Abstract
In addition to its well-known role in the control of saccades, the primate superior colliculus (SC) has been implicated in the processes of target choice for overt orienting movements and for covert spatial attention. We focally inactivated the SC, by muscimol injection, while monkeys selected the target of a smooth pursuit, saccade, or button press response from two competing stimuli. The choice stimuli were placed so that one appeared within and the other appeared outside the affected visual field. SC inactivation biased the subject to choose stimuli out of the affected visual field for all three types of responses, although the effects on target choice were significantly smaller for button presses. Inactivation caused no changes in the selection of single stimuli within or out of the affected visual field, indicating the choice bias was not caused by deficits in response execution. The inactivation-induced bias for smooth pursuit and button press responses indicates SC activity is important for selecting the target, independent of any role in saccade preparation.
INTRODUCTION
Primates coordinate two types of voluntary eye movements when tracking visual objects. Saccades quickly orient gaze to the target position, and smooth pursuit matches eye rotation with the target velocity, stabilizing the image on the retina. Although historically viewed as distinct systems, recent studies show that saccades and smooth pursuit are coordinated by a shared target selection process (Case and Ferrera 2007; Liston and Krauzlis 2003, 2005; see Krauzlis 2004, 2005 for review).
The superior colliculus (SC) is one of the principal structures implicated in saccade selection. The SC is a retinotopically organized midbrain structure long known to be important for the generation of saccades (Hikosaka and Wurtz 1985a; Robinson 1972; Schiller and Stryker 1972; Wurtz and Albano 1980). The activity of some SC neurons indicates whether a stimulus within their response field will be chosen as the saccade target (Glimcher and Sparks 1992; Horwitz and Newsome 1999; McPeek and Keller 2002). Furthermore, focal inactivation of the SC biases saccade selection away from stimuli located within the affected region (McPeek and Keller 2004) and microstimulation, subthreshold for evoking saccades, biases saccade choice toward stimuli at the activated location (Carello and Krauzlis 2004).
Evidence from experiments requiring target selection for smooth pursuit indicates that the SC is involved in choosing the target of the next eye movement, distinct from its role in the production of saccades. SC activity indicates the location of objects tracked by smooth pursuit (Krauzlis et al. 1997, 2000), and neurons that predict the saccade target also predict the pursuit target (Krauzlis and Dill 2002). Moreover, subthreshold microstimulation in the SC is sufficient to bias choice of the stimulus at the retinotopic location, even if this requires a pursuit eye movement directed away from that location (Carello and Krauzlis 2004) counter to what would be expected of a saccade motor map but consistent with a role in target choice for saccades and pursuit. Although these studies indicate that the SC is connected to structures responsible for choosing the target of an eye movement, they have not determined whether SC activity is crucial for making the target choice.
We tested how important the SC is for the selection of visual objects by reversibly inactivating the SC of monkeys trained to report their choices with saccade, smooth pursuit, and button press responses. Inactivation of the SC biased the monkeys' selections for all three types of response; however, the effects were much stronger for eye movements than for pressing buttons. Primarily, these results indicate that the SC is crucial for selecting visual targets of eye movements. Second, these results show the SC also plays some role in selecting visual targets used to inform button press responses.
METHODS
Our experiments were controlled by a computer using the Tempo software package (Reflective Computing), and a second computer running the Psychophysics Toolbox (Brainard 1997; Pelli 1997) in Matlab (MathWorks) acted as a server device for presenting the visual stimuli. Stimuli were presented with a video monitor (75 Hz, ∼20 pixels/°) at a viewing distance of 41 cm. Eye movements were recorded using scleral search coils (Judge et al. 1980) and the electromagnetic induction technique (Fuchs and Robinson 1966) using standard phase detector circuits (Riverbend Instruments). All data and events related to the onset of stimuli were stored on disk during the experiment (1 kHz sampling rate) for additional off-line analysis.
