detection of conflict on a trial will result in an adjustment of response time on the subsequent trial. The data in this study were not consistent with this aspect of the hypothesis because we found no consistent relationship between the putative conflict signal in the LFP and changes in saccade latency across trials. However, this result must be interpreted with caution because no clear contingency of response time on trial history was seen in the data used for this study, unlike in other data sets (e.g., Emeric et al. 2007; Nelson et al., unpublished data).
Comparison of SEF and ACC
We now can systematically compare the results of this study with those of a previous report of LFP signals in the ACC of monkeys performing the saccade stop signal task (Emeric et al. 2008) (Table 1). First, the LFPs in the SEF exhibit more clear stimulus-evoked polarization with earlier latencies than the LFPs in ACC. Second, in the interval immediately preceding the saccade, the LFPs in SEF exhibited larger magnitude negative polarization than those in ACC and this presaccadic polarization was more frequently observed in the SEF than that in the ACC. These results are consistent with the general view that SEF is more involved in sensorimotor processes than is ACC. However, neither SEF nor ACC produced LFP signals that were sufficient to control gaze when analyzed through the race model when applied to a countermanding task. The results indicate other differences related to the roles of SEF and ACC in performance monitoring and executive control. First, the fraction of sites with postsaccadic polarization was not different between SEF and ACC. However, the incidence and amplitude of error-related potentials were notably greater in ACC than those in SEF. The error-related negativity in SEF had a significantly shorter latency than that in ACC. Second, LFPs modulated by reward prediction error were observed in ACC but not in SEF. Third, a pattern of LFP polarization consistent with signaling response conflict was observed in SEF but not in ACC (see also Dias et al. 2006).
Table 1.
Summary of ACC and SEF single-unit and LFP properties in the countermanding saccade task
| ACC |
SEF |
|||
|---|---|---|---|---|
| Modulation Type | Single Units | LFP | Single Units | LFP |
| Sensory | + | − | ++ | ++ |
| Movement | − | − | ++ | ++ |
| Error | + | ++ | + | + |
| Reward prediction error | + | + | ? | − |
| Reward anticipation | + | ? | + | ? |
| Response conflict | − | − | + | + |
“++” indicates the pronounced presence of indicated modulation; “+” indicates simple presence; “−” indicates absence; and “?” indicates lack of testing.
Overall, the results are consistent with the general conclusion that the SEF responds to visual and auditory stimuli, signal errors, and response conflict and only indirectly influences movement preparation whereas the ACC monitors the consequences of actions. Beyond improving our understanding of the functions of these different cortical areas, these qualitative and quantitative differences between SEF and ACC have important implications for their respective contributions as the source of event-related potentials recorded from the scalp.
Source localization of the ERN: SEF and ACC
Dipole localization solutions have led to the conclusion that the ACC is the major source of the ERN (e.g., Dehaene et al. 1994; Miltner et al. 1997; reviewed by Gehring et al. 2010). Being an inverse problem, an effectively infinite number of dipoles can account for a given scalp potential topography (Helmholtz 1853). However, converging evidence from functional brain imaging and intracranial recordings in humans has provided additional evidence identifying the ACC as the source of the ERN (e.g., Brázdil et al. 2002; Carter et al. 1998; Wang et al. 2005). The presence of error-related and feedback-related LFP in the ACC of macaque monkeys reinforces this localization. However, other structures are active when errors are produced (reviewed by Hester et al. 2004). Our finding of an error-related LFP in SEF demonstrates that the ACC is not the only contributor to this surface potential.
Due to superposition, electrical potentials generated by local and remote sources and sinks add algebraically to produce the potential recorded on the scalp. Obviously, the morphology of the cortex dictates the geometry of this algebraic summation. As far as we know, no one has investigated how the specific scalp distribution of the ERN relates to individual differences of morphology in the human medial frontal cortex. Such an analysis would be complex because one third to one half of the population possesses a second cingulate sulcus (Paus et al. 1996a,b). The morphology of the macaque monkey frontal lobe is simpler and therefore more amenable to an analysis of this type. In the macaque frontal lobe, the SEF is located on the dorsal convexity with its pyramidal cell apical dendrites directed dorsally. The error-related LFPs we found in ACC were restricted to the dorsal bank with its pyramidal cell apical dendrites directed ventrally. Thus the dipoles in SEF and ACC are oriented in opposite directions. Recall that the error-related LFP is present at more sites in ACC and is of greater magnitude but arises later than that present in SEF. Thus an ERN recorded on the scalp of monkeys performing this task must at least be the product of the temporal and spatial summation of fewer, earlier, dorsally oriented dipoles in the SEF with more numerous, later, ventrally oriented dipoles in the ACC. This observation leads to two interesting conclusions. First, the ERN recorded on the scalp may underestimate the total magnitude of error-related field potentials in the brain. Second, the time course of the ERN recorded on the scalp may not correspond to the time course of any of the individual error-related field potentials in the brain. The next step in this program of research bridging human electrophysiology and monkey neurophysiology is to determine whether an ERN can be recorded from the scalp or skull of macaque monkeys performing the saccade countermanding task.
