Abstract
Somatostatin (somatotropin release-inhibiting factor [SRIF]) is known to modulate the excitability of retinal ganglion cells, but the membrane mechanisms responsible and the extent to which intracellular calcium signaling is affected have not been determined. We show that somatostatin receptor subtype 4 (sst4) is expressed specifically in rat ganglion cells and that the generation of repetitive action potentials by isolated ganglion cells is reduced in the presence of L-803,087, a selective sst4 agonist (10 nM). Under voltage clamp, L-803,087 increased outward K+ currents by 51.1 ± 13.1% at 0 mV and suppressed Ca2+ channel currents by 32.5 ± 9.4% at −10 mV in whole cell patch-clamped ganglion cells. The N-type Ca2+ channel blocker ω-conotoxin GVIA (CTX, 1 μM) reduced L-type Ca2+ current (ICa) in ganglion cells by 43.5 ± 7.2% at −10 mV, after which addition of L-803,087 further reduced ICa by 28.0 ± 16.0% . In contrast, ganglion cells treated first with nifedipine (NIF, 10 μM), which blocked 46.1 ± 3.5% of the control current at −10 mV, did not undergo any further reduction in ICa in the presence of L-803,087 (−3.5 ± 3.8% vs. NIF), showing that stimulation of sst4 reduces Ca2+ influx through L-type Ca2+ channels. To assess the effects of sst4 stimulation on intracellular Ca2+ levels ([Ca2+]i) in ganglion cells, fura-2 was used to measure changes in [Ca2+]i in response to depolarization induced by elevated [K+]o. [Ca2+]i was increased to a lesser extent (86%) in the presence of L-803,087 compared with recordings made in the absence of the sst4 agonist and this effect was blocked by NIF (10 μM). Suppression of spiking and Ca2+ signaling via sst4 may contribute to the reported neuroprotective actions of somatostatin and promote ganglion cell survival following ischemia and axonal trauma.
INTRODUCTION
Somatostatin (somatotropin release-inhibiting factor [SRIF]) is a neuropeptide transmitter in many brain regions including the retina. Somatostatin is a cyclic tetradecapeptide with multiple cellular actions mediated by five G protein-coupled receptors designated sst1, sst2A/sst2B, sst3, sst4, and sst5 (Hoyer et al. 1995; Møller et al. 2003; Vanetti et al. 1992). Somatostatin signal transduction cascades may lead to activation of voltage-gated ion currents (Akopian et al. 2000; Ikeda and Schofield 1989; Inoue et al. 1988; Ishibashi and Akaike 1995; Mihara et al. 1987; Wang et al. 1989) and modulation of glutamate-induced currents in neurons (Moneta et al. 2002; Viollet et al. 1997).
In mammalian retina, somatostatin is predominantly, if not exclusively, expressed by a limited number of widely ramifying and sparsely distributed amacrine cells (Brecha 1983, 2003; Cristiani et al. 2002). In contrast, the sst receptors, all subtypes of which are expressed in the retina, are distributed to specific retinal cell populations, including subtypes of photoreceptor, bipolar, amacrine, and ganglion cells (Brecha 1983, 2003; Thermos 2003). Sst4 receptors are found only in ganglion cells of the rat retina (Vasilaki et al. 2002), potentially making them a selective therapeutic target in retinal diseases.
Somatostatin has several known actions on retinal neurons. In photoreceptor terminals, somatostatin elicits an increase in a delayed rectifier K+ current (IKV), a decrease in L-type Ca2+ current (ICa) in rods, and an increase in ICa in cones (Akopian et al. 2000). Somatostatin also inhibits calcium-activated K+ (BK) channels in rabbit bipolar cells via inhibition of L-type Ca2+ channels (Petrucci et al. 2001) and high K+-evoked increases of intracellular Ca2+ concentration ([Ca2+]i) in rat rod bipolar cells (Johnson et al. 2001). Ganglion cells recorded in rabbit eyecup show that somatostatin evokes long-lasting increases in both spontaneous and light-evoked spike activity, increases the signal-to-noise ratio of light-evoked responses, and produces a shift in the center–surround balance toward a more dominant center for all ganglion cell receptive fields (Zalutsky and Miller 1990).
Studies have shown that somatostatin analogues may be effective therapeutic agents in retinal diseases (Vasilaki and Thermos 2009). Excessive Ca2+ influx into cells is thought to be a major contributor to cell death in ischemic and excitotoxic models of neuronal injury (Smaili et al. 2009). Therefore mechanisms of Ca2+ entry are prime candidates as targets for potential neuroprotective effects. Most sst subtypes have been shown to modulate voltage-gated Ca2+ channels as well as glutamate receptor channels in several organ systems (reviewed by Cervia and Bagnoli 2007). For example, sst2 has been most extensively studied and has been shown to interact with Gi/o proteins, resulting in a reduction in intracellular Ca2+ levels in a variety of cell types (Cervia and Bagnoli 2007). However, relatively little is known about the signaling pathways and Ca2+ modulatory effects of the other sst receptor subtypes. In particular, there have been no prior reports, to our knowledge, of sst4 receptor stimulation influencing voltage-gated Ca2+ channels.
The objective of this study was to investigate the actions of sst4 signaling on membrane excitability in rat retinal ganglion cells (RGCs) in terms of the involvement and modulation of specific ionic currents and to define effects of sst4 signaling on intracellular calcium signaling.
METHODS
Experimental protocols for electrophysiological and imaging experiments were approved by the Dalhousie University Committee on Laboratory Animals and performed in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals (CCAC, Ottawa, Canada, 1984 and 1993). Procedures for immunohistochemical experiments were approved by the University of California, Los Angeles Animal Research Committee in accordance with the National Institute of Health guidelines.