Animal preparation
We collected data from two (P and V) adult male rhesus monkeys (Macaca mulatta) ages 8 and 9 weighing 9–11 kg. All experimental protocols for the monkeys were approved by the Institute Animal Care and Use Committee and complied with Public Health Service policy on the humane care and use of laboratory animals. The monkeys were prepared and studied using standard surgical techniques that have been described in detail previously (Krauzlis 2003).
Behavioral tasks
We trained the monkeys to select one of two isoluminant stimuli as the target of a saccadic, smooth pursuit, or button press response (Fig. 1). At the start of each trial, the monkeys were required to fix gaze on a white 0.2 × 0.2° spot at the center of visual display, with gray background (16 cd/m2) for 1.5–2 s. After 500 ms, a 0.4 × 0.4° color cue was displayed for 500 ms (either blue or green, luminance 46 cd/m2), indicating the identity of the upcoming target. At the end of the fixation period, two 0.4 × 0.4° stimuli (1 blue and 1 green) were displayed on opposite sides of the extinguished fixation spot at a fixed eccentricity (offset 0.3° vertically and 3.5–7.2° horizontally, depending on SC site). The stimulus that matched the cue color was defined as the target, and the other stimulus was the distracter. Each color was assigned to the cue, and each stimulus, on an equal number of trials.
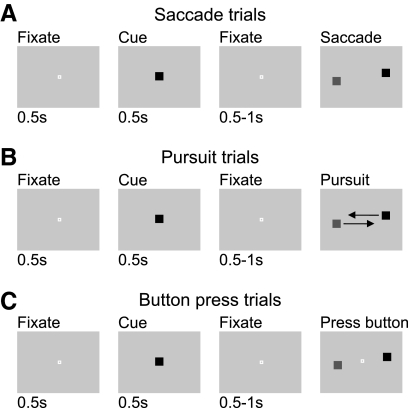
Fig. 1.
Schematic of target selection tasks. Each box depicts the monitor display at a point in the trial. On each trial, the monkey fixated the central stimulus while a color cue was presented and committed that color cue to memory. A: on saccade trials, the monkey made a saccade to fixate the stimulus that matched the cue to earn a juice reward. B: on smooth pursuit trials, the monkey tracked the matching stimulus without making a saccade during the 1st 250 ms of stimulus presentation. C: on button press trials, the monkey maintained fixation on the central white spot, and selected the matching stimulus by pressing a left or right button to indicate his choice.
The task of the monkey was to correctly select the target to receive a small juice reward at the end of each trial. In three separate blocks, monkeys were required to select the target with either a saccadic eye movement, smooth pursuit eye movement, or a button press response. Trials for each type of response (saccade, pursuit, button press) were blocked into groups of 48 or 56 trials, and these blocks were regularly interleaved during experiments. In addition, eight trials in each block included only a target, and no distracter, to evaluate response execution independent of target selection. Experiments typically included three or four blocks for each response type.
On saccade trials, the target and distracter stimuli remained stationary (Fig. 1A, saccade trials), and choice was indicated by the first saccade (70°/s threshold). Selection of the target required the saccade endpoint to land within 2 or 3° of the target depending on target eccentricity.
On pursuit trials, the target and distracter stimuli traveled at constant velocity toward the vertical midline of the display, such that they approached the midline close to pursuit onset, resulting in saccade-free initiation of smooth pursuit (Rashbass 1961) (Fig. 1B, pursuit trials 17.6–35.2°/s, depending on stimulus eccentricity). The monkey was allowed 280 ms to get within 2 or 4° of the target (depending on target speed) and required to stay within 2 or 3° of the target for at least another 200 ms.
On button press trials, the fixation spot was presented along with the stimuli, which remained stationary (Fig. 1C, button press trials). The monkey was required to maintain central fixation (within 1.5° position, 70°/s velocity threshold) until one stimulus was selected by pressing one of two buttons. The buttons were located in a response box outside the monkey's field of view, within 6 in of the normal resting position of their right hand (both monkeys used only their right hands) with one button on either side of the monkey's midline. The buttons were spatially associated to each stimulus so that pushing the button on the right selected the stimulus on the right side of the visual display and pushing the button on the left selected the stimulus on the left. The choice stimuli were either extinguished or masked by white squares after 180 ms to keep the amount of time available to visually inspect the stimuli comparable to the saccade task, and a response was required within 1 s of stimuli onset.