GRANTS
This work was supported by National Institutes of Health Grants F32-EY-017765, T32-MH-065782, R01-MH-55806, P30-EY-08126, and P30-HD-015052 and by Robin and Richard Patton through the E. Bronson Ingram Chair in Neuroscience.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank E. Crowder, A. Garr, C. Segovis, and M. Feurtado for help with animal care and A. Garr, D. Godlove, and G. Woodman for helpful comments on the manuscript.
REFERENCES
- Akkal D, Bioulac B, Audin J, Burbaud P. Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur J Neurosci 15: 887–904, 2002 [DOI] [PubMed] [Google Scholar]
- Amador N, Fried I. Single-neuron activity in the human supplementary motor area underlying preparation for action. J Neurosurg 100: 250–259, 2004 [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol 84: 2166–2170, 2000 [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91: 1672–1689, 2004 [DOI] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci 21: 3447–3452, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Procyk E, HonorÉ J, Sequeira H, Joseph JP. Reward anticipation, cognition, and electrodermal activity in the conditioned monkey. Exp Brain Res 149: 267–275, 2003 [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27: 3743–3752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GPH, Ridderinkhof KR, van der Molen MW. Speed–accuracy modulation in case of conflict: the roles of activation and inhibition. Psychol Res 67: 266–279, 2003 [DOI] [PubMed] [Google Scholar]
- Bekker EM, Overtoom CC, Kenemans JL, Kooij JJ, Noord ID, Buitelaar JK, Verbaten MN. Stopping and changing in adults with ADHD. Psychol Med 35: 807–816, 2005 [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol 112: 2224–2232, 2001 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev 108: 624–652, 2001 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8: 539–546, 2004 [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev 114: 376–397, 2007 [DOI] [PubMed] [Google Scholar]
- Boucher L, Stuphorn V, Logan GD, Schall JD, Palmeri TJ. Stopping eye and hand movements: are the processes independent? Percept Psychophys 69: 785–801, 2007 [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple go/no-go task. J Psychophysiol 19: 244–255, 2005 [Google Scholar]
- Brázdil M, Roman R, Falkenstein M, Daniel P, Jurák P, Rektor I. Error processing: evidence from intracerebral ERP recordings. Exp Brain Res 146: 460–466, 2002 [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci 7: 266–277, 2007 [DOI] [PubMed] [Google Scholar]
- Brown JW, Hanes DP, Schall JD, Stuphorn V. Relation of frontal eye field activity to saccade initiation during a countermanding task. Exp Brain Res 190: 135–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749, 1998 [DOI] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Schall JD, Woodman GF. On the origin of event-related potentials indexing covert attentional selection during visual search. J Neurophysiol 102: 2375–2386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Fournier L. Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol (Amst) 90: 129–144, 1995 [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M. Canceling planned action: an fMRI study of countermanding saccades. Cereb Cortex 15: 1281–1289, 2005 [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25: 11730–11737, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner M, Tucker D. Localization of a neural system for error detection and compensation. Psych Sci 5: 303–305, 1994 [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform 21: 498–511, 1995 [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. J Exp Psychol Hum Percept Perform 16: 164–182, 1990 [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res 174: 279–291, 2006 [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain Cogn 62: 98–112, 2006 [DOI] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Boucher L, Carpenter RHS, Hanes DP, Harris R, Logan GD, Mashru RN, Paré M, Pouget P, Stuphorn V, Taylor TL, Schall JD. Influence of history on saccade countermanding performance in humans and macaque monkeys. Vision Res 47: 35–49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V, Schall JD. Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex. J Neurophysiol 99: 759–772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeric EE, Stuphorn V, Schall JD. Error-related local field potentials in medial frontal lobe of macaques during saccade countermanding. Soc Neurosci Abstr 79.20, 2003 [Google Scholar]
- Endrass T, Franke C, Kathmann N. Error awareness in a saccade countermanding task. J Psychophysiol 19: 275–280, 2005 [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur J Neurosci 26: 1714–1720, 2007 [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Liakopoulos D, Papageorgiou C. Cortical potentials preceding centrifugal and centripetal self-paced horizontal saccades. Electroencephalogr Clin Neurophysiol 79: 503–505, 1991 [DOI] [PubMed] [Google Scholar]
- Everling S, Krappmann P, Preuss S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp Brain Res 111: 289–295, 1996 [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Psychophysiological Brain Research, edited by Brunia CHM, Gaillard AWK, Kok A. Tilburg, The Netherlands: Tilburg Univ. Press, 1990, p. 192–195 [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78: 447–455, 1991 [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain 128: 788–796, 2005 [DOI] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui KI. Segregated and integrated coding of reward and punishment in the cingulate cortex. J Neurophysiol 101: 3284–3293, 2009 [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. NeuroImage 20: 1132–1139, 2003 [DOI] [PubMed] [Google Scholar]