Purified RGC culture
The two-step panning procedure to purify the ganglion cells has been previously described (Barres et al. 1988; Hartwick et al. 2004). Briefly, litters of Long–Evans rats (Charles River Laboratories, Montreal, Canada) were killed at postnatal day 7 by overexposure to halothane and decapitation. Dissected eyecups were immersed in Hibernate-A (BrainBits, Springfield, IL) with 2% B27 supplements and 10 μg/ml gentamicin. Retinas were removed and treated as described in Hartwick et al. (2004). Purified ganglion cells were plated onto poly-d-lysine/laminin-coated Biocoat glass coverslips (12 mm round; BD Biosciences, Bedford, MA) in four-well tissue culture plates at a density of 2.5 × 104 cells per well. Cultures were maintained at 37°C in a humidified 5% CO2-air atmosphere. Patch recordings were made on the second day following cell dissociation and panning.
Immunohistochemical procedures
Sprague–Dawley rats (of either sex, 150–250 g; Harlan, Indianapolis, IN) were maintained on a 12-h/12-h light–dark schedule. Following an overdose of isoflurane, the eyes were removed, the anterior segment was dissected, and the posterior eyecup containing the retina was fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) for 1 h at room temperature. The eyecup was then stored in 25% sucrose in 0.1 M PB at 4°C. Sections of the retina were cut perpendicular to the vitreal surface with a cryostat at 12 μm, mounted on gelatin-coated slides, air-dried, and stored at −20°C. Purified ganglion cells were fixed in PFA (0.01 M PB, pH 7.4) for 10 min at room temperature and stored in 0.01 M PB (4°C) until immunostaining.
The polyclonal antibody used in the present study was developed using a synthetic peptide directed to the C-terminus of the rat sst4 receptor (amino acids 362–384), as previously described by Vasilaki et al. (2002). Specificity of the antibody used here was evaluated by direct comparison with immunostaining by an antibody used previously (Brecha et al. 2002; Vasilaki et al. 2002). Sst4 immunostaining of rat retinal sections using either antibody produced an identical pattern of localization.
Retinal sections and purified ganglion cells were washed in 0.1 M PB, incubated for 12–36 h in sst4 primary antibody (1:3000) with 0.5% Triton X-100 and 10% normal goat serum at 4°C, then washed in 0.1 M PB. Samples were incubated in Alexa 488 goat anti-rabbit antibodies (Invitrogen, Carlsbad, CA) for 2 h at room temperature and then coverslipped using the ProLong Antifade Kit (Vector Laboratories, Burlingame, CA).
Electrophysiological recordings
To record outward K+ channel currents and action potentials in isolated ganglion cells, the extracellular bathing solution contained (in mM): 137 NaCl, 5 KCl, 1 MgCl2, 2.5 CaCl2, 22.2 glucose, and 5 HEPES, adjusted to pH 7.2 with NaOH. The intracellular pipette solution contained (in mM): 140 KCl, 2 MgCl2, 1 CaCl2, 1.5 EGTA, and 10 HEPES, adjusted to pH 7.2 with KOH. To isolate Ca2+ channel currents the extracellular solution contained (in mM): 115 NaCl, 2.5 KCl, 5 CsCl, 10 BaCl2, 15 TEA-Cl, 10 glucose, and 15 HEPES, adjusted to pH 7.6 with NaOH, whereas the intracellular pipette solution contained (in mM): 140 CsCl, 0.8 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES, adjusted to pH 7.2 with CsOH. Tetrodotoxin (TTX, 1 μM) was added to block Na channels. Room-temperature (21–25°C) solutions were superfused via a fast perfusion system (VC8-S; ALA Scientific, Albany, NY). Patch electrodes with 5 to 10 MΩ tip resistance were pulled from fire-polished borosilicate glass capillary tubes using a micropipette puller (Sutter Instrument, Novato, CA). The bath reference electrode consisted of an agar bridge with an AgCl wire. Cell voltage was clamped with an Axopatch-1D amplifier (Axon Instruments, Foster City, CA) using whole cell capacitance and series resistance compensation. The current signal was filtered at 0.5 Hz (4302 Dual 24dB/octave filter; Ithaco, Ithaca, NY) and digitized at 1 kHz with an Indec BioSystems interface (Santa Clara, CA) for storage on the hard disk of a computer running BASIC-FASTLAB acquisition software. Holding potential was set at −60 mV. In current-clamp experiments, a 150 pA step for 150 ms elicited action potentials. In voltage-clamp experiments, voltage steps (150 ms, 1 Hz) were made in 10 mV increments from −60 to +40 mV. Steady-state currents, measured as the mean of the last 10 ms of each voltage step, were used to construct current–voltage (I–V) relations. All recordings made in the presence of drugs were taken after cells had been exposed to the drug for 2.5 min.
Ca2+ imaging in retinal flat mounts
Fura-2 pentasodium solution (2 μL, 60 μM) was injected into the vitreous via the optic nerve stump of eyes removed from Long–Evans rats (100–150 g; Charles River Laboratories) killed via cervical dislocation following isoflurane anesthesia. The fura-2 was electroporated into cells of the ganglion cell layer with 50 ms, 40 mV pulses at 1 Hz for 5 s with the anode on the cornea and the cathode at the optic nerve stump. The retina was flat-mounted ganglion cell side up on nitrocellulose paper and superfused at a rate of 1 ml/min with oxygenated Ringer solution containing (in mM) 145 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (adjusted to pH 7.4 with NaOH). The flat mounts were imaged using a SenSys cooled charge-coupled device camera on a Nikon UM-2 microscope with a ×40 water-immersion objective. Cells were depolarized by superfusing high K+ solution (50 mM added KCl, NaCl reduced by the same) for 30 s. Fura-2 fluorescence was produced by excitation from a 100 W xenon arc lamp with filter sets for excitation at 340 and 380 nm and emission at 510 nm (Lambda 10; Sutter Instrument); ratio measurements were performed every 5 or 10 s. Ganglion cell fluorescence was converted to ratiometric values by Imaging Workbench 5.1 (Indec BioSystems) and saved to a hard disc. The mean fura-2 ratio at each time point was calculated over a large area of each ganglion cell body.