In addition to the target selection task, monkeys also performed a visually guided saccade task to measure the spatial extent of each SC inactivation. In this task, monkeys were required to fixate a centrally located 0.2 × 0.2° white spot for 500-1,000 ms, after which the spot stepped to another location on the display. The monkeys were given a small juice reward for making a saccade to the white spot within 500 ms.
Reversible SC inactivation
We inactivated portions of the SC of monkeys V and P using local muscimol injections (Chen et al. 2001; Hikosaka and Wurtz 1985a,b) (0.5 μl, 5 μg/μl). In 18 experiments, injections were aimed at the intermediate and deep layers of the SC (1.8–3.0 mm below surface). Additionally, in two control sessions, we injected sterile saline solution at sites previously inactivated with muscimol.
We identified our injection sites (Table 1) as follows. The day before each experimental session, we identified an injection site and depth within the SC using single-unit recording and electrical microstimulation to evoke saccades consistent with metrics encoded by the visited site (microstimulation parameters: 400 ms, 500 Hz <30 μA, biphasic pulses). During the session, we usually confirmed our site by observing multi- or single-unit saccade-related activity and/or evoking saccades with microstimulation. Finally, after injecting muscimol, using a custom-made apparatus modified from Chen et al. (2001), we verified our site by observing reductions in peak velocity of visually guided saccades (Hikosaka and Wurtz 1985a,b). These decreases in peak velocity were localized to the region of retinotopic space affected by muscimol injection (e.g., see Fig. 2, A and B); no change in peak velocity was observed following saline injection. Injection of the entire volume of muscimol or saline was done over a period of 16–24 min.
Table 1.
Summary of injection experiments
| Injection Site |
Stimulus Location |
Strength of Inactivation |
Fixation Offset |
Change in Bias |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Injection No. | Injection Volume, μl | Injection Depth, mm | H,° | V,° | H,° | V,° | S1 | S2 | H,° | V,° | Pursuit | Saccade | Button |
| V | 1† | 0.5 | 2.70 | −3.7 | −1.6 | −3.5 | −0.3 | 0.83 | 0.71 | 0.08 | 0.03 | 1.47* | 0.72* | 0.51* |
| 2 | 0.5 | 1.90 | 1.1 | 1.1 | 3.5 | 0.3 | 0.97 | 0.85 | −0.02 | −0.04 | 0.58* | 0.24 | 0.25 | |
| 3† | 0.5 | 2.50 | 0.6 | −0.1 | 3.5 | 0.3 | 0.91 | 0.67 | −0.11 | −0.01 | 1.27* | 0.74* | 0.34* | |