- Garr AK, Emeric EE, Schall JD. Extracranial error-related potential in macaque monkeys during saccade countermanding. Program No. 211.11. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 21: 9430–9437, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci 4: 385–390, 1993 [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne). In: Oxford Handbook of Event-Related Potential Components, edited by Luck SJ, Kappenman E. New York: Oxford Univ. Press, 2010, p. 100–150 [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295: 2279–2282, 2002a [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Medial prefrontal cortex and error potentials: response. Science 296: 1610–1611, 2002b [DOI] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Brooks VB. “Error” potentials in limbic cortex (anterior cingulate area 24) of monkeys during motor learning. Neurosci Lett 70: 223–227, 1986 [DOI] [PubMed] [Google Scholar]
- Godlove DC. Eye movement artifact may account for putative frontal feedback-related potentials in nonhuman primates. J Neurosci 30: 4187–4189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol 79: 817–834, 1998 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Countermanding saccades in macaque. Vis Neurosci 12: 929–937, 1995 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science 274: 427–430, 1996 [DOI] [PubMed] [Google Scholar]
- Hanes DP, Thompson KG, Schall JD. Relationship of presaccadic activity in frontal eye field and supplementary eye field to saccade initiation in macaque: Poisson spike train analysis. Exp Brain Res 103: 85–96, 1995 [DOI] [PubMed] [Google Scholar]
- Helmholtz H. Ueber einige gesetze der vertheilung elektrischer ströme in körperlichen leitern mit anwendung auf die thierisch-elektrischen versuche. Ann Physik Chem 89: 211–233, 354–377, 1853 [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the go/nogo task. Cereb Cortex 14: 986–994, 2004 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 109: 679–709, 2002 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett 242: 65–68, 1998 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MGH, Cohen JD. A mechanism for error detection in speeded response time tasks. J Exp Psychol Gen 134: 163–191, 2005 [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299–330, 1990 [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol 265: 332–361, 1987 [DOI] [PubMed] [Google Scholar]
- Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nat Neurosci 6: 117–118, 2003 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, Nagamine T, Taki W, Kimura J, Shibasaki H. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain 122: 915–931, 1999 [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10: 240–248, 2007 [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science 302: 120–122, 2003 [DOI] [PubMed] [Google Scholar]
- Kay RF, Ross C, Williams BA. Anthropoid origins. Science 275: 797–804, 1997 [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026, 2004 [DOI] [PubMed] [Google Scholar]
- Kok A, Ramautar JR, De Ruiter MB, Band GP, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 41: 9–20, 2004 [DOI] [PubMed] [Google Scholar]
- Lins O, Picton T, Berg P, Scherg M. Ocular artifacts in EEG and event-related potentials. I: Scalp topography. Brain Topogr 6: 51–63, 1993 [DOI] [PubMed] [Google Scholar]
- Lo CC, Boucher L, Paré M, Schall JD, Wang XJ. Proactive inhibitory control and attractor dynamics in countermanding action: a spiking neural circuit model. J Neurosci 29: 9059–9071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psych Rev 91: 295–327, 1984 [Google Scholar]
- Luppino G, Matelli M, Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp Brain Res 82: 214–218, 1990 [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, Rushworth MF. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci 27: 6926–6931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RD. Primate origins: plugging the gaps. Nature 363: 223–234, 1993 [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311: 445–462, 1991 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci 10: 647–656, 2007 [DOI] [PubMed] [Google Scholar]
- Miltner W, Baum C, Coles M. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a generic neural system for error detection. J Cogn Neurosci 9: 788–798, 1997 [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci 28: 14000–14005, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov IE, Trageser JC, Thompson KG. Measurements of simultaneously recorded spiking activity and local field potentials suggest that spatial selection emerges in the frontal eye field. Neuron 57: 614–625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Matsumura M. Movement-related potentials associated with motor inhibition as determined by use of a stop signal paradigm in humans. Brain Res Cogn Brain Res 2: 139–146, 1994 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol 93: 884–908, 2005 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38: 752–760, 2001 [PubMed] [Google Scholar]
- Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171: 213–224, 1979 [DOI] [PubMed] [Google Scholar]
- Olson CR. Brain representation of object-centered space in monkeys and humans. Annu Rev Neurosci 26: 331–354, 2003 [DOI] [PubMed] [Google Scholar]