Drugs and chemicals
All chemicals and reagents, unless otherwise noted, were obtained from Sigma–Aldrich (Oakville, Canada). L-803,087 trifluoroacetate was purchased from Tocris Bioscience (Ellisville, MO). Tetrodotoxin (TTX), charybdotoxin (ChTX), and ω-conotoxin GVIA (CTX) were obtained from Ascent Scientific (Princeton, NJ). Fura-2 was purchased from Invitrogen (Burlington, Canada). L-803,087 (0.001%) and nifedipine (NIF, 0.1%) were prepared as DMSO stock solutions, frozen at −20°C, and thawed immediately before experiments (numbers in parentheses indicate percentage of DMSO in the final working solution). Vehicle control experiments (0.1% DMSO) were used where appropriate for comparisons between treatments. TTX was prepared as a stock in 0.4 mM citrate buffer solution and frozen at −20°C until use. All other drugs and reagents were prepared in double-distilled water either as stock solutions (frozen at −20°C) or prepared fresh before performing experiments.
Data analysis
All data are reported as the means ± SE. Data analysis for electrophysiology and Ca2+ imaging experiments was performed using Clampfit 10 software (Molecular Devices, Sunnyvale, CA). Graphing and statistical analyses were performed using SigmaPlot 11.0 software (Systat Software, San Jose, CA). Specific statistical tests used are noted in the figure legend in the appropriate results sections. Values of P < 0.05 were considered statistically significant. All pairwise multiple comparison procedures were performed in SigmaPlot, using the Holm–Šidák method.
RESULTS
Localization of sst4 to the ganglion cells of rat retina
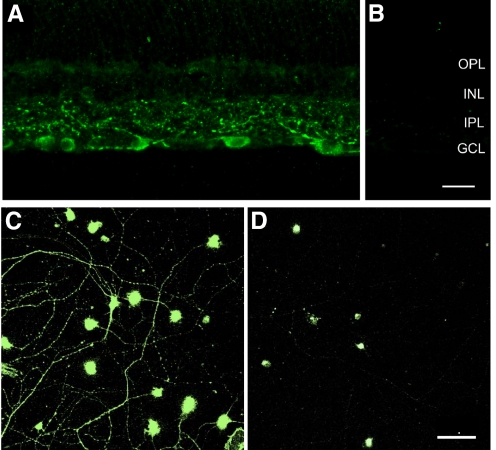
Since the aim of the present study was to determine the effects of selective sst4 stimulation on voltage-gated ion channels in mammalian ganglion cells, we first examined the expression pattern of sst4 in the mammalian retina using an affinity-purified polyclonal antibody directed to the C-terminus of rat sst4 (see methods). Figure 1A shows sst4 immunoreactivity in a rat retinal section. sst4 is localized to multistratified dendrites in the inner plexiform layer (IPL) and to numerous cell bodies in the ganglion cell layer (GCL), similar to a previous report (Brecha et al. 2002). Soma staining in the GCL is characterized by a granular appearance in the cytoplasm, suggesting association with the Golgi complex and endoplasmic reticulum. Robust immunostaining was observed in the optic nerve fiber layer and optic nerve (not shown). Preadsorbing the antibody with the synthetic peptide (10−6 M sst4(362–384)) produced no immunostaining in the retina (Fig. 1B). In purified ganglion cell cultures, immunostaining was evident over the somata and dendrites (Fig. 1C). Also, 100% of purified RGCs stained positive for sst4. Preadsorption control experiments in cultures showed weak secondary staining of only the ganglion cell somata (Fig. 1D).
Fig. 1.
Somatostatin receptor subtype 4 (sst4) immunoreactivity in ganglion cells of the rat retina. A: immunostaining was found in medium to large cell bodies in the ganglion cell layer and in dendrites that ramify throughout the inner plexiform layer (IPL). B: staining was not seen in sections incubated with sst4 antibody preadsorbed with the sst4(362–384) peptide (10−6 M). Scale bar for A and B is 20 μm. C: purified retinal ganglion cells (RGCs) exhibited strong immunostaining over cell somata and all dendrites. D: preadsorption experiments showed weak staining of the secondary antibody confined to the somata of panned cells, with no staining detectable over the dendrites. Scale bar for C and D is 50 μm.
sst4 was expressed by many ganglion cells in rat retina, the identity of which was based on the size of immunoreactive cells. Immunostained cells ranged from 12 to 25 μm in diameter in the rat retina and, in general, matched the known spectrum of ganglion cell diameters, although the smallest ganglion cells are underrepresented compared with the general population of ganglion cells (Raymond et al. 2008). In retinal sections, the primary dendrites were seen to branch at several levels in the IPL and an immunoreactive axon was often observed at the base of the cell body. These axons formed fascicles within the nerve fiber layer.