| 4 | 0.5 | 2.00 | −4.4 | −0.5 | −3.5 | −0.3 | 0.79 | 0.67 | 0.06 | 0.06 | 1.65* | 0.79* | 0.14 | |
| 5† | 0.5 | 2.00 | 1.9 | 2.2 | 3.5 | 0.3 | 1.02 | 0.79 | −0.11 | −0.02 | 1.20* | 1.02* | −0.41 | |
| 6† | 0.5 | 2.05 | 1.5 | 0.5 | 3.5 | 0.3 | 0.97 | 0.68 | −0.01 | −0.04 | 2.04* | 0.34* | 0.00 | |
| 7 | 0.5 | 1.80 | −4.5 | −0.3 | −3.5 | −0.3 | 0.84 | 0.65 | 0.10 | 0.01 | 2.33* | 1.18* | 0.28 | |
| 8 | 0.5 | 2.00 | −2.9 | −0.3 | −3.5 | −0.3 | 0.72 | 0.55 | 0.09 | 0.06 | 2.64* | 1.25* | 0.39* | |
| 9† | 0.5 | 2.20 | 0.2 | 0.8 | 3.5 | 0.3 | 1.06 | 0.70 | −0.05 | 0 | 1.34* | 0.41* | 0.21 | |
| 10‡ | 0.5 | 2.10 | −4.8 | 0.5 | −3.5 | −0.3 | 1.09 | 1.11 | 0.03 | 0.03 | −0.05 | 0.20 | −0.43 | |
| 11 | 0.45 | 2.00 | 4.0 | 0.2 | 7.5 | 0.3 | 0.66 | 0.55 | −0.15 | −0.03 | 2.81* | 1.25* | 0.94* | |
| 12 | 0.5 | 2.00 | −3.4 | −0.3 | −7.5 | −0.3 | 0.90 | 0.60 | 0 | 0 | 2.02* | 0.79* | 0.12 | |
| 13 | 0.4 | 2.00 | 4.2 | 0.3 | 7.5 | 0.3 | 0.81 | 0.53 | −0.17 | −0.01 | 2.02* | 0.75* | 0.20 | |
| P | 14 | 0.5 | 2.45 | −1.6 | −0.3 | −4.5 | −0.3 | 0.76 | 0.51 | 0.39 | 0.29 | 3.29* | 1.76* | 0.53* |
| 15 | 0.5 | 2.00 | 4.0 | 0.3 | 4.5 | 0.3 | 0.91 | 0.72 | 0.29 | 0.60 | 1.66* | 2.02* | 0.17 | |
| 16 | 0.5 | 2.75 | 3.3 | 0.6 | 4.5 | 0.3 | 0.59 | 0.61 | −0.56 | 0.07 | 1.54* | 2.00* | 0.24 | |
| 17 | 0.25 | 2.25 | −1.7 | −0.4 | −4.5 | −0.3 | 0.62 | 0.58 | 1.11 | 0.30 | 2.71* | 1.73* | 0.45* | |
| 18‡ | 0.5 | 2.30 | 2.5 | −0.1 | 4.5 | 0.3 | 0.95 | 1.08 | −0.13 | 0 | 0.01 | 0.04 | −0.04 | |
| 19 | 0.5 | 2.40 | −2.3 | −0.1 | −6.5 | −0.3 | 0.87 | 0.72 | 0 | −0.04 | 0.62* | 1.25* | −0.01 | |
| 20 | 0.5 | 2.25 | 1.2 | 0.4 | 6.5 | 0.3 | 0.57 | 0.49 | −0.16 | 0.04 | 1.30* | 1.50* | 0.31* | |
Summary of injection experiments. The injection site was measured by the mean horizontal (H) and vertical (V) amplitude of evoked saccades at the injection depth. The location of the stimulus targeted by each injection is provided, and the strength of inactivation is given by the peak velocity of saccades to that location shortly after injection (S1) and after data collection (S2), normalized to pre-injection saccades. The fixation offset indicates the mean displacement in eye position during fixation caused by SC inactivation.
, the change in bias was statistically significant at the 0.05 level;
, the injection site was measured from the same site and depth on the previous day due to failure to evoke saccades with the injectrode;
, saline was injected instead of muscimol.