- Paré M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci 23: 6480–6489, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, Morgan PS, Jackson S, Rees G, Husain M. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia 45: 997–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D'Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 376: 664–673, 1996a [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex 6: 207–214, 1996b [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Koppell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol 60: 423–434, 1985b [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Ploner CJ, Muri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic: eye movements in humans. Ann NY Acad Sci 956: 216–229, 2002 [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry 48: 238–246, 2000 [DOI] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis K, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field, and anterior cingulate cortex. J Neurophysiol 94: 2086–2092, 2005 [DOI] [PubMed] [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci 3: 502–508, 2000 [DOI] [PubMed] [Google Scholar]
- Pujol J, López A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage 15: 847–855, 2002 [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain Cogn 56: 234–252, 2004 [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Kok A, Ridderinkhof KR. Effects of stop-signal modality on the N2/P3 complex elicited in the stop-signal paradigm. Biol Psychol 72: 96–109, 2006a [DOI] [PubMed] [Google Scholar]
- Ramautar JR, Slagter HA, Kok A, Ridderinkhof KR. Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Res 1105: 143–154, 2006b [DOI] [PubMed] [Google Scholar]
- Ray S, Pouget P, Schall JD. Functional distinction between visuomovement and movement neurons in macaque frontal eye field during saccade countermanding. J Neurophysiol 102: 3091–3100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Gemba H, Tsujimoto T. Suppression of visually initiated hand movement by stimulation of the prefrontal cortex in the monkey. Brain Res 495: 100–107, 1989 [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol 66: 530–558, 1991a [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol 66: 559–579, 1991b [DOI] [PubMed] [Google Scholar]
- Schall JD, Boucher L. Executive control of gaze by the frontal lobes. Cogn Affect Behav Neurosci 7: 396–412, 2007 [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, Kaas JH. Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Vis Neurosci 10: 385–393, 1993 [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology 33: 42–53, 1996 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci 1: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Chou IH. The effects of anterior arcuate and dorsomedial frontal cortex lesions on visually guided eye movements in the rhesus monkey: 1. Single and sequential targets. Vision Res 40: 1609–1626, 2000 [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Unit activity related to spontaneous saccades in frontal dorsomedial cortex of monkey. Exp Brain Res 58: 208–211, 1985 [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol 57: 179–200, 1987 [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia 44: 384–395, 2005 [DOI] [PubMed] [Google Scholar]
- Stahl J, Gibbons H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clin Neurophysiol 118: 581–596, 2007 [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to the frontal lobe from the macaque frontal eye fields. J Comp Neurol 330: 286–301, 1993 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Brown JW, Schall JD. Role of supplementary eye field in saccade initiation: executive not direct control. J Neurophysiol 103: 801–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9: 925–931, 2006 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2000 [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist 13: 160–172, 2007 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev 32: 413–448, 2000 [DOI] [PubMed] [Google Scholar]
- van Boxtel GJ, van der Molen MW, Jennings JR, Brunia CH. A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biol Psychol 58: 229–262, 2001 [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston, MA: Birkhauser, 1993, p. 249–284 [Google Scholar]
- Van't Ent D, Apkarian P. Motoric response inhibition in finger movement and saccadic eye movement: a comparative study. Clin Neurophysiol 110: 1058–1072, 1999 [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77: 477–482, 2002 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezoli J, Procyk E. Frontal feedback-related potentials in nonhuman primates: modulation during learning and under haloperidol. J Neurosci 29: 15675–15683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci 25: 604–613, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport 15: 1559–1563, 2004 [DOI] [PubMed] [Google Scholar]
- Wong-Lin K, Eckhoff P, Holmes P, Cohen JD. Optimal performance in a countermanding saccade task. Brain Res 1318: 178–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Kang MS, Rossi AF, Schall JD. Nonhuman primate event-related potentials indexing covert shifts of attention. Proc Natl Acad Sci USA 104: 15111–15116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Ikeda A, Satow T, Matsuhashi M, Baba K, Yamane F, Miyamoto S, Mihara T, Hori T, Taki W, Hashimoto N, Shibasaki H. Human eye fields in the frontal lobe as studied by epicortical recording of movement-related cortical potentials. Brain 127: 873–887, 2004 [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 111: 931–959, 2004 [DOI] [PubMed] [Google Scholar]