Patch-clamp analysis of somatostatin and a selective sst4 agonist, L-803,087, on ion channels in isolated rat ganglion cells
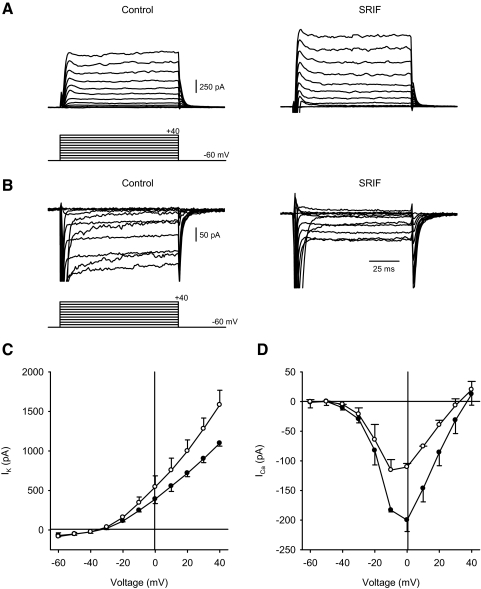
In preliminary experiments, we determined the effects of nonselective sst receptor stimulation using the endogenous agonist, somatostatin, in purified RGC cultures. Figure 2A shows representative voltage-clamp recordings of outward K+ current (IK) in the absence (left) and presence (right) of SRIF (1 μM). Administration of somatostatin increased IK compared with the control recording. Figure 2B shows examples of inward Ca2+ channel current (ICa) recorded in the presence of K+-channel blockers in the absence (left) and presence (right) of somatostatin. Mean I–V relations (Fig. 2, C and D) show that administration of somatostatin to ganglion cells increased IK, whereas ICa was decreased compared with control recordings. No TTX was included in these experiments so large unclamped sodium currents appear at the initiation of the depolarizing steps.
Fig. 2.
Effects of nonselective sst stimulation, using somatostatin (somatotropin release-inhibiting factor [SRIF]), on transmembrane currents in isolated ganglion cells. A: representative outward K+ current (IK) recordings from a ganglion cell in the absence (left) and presence (right) of 1 μM SRIF. Voltage command protocol shown below. B: examples of Ca2+ channel currents in the absence (left) and presence (right) of SRIF. Voltage command protocol shown below. The recordings were made without tetrodotoxin (TTX). C: steady-state current–voltage (I–V) relationships for IK in the absence (filled symbols) and presence of 1 μM somatostatin (hollow symbols). D: steady-state Ca2+ channel I–V relationships in the absence (filled symbols) and presence of 1 μM somatostatin (hollow symbols). n = 2 cells/group, statistics not performed.
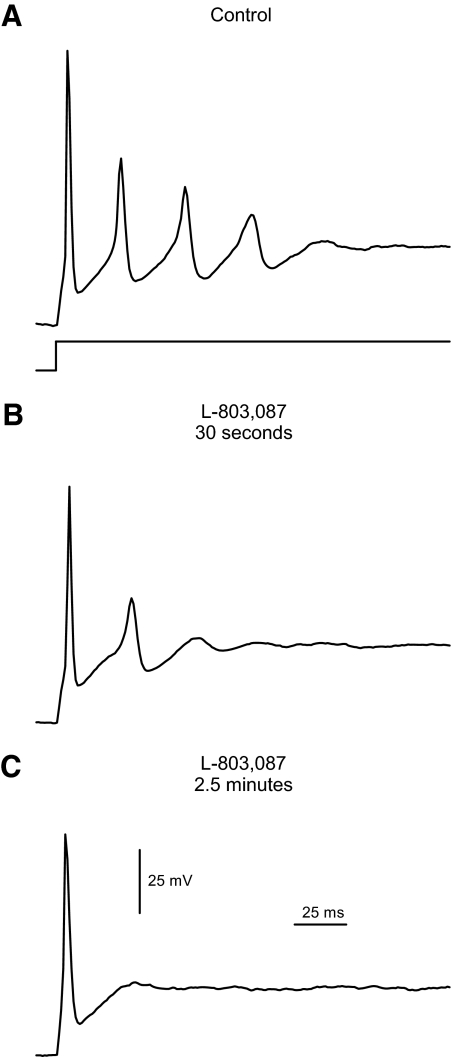
Next, the selective sst4 agonist L-803,087 (10 nM) was administered to ganglion cells. Figure 3 shows examples of action potentials recorded from a ganglion cell under current-clamp conditions. In the absence of drug (Fig. 3A), repetitive action potentials were generated when current was injected through the microelectrode. Administration of L-803,087 reduced the amplitude and frequency of these action potentials (Fig. 3B) and, eventually, only a single action potential was generated in response to the current injection (Fig. 3C). This inhibitory effect on action potential generation was partially reversible on removal of the drug. In seven cells, the resting potential was unchanged by L-803,087, being −64.9 ± 1.4 mV in control and −63.9 ± 1.9 mV after administration of the drug. The amplitude of action potentials (second in train) was reduced from 35.9 ± 11.4 mV before drug to 19.9 ± 10.5 mV in L-803,087 (P < 0.05, paired t-test, n = 6). The rate of action potential repolarization was reduced in five cells by 68 ± 9.3% by the addition of L-803,087.
Fig. 3.
Action potential generation was inhibited by selective sst4 stimulation in isolated ganglion cells. A: example of a current-clamp recording, in which injection of depolarizing current (150 pA, timing per trace shown below) generated repetitive action potentials in an isolated ganglion cell. The cell's resting potential was set near −60 mV with continuous injection of hyperpolarizing current. B: following administration of L-803,087 (10 nM) for 30 s, the frequency of action potentials was reduced in the same cell. C: continued treatment with L-803,087 inhibited the repetitive action potentials all together.