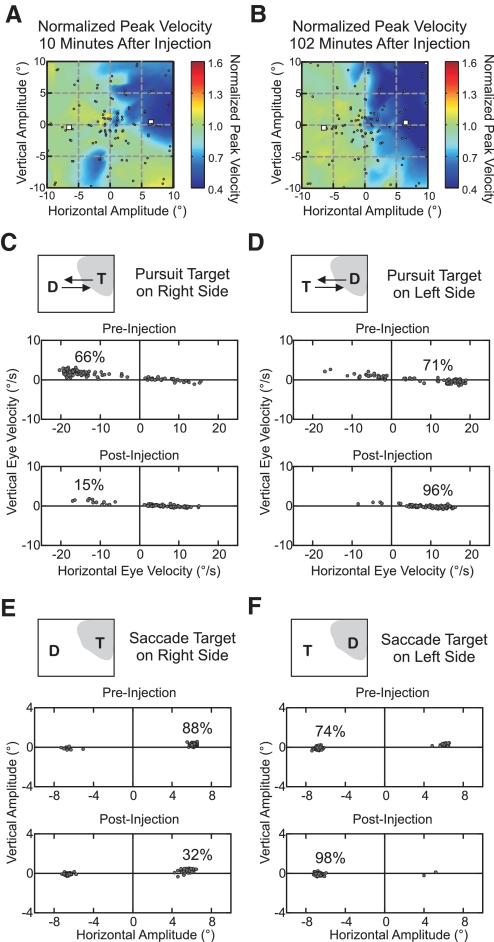
Fig. 2.
Sample injection experiment demonstrating the effects of focal superior colliculus (SC) inactivation on target selection (20). A and B: the extent of SC inactivation was measured by plotting peak velocity of visually guided saccades normalized to saccades made prior to inactivation, shortly after muscimol injection (A), and after the collection of target selection behavior (B). ●, the amplitude of visually guided saccades; □, the starting locations of the target selection stimuli. C and D: for smooth pursuit target selection, the distribution of choices is illustrated by plotting the mean horizontal eye velocity against mean vertical eye velocity over the 1st 100 ms of smooth pursuit. The changes in the choice distributions caused by muscimol injection are shown separately for trials in which the target appeared on the right (C) or left (D) side. E and F: for saccade target selection, the distribution of choices is illustrated by plotting the horizontal saccade amplitude against the vertical saccade amplitude with choice distributions shown separately for trials in which the target appeared on the right (E) or left (F) side.
We typically collected behavior from three or four blocks (144–224 trials) of the target selection task for all three response types (saccade, pursuit, and button press) before injection (preinjection) and three or four blocks of each response afterward (postinjection). Our preinjection data were collected with our injection apparatus resting >2 mm above our estimate of SC surface obtained from the previous day's recording session, ensuring that no muscimol leaked into the SC while we collected this data. Also for both the pre- and postinjection data sets, all experimental parameters were identical although we rewarded both subjects somewhat more generously as the session progressed to maintain motivation.
On the day following each inactivation experiment, we collected recovery data using the same stimulus parameters (data not shown). In addition to confirming that the monkeys recovered, we did this to confirm that the monkeys' preinjection target selection behavior was stationary (i.e., that changes observed with inactivation were not caused by natural variability in behavior).
Data analysis
Eye movements were sampled at 1 kHz. Saccades were detected using velocity and acceleration thresholds (Krauzlis and Miles 1996) using 40°/s velocity and 800°/s2 acceleration thresholds except prior to pursuit onset when we used more stringent thresholds of 8°/s velocity and 250°/s2 acceleration. Marked saccades were individually inspected and verified. The onset of pursuit was estimated from traces of horizontal eye velocity on individual trials by fitting a “hinge model,” a linear regression technique described previously (Adler et al. 2002) with two modifications: the response interval was 100 ms and hinge placements ranged ± 25 ms from an initial subjective estimate of pursuit latency. If a saccade occurred within 50 ms of pursuit latency, the trial was discarded. To confirm that this technique provided reliable estimates of pursuit onset, it was corroborated by a second test in which we compared the velocity at pursuit onset (the 1st 50 ms after pursuit latency) to the velocity during fixation (the final 50 ms prior to stimuli onset). We accepted the pursuit latency estimate only if the median velocity during pursuit onset was significantly greater than during fixation (rank sum test, P < 0.01). All pursuit or button press trials with saccade amplitudes greater than the typical range for microsaccades (≤0.33°) prior to response onset were discarded.