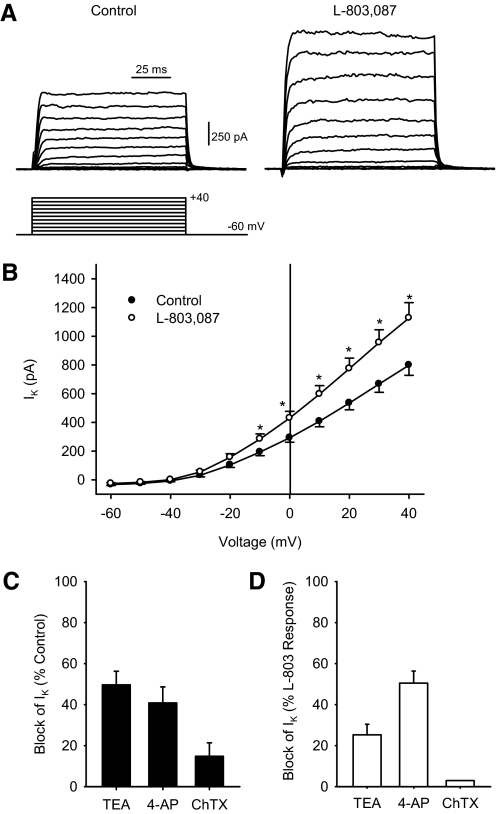
To examine the effects of sst4 stimulation on membrane currents, ganglion cells were voltage-clamped in the absence and presence of L-803,087. Figure 4A shows representative recordings of IK in the absence (left) and presence (right) of L-803,087. Mean data show that the administration of L-803,087 significantly enhanced IK at positive membrane potentials (Fig. 4B). Next, to determine which K+ channels were involved in this increase in IK, known K+ channel blockers were applied in the absence and presence of L-803,087. Figure 4, C and D shows the percentage of IK blocked when different K+ channel blockers were applied to ganglion cells in the absence and presence of L-803,087 at 0 mV. In the absence of L-803,087, tetraethylammonium (TEA+) blocked 49.6 ± 6.6% of the steady-state IK, whereas 4-aminopyridine (4-AP) and charybdotoxin (ChTX) blocked 40.8 ± 7.8 and 14.8 ± 6.5%, respectively (Fig. 4C). However, when the same blockers were applied after L-803,087 administration, the majority of IK was sensitive to 4-AP (50.5 ± 5.8%), whereas TEA+ and ChTX blocked only 25.2 ± 5.2 and 3.0%, respectively (Fig. 4D).
Fig. 4.
K+ currents in isolated ganglion cells were enhanced following L-803,087 administration. A: representative examples of IK recordings from an isolated ganglion cell under voltage-clamp conditions in the absence (left) and presence (right) of L-803,087 (10 nM). Voltage command protocol shown below. B: mean I–V curve data show that administration of L-803,087 significantly increased IK at more positive membrane potentials (n = 12; *P < 0.05, 2-way repeated-measures ANOVA vs. control). C: in the absence of L-803,087, administration of K+ channel blockers, tetraethylammonium (TEA+, 20 mM), 4-aminopyridine (4-AP, 5 mM), and charybdotoxin (ChTX, 100 nM) reduced IK compared with control recordings (recorded at 0 mV, n = 7–10 cells/group). D: following administration of L-803,087, TEA+ and ChTX blocked smaller proportions of IK compared with the control conditions, whereas 4-AP blocked a larger proportion of IK compared with control (recorded at 0 mV, n = 1–5 cells/group).
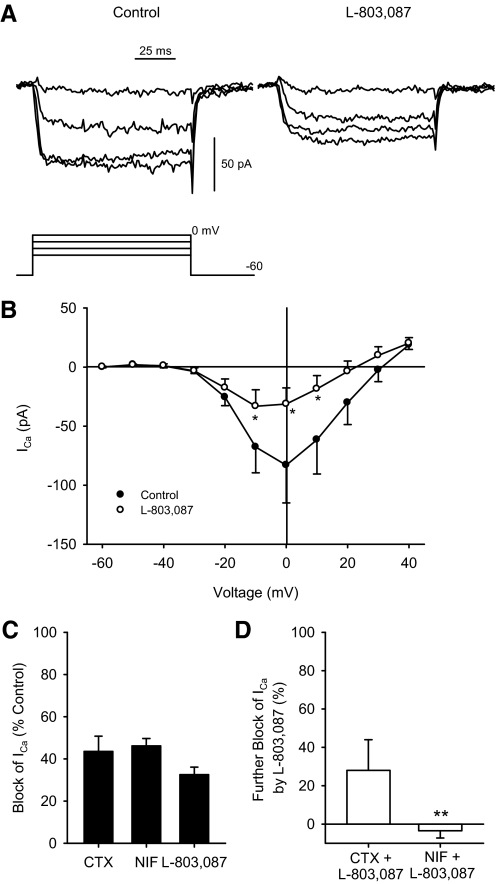
Next, Ca2+ channel currents were isolated and recorded in the absence and presence of L-803,087 in ganglion cells. Figure 5A shows examples of Ca2+ channel currents in the absence (left) and presence (right) of L-803,087. Mean data show that L-803,087 significantly reduced Ca2+ channel currents (Fig. 5B). Next, selective Ca2+ channel blockers were administered in the absence and presence of L-803,087 to determine which Ca2+ channel subtypes may be involved in the sst4 signaling pathway. Figure 5C shows the percentage of Ca2+ channel current blocked by the administration of nifedipine (NIF, L-type Ca2+ channel blocker), ω-conotoxin GVIA (CTX, N-type Ca2+ channel blocker), and L-803,087. Mean data show that NIF and CTX blocked 46.1 ± 3.5 and 43.5 ± 7.2% of the Ca2+ channel current, respectively, whereas L-803,087 blocked 32.5 ± 9.4% of the Ca2+ channel current. Figure 5D shows that when L-803,087 was administered after NIF, there was no additional block of the Ca2+ channel current (−3.5 ± 3.8%); however, when L-803,087 was applied after CTX, there was a further 28.0 ± 16.0% block of the Ca2+ channel current.
Fig. 5.
Ca2+ channel currents in isolated ganglion cells were reduced by L-803,087. A: examples of Ca2+ channel currents recorded from an isolated ganglion cell under voltage-clamp in the absence (left) and presence (right) of L-803,087 (10 nM). Voltage command protocol shown below. B: mean data show that administration of L-803,087 significantly reduced Ca2+ channel current at intermediate membrane potentials (n = 4; *P < 0.05, 2-way repeated-measures ANOVA vs. control). C: in the absence of L-803,087, administration of Ca2+ channel blockers, NIF (10 μM) and ω-conotoxin GVIA (CTX, 1 μM) reduced Ca2+ channel currents compared with control recordings (recorded at −10 mV, n = 3–4 cells/group). D: following administration of NIF, L-803,087 had no additional blocking effect on Ca2+ channel current, whereas following administration of CTX, L-803,087 further reduced Ca2+ channel current (−10 mV, n = 3–7 cells/group; **P < 0.05, ANOVA vs. L-803,087).
Calcium signal imaging in retinal flat mounts
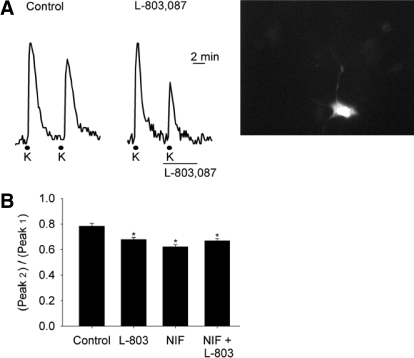
Application of high extracellular K+ solution (52.5 mM K+; see methods for full composition) for 30 s produced transient increases in intracellular Ca2+ levels that declined in amplitude with each subsequent high K+ application. As seen in Fig. 6A, in the absence of drug (left) the second of two high K+ evoked Ca2+ transients was smaller than the first. However, when L-803,087 (100 nM) was administered during high K+ application (right), the second Ca2+ transient was reduced more than the control example. Mean data showed that under control conditions the second Ca2+ transient was routinely smaller than the first (Fig. 6B; second transient/first transient: 78.2 ± 2.3%). Administration of L-803,087 reduced Ca2+ transients compared with the first recorded Ca2+ transient in the absence of drug (67.9 ± 1.4%; P < 0.05 vs. control). To test whether the results of the patch-clamp experiments would be supported in Ca2+ imaging, we applied nifedipine (NIF) before and during the treatment with L-803,087. When NIF (10 μM) was applied during the second high K+ application, mean data show that NIF reduced Ca2+ transient amplitudes to 62.2 ± 1.8% compared with the first transient (P < 0.05 vs. control). However, when NIF and L-803,087 were applied together, there was no further reduction in Ca2+ transient amplitude compared with either drug alone (66.9 ± 1.8%; P < 0.05 vs. control; NS vs. NIF or L-803,087 alone). Thus blockade of L-type Ca2+ channels by NIF occluded further block by L-803,087.
Fig. 6.
Intracellular Ca2+ signaling was reduced by L-803,087 in ganglion cells in retinal flat mount. A: applications of 52 mM K+ solution for 30 s depolarized ganglion cells and produced transient increases in intracellular Ca2+ concentration, measured with the dye fura-2. Traces (left) show that, in the absence of drug, the second of paired K+ applications produced a smaller peak Ca2+ signal. On the right, the paired K+ pulses, with L-803,087 (100 nM) applied 30 s prior to the second K+ application, show greater reduction of the Ca2+ signal compared with control. Far right panel shows a fluorescent image (stimulated at 380 nm) of a fura-2-containing ganglion cell in the flat-mount retina. B: mean data show amplitudes of the second high K+ response expressed as a percentage of first peak in control, L-803,087 (L-803, 100 nM), nifedipine (NIF, 10 μM), and nifedipine plus L-803,087. The mean Ca2+ transient amplitudes of all 3 drug treatment groups (L-803, NIF, L-803 + NIF) were significantly smaller than those of the control group, but they did not differ among themselves (n = 21–89 cells/group; *P < 0.05, ANOVA).
DISCUSSION
Summary of key findings
Results of the present study show that in the rat retina, ganglion cells are the only cell type expressing sst4. Thus the aim of this study was to investigate the effects of an sst4 agonist (L-803,087) on voltage-gated ion channels as potential therapeutic avenues to selectively target RGCs. To our knowledge, this is the first study showing that selective activation of sst4 in isolated RGCs enhanced outward K+ current and reduced inward Ca2+ current. In addition, we show that administration of L-803,087 inhibited L-type, but not N-type, Ca2+ channels, suggesting a preferential signaling pathway interaction between sst4 and L-type Ca2+ channels in RGCs.
sst4 receptor distribution in ganglion cells
As shown in other studies of mammalian retina, sst4 immunoreactivity was found in many rat RGC bodies, but in no other retinal cell somata (Brecha et al. 2002; Cristiani et al. 2002; Dal Monte et al. 2003; Thermos 2003; Vasilaki et al. 2002). The dendrites of immunopurified ganglion cells showed strong punctate staining, suggesting the possibility of localized sst4 signaling in the dendrites. Since all purified RGCs exhibited strong immunostaining over the cell somata and all dendrites, these results suggest an important role for sst4 in a significant population of ganglion cells. Further studies are warranted to determine the extent of sst4 expression in ganglion cells of the rat retina.
Previous work showing localization of somatostatin to wide-field amacrine cells suggests that this peptide has a broad modulatory influence via the activation of different sst receptors. As we know, somatostatin acts on specific cell types via distinct sst receptors. Thus results of this study, together with previous studies, suggest that sst4 is a target that is found only on ganglion cells (Brecha et al. 2002; Cristiani et al. 2002; Dal Monte et al. 2003; Vasilaki et al. 2002).
Inhibitory electrophysiological actions mediated by sst4 receptors
Electrophysiological results of this study showed that selective stimulation of sst4 receptors by L-803,087 in rat ganglion cells most prominently enhanced steady-state IK and reduced L-type Ca2+ channel current. Generation of repetitive action potentials in ganglion cells was inhibited by L-803,087 and Ca2+ imaging showed that intracellular levels of Ca2+ were increased to a lesser extent in response to depolarization in the presence of L-803,087. Together, these data suggest that stimulation of sst4 reduces the excitability of ganglion cells and limits Ca2+ influx. Thus the present study on the effects of sst4 stimulation in isolated ganglion cells motivates elucidation of a potential role of sst4 as a therapeutic target.
The inhibition of spiking shown in the present study could be due to the enhancement of K+ channel activity produced by sst4 receptor activation. Indeed, patch-clamp studies of whole cell currents showed that application of L-803,087 greatly enhanced outward currents. In addition, the K+ channels involved appear to be sustained 4-AP-sensitive voltage-gated K+ channels, the activity of which is known to be involved in repolarizing the membrane after the upstroke of an action potential. Depending on the exact activation range of these K+ channels, their modulation may also affect cell resting potential, which in turn can affect steady-state levels of sodium channel inactivation. In the present study, resting potential, which was set to a value near −60 mV with the injection of hyperpolarizing current under control conditions, was not affected by L-803,087. However, because this study did not address the potential of sodium channel modulation by sst4 stimulation, this would be an important topic for future investigation.
Results of the present study showed that administration of L-803,087 reduced Ca2+ currents in isolated RGCs. In addition, we show that this effect of L-803,087 was unique to L-type (and not N-type) Ca2+ channels. Although the reduced Ca2+ channel activity induced by L-803,087 likely had little effect on ganglion cell excitability, lowering intracellular Ca2+ is generally accepted as a mechanism to reduce cellular toxicity and death. Given that the primary aim of this study was to determine the effects of sst4 stimulation on voltage-gated ion channels, we did not investigate cell death or apoptosis. Direct evaluation of the effects of specific sst4 agonists on cell death will be an important area to pursue in future studies.
The actions of somatostatin on voltage-gated ion channels in ganglion cells, shown here, share some features of somatostatin action mediated by stimulation of other sst receptor subtypes in other retinal neurons: in rod photoreceptors, somatostatin elicits an increase in delayed rectifier K+ current (IKV) and decreases L-type Ca2+ current (but not in cones, where it produces increases in both K+ and Ca2+ currents; Akopian et al. 2000). In bipolar cells, somatostatin inhibits L-type Ca2+ channels, which leads to inhibition of calcium-activated K+ channels (Petrucci et al. 2001). In isolated rod bipolar cells somatostatin inhibits high K+-evoked increases of [Ca2+]i, presumably by inhibiting Ca2+ currents (Johnson et al. 2001). In ganglion cells in the intact retina, however, somatostatin increases spontaneous and light-evoked spike activity (Zalutsky and Miller 1990). The results of the present study, from isolated ganglion cells, neither replicate nor provide an explanation for this action. Rather, it is likely that in the eyecup, under dark-adapted conditions, somatostatin increased the excitatory drive provided to ganglion cells from bipolar cells, possibly by suppressing inhibitory circuits and/or by directly suppressing inhibitory input onto ganglion cells. Indeed, amacrine cell inhibitory signaling was shown to be suppressed by somatostatin as the antagonistic surround of ganglion cells was reduced (Zalutsky and Miller 1990). Thus somatostatin signaling pathways in the mammalian retina are complex and require further studies to fully elucidate and understand their mechanisms.
Potential neuroprotective uses of sst4 agonists
The results of the present study show that sst4 stimulation reduced L-type Ca2+ channel current, reduced depolarizing membrane potential excursions, and suppressed intracellular Ca2+ levels. These findings suggest that sst4 agonists have the potential to act as selective neuroprotective agents by targeting ion channels via sst4 signaling pathways to reduce excitability and Ca2+ influx. The results are consistent with previous work suggesting that, in general, somatostatin may prevent proapoptotic signaling events (Cervia and Bagnoli 2007). The targeting of sst4 receptors to suppress Ca2+ signaling provides a potential new therapeutic avenue in the treatment of neurodegenerative diseases of the retina. In spite of potentially interfering with the normal actions of somatostatin released endogenously in the retina, it is possible that selective sst4 receptor activation could sufficiently reduce ganglion cell excitability to counteract disease-induced elevations in [Ca2+]i and delay or prevent cell degeneration.
GRANTS
This work was supported by a Plum Foundation grant to S. Barnes and N. Brecha, National Eye Institute Grant EY-15573 to N. Brecha, a Veterans Administration Senior Career Scientist Award to N. Brecha, and Canadian Institutes for Health Research Grant MT-10968 to S. Barnes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. William Baldridge and J. Nason of the Department of Anatomy and Neurobiology at Dalhousie University for providing the purified cultures of RGCs.
REFERENCES
- Akopian A, Johnson J, Gabriel R, Brecha N, Witkovsky P. Somatostatin modulates voltage-gated K(+) and Ca(2+) currents in rod and cone photoreceptors of the salamander retina. J Neurosci 20: 929–936, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1: 791–803, 1988 [DOI] [PubMed] [Google Scholar]
- Brecha NC. Retinal neurotransmitters: histochemical and biochemical studies. In: Chemical Neuroanatomy, edited by Emson IPC.New York: Raven Press, 1983, p. 85–129 [Google Scholar]
- Brecha NC. Peptide and peptide receptor expression and function in the vertebrate retina. In: Visual System.Cambridge, MA: MIT Press, 2003, p. 3–28 [Google Scholar]
- Brecha NC, Vila A, Allen J. Somatostatin 4 receptor in mouse and rat retina (E-Abstract). Invest Ophthalmol Vis Sci 43: 2768, 2002 [Google Scholar]
- Cervia D, Bagnoli P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther 116: 322–341, 2007 [DOI] [PubMed] [Google Scholar]
- Cristiani R, Petrucci C, Dal Monte M, Bagnoli P. Somatostatin (SRIF) and SRIF receptors in the mouse retina. Brain Res 936: 1–14, 2002 [DOI] [PubMed] [Google Scholar]
- Dal Monte M, Petrucci C, Vasilaki A, Cervia D, Grouselle D, Epelbaum J, Kreienkamp HJ, Richter D, Hoyer D, Bagnoli P. Genetic deletion of somatostatin receptor 1 alters somatostatinergic transmission in the mouse retina. Neuropharmacology 45: 1080–1092, 2003 [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Lalonde MR, Barnes S, Baldridge WH. Adenosine A1-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 45: 3740–3748, 2004 [DOI] [PubMed] [Google Scholar]
- Hirooka K, Kourennyi DK, Barnes S. Calcium channel activation facilitated by nitric oxide in retinal ganglion cells. J Neurophysiol 83: 198–207, 2000 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci 16: 86–88, 1995 [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol 409: 221–240, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Nakajima S, Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol 407: 177–198, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Akaike N. Somatostatin modulates high-voltage-activated Ca2+ channels in freshly dissociated rat hippocampal neurons. J Neurophysiol 74: 1028–1036, 1995 [DOI] [PubMed] [Google Scholar]
- Johnson J, Caravelli ML, Brecha NC. Somatostatin inhibits calcium influx into rat rod bipolar cell axonal terminals. Vis Neurosci 18: 101–108, 2001 [DOI] [PubMed] [Google Scholar]
- Lalonde MR, Jollimore CAB, Stevens K, Barnes S, Kelly MEM. Cannabinoid receptor-mediated inhibition of calcium signaling in rat retinal ganglion cells. Mol Vis 12: 1160–1166, 2006 [PubMed] [Google Scholar]
- Møller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta 1616: 1–84, 2003 [DOI] [PubMed] [Google Scholar]
- Moneta D, Richichi C, Aliprandi M, Dournaud P, Dutar P, Billard JM, Carlo AS, Viollet C, Hannon JP, Fehlmann D, Nunn C, Hoyer D, Epelbaum J, Vezzani A. Somatostatin receptor subtypes 2 and 4 affect seizure susceptibility and hippocampal excitatory neurotransmission in mice. Eur J Neurosci 16: 843–849, 2002 [DOI] [PubMed] [Google Scholar]
- Petrucci C, Resta V, Fieni F, Bigiani A, Bagnoli P. Modulation of potassium current and calcium influx by somatostatin in rod bipolar cells isolated from the rabbit retina via sst2 receptors. Naunyn Schmiedebergs Arch Pharmacol 363: 680–694, 2001 [DOI] [PubMed] [Google Scholar]
- Raymond ID, Vila A, Huynh UC, Brecha NC. Cyan fluorescent protein expression in ganglion and amacrine cells in a thy1-CFP transgenic mouse retina. Mol Vis 14: 1559–1574, 2008 [PMC free article] [PubMed] [Google Scholar]
- Smaili S, Hirata H, Ureshino R, Monteforte PT, Morales AP, Muler ML, Terashima J, Oseki K, Rosenstock TR, Lopes GS, Bincoletto C. Calcium and cell death signaling in neurodegeneration and aging. Ann Acad Bras Cienc 81: 467–475, 2009 [DOI] [PubMed] [Google Scholar]
- Sun X, Barnes S, Baldridge WH. Adenosine inhibits calcium channel currents via A1 receptors on tiger salamander retinal ganglion cells in a mini-slice preparation. J Neurochem 81: 550–556, 2002 [DOI] [PubMed] [Google Scholar]
- Thermos K. Functional mapping of somatostatin receptors in the retina: a review. Vision Res 43: 1805–1815, 2003 [DOI] [PubMed] [Google Scholar]
- Vanetti M, Kouba M, Wang X, Vogt G, Höllt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett 311: 290–294, 1992 [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Mouratidou M, Schulz S, Thermos K. Somatostatin influences nitric oxide production in the rat retina. Neuropharmacology 43: 899–909, 2002 [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Thermos K. Somatostatin analogues as therapeutics in retinal disease. Pharmacol Ther 122: 324–333, 2009 [DOI] [PubMed] [Google Scholar]
- Viollet C, Bodenant C, Prunotto C, Roosterman D, Schaefer J, Meyerhof W, Epelbaum J, Vaudry H, Leroux P. Differential expression of multiple somatostatin receptors in the rat cerebellum during development. J Neurochem 68: 2263–2272, 1997 [DOI] [PubMed] [Google Scholar]
- Wang HL, Bogen C, Reisine T, Dichter M. Somatostatin-14 and somatostatin-28 induce opposite effects on potassium currents in rat neocortical neurons. Proc Natl Acad Sci USA 86: 9616–9620, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Miller RF. The physiology of somatostatin in the rabbit retina. J Neurosci 10: 383–393, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]