Performance on the target selection task was scored using the following criterion. Saccade trials were scored correct if the horizontal saccade amplitude was directed toward the target. Most of the time the response landed within 1° of the stimulus (>99% baseline, >90% inactivation), but because SC inactivation can reduce the accuracy of saccades (Lee et al. 1988), choices were still assigned for saccades that landed further away. Pursuit trials were scored correct if the initial horizontal pursuit velocity (mean over 1st 100 ms after onset) was in the same direction as the target velocity (which was always exclusively horizontal, either left- or rightward). Button press responses were scored correct if the button corresponding to the target location was pressed. Trials in which the response was directed to the distracter and not the target were scored as incorrect, and trials without a response to the target or the distracter were discarded.
To test for differences in target selection performance between different conditions, we compared binomial distributions calculated from the fraction of correct target selections. In addition, we evaluated changes in performance using methods from signal detection theory. Bias was calculated in units of d′ assuming a constant criterion (MacMillan and Creelman 2005). In brief, correct selection of a target contralateral to injection (corresponding to the affected visual field), was scored as a “hit” and incorrect selection of a contralateral distracter was scored as a “false alarm.” Bias, c, was then defined as the sum of the z-transformed hit rate (H) and false alarm rate (FA) values, divided by −2: c = −0.5[z(H) + z(FA)].
Similarly, sensitivity was measured in units of d′ using the same hit rate and false alarm rate values. Sensitivity (d′) was defined as the difference of the z-transformed hit rate and false alarm rate, divided by the square root of 2: d′ = [1/sqrt(2)][z(H) −z(FA)].
Confidence intervals for bias and sensitivity were generated from individual experiments using a bootstrap procedure (Efron and Tibshirani 1998).
All group comparisons among values of latency, fraction correct, bias, sensitivity, or error rate were tested for significance using nonparametric signed rank or rank sum tests in difference of medians because these data typically did not fit a normal distribution. To limit the number of comparisons, we only made group comparisons across factors that were found to be significant using multi-way ANOVAs. Furthermore, we controlled for the rate of false positives by correcting for multiple group comparisons using a Holm-Sidak step-down procedure (Ludbrook 1998). Tests for control experiments (saline injections and fixation offset controls) were not subject to correction for multiple comparisons to ensure the detection of any confounding factors.
We found small offsets in the eye position of central fixation away from the affected visual field (see Table 1), similar to offsets in eye position reported during smooth pursuit (Hafed et al. 2008). For monkey P, we measured the difference between eye position at central fixation before muscimol injection and following the collection of postinjection data. Movement of equipment in and out of the magnetic field after collection of preinjection data prevented use of the same method to estimate shifts in eye position for monkey V. Instead we took the difference between saccade amplitudes directed into the unaffected region, before and after SC inactivation, reasoning that any decrease in amplitude was due to an offset in eye position toward the stimulus. The shifts in eye position for monkey V were similar to measurements made by Hafed et al. (2008) for a stimulus of the same size, whereas the shifts in eye position for monkey P were larger and had greater variance. These measurements were used for control experiments in which we offset the position of fixation by the measured amount instead of performing an injection. For these experiments, ANOVA did not find that subject (and thus method of offset measurement) was a significant factor for the shifts in choice bias; therefore the results from these control experiments were pooled across the two monkeys for group comparisons.
RESULTS
We measured the target selection behavior of two monkeys (P and V) before and after 20 SC injections (Table 1). Muscimol was injected into the intermediate and deep layers of the SC in 18 of the experiments, 6 in P and 12 in V. In addition, each monkey received one saline injection into the SC as a control.
Sample inactivation experiment
Injection of muscimol into the intermediate and deep layers of the SC caused large pursuit and saccade selection deficits for targets placed within the affected visual field but only caused a small deficit in button press target selection. The affected visual field for a representative muscimol injection (injection 20) is shown shortly after injection (Fig. 2A) and after the collection of postinjection behavior (Fig. 2B); the spread of the inactivation is indicated by the reduction in peak velocity of visually guided saccades, with saccade endpoints indicated by small circles. The white squares (Fig. 2, A and B) indicate the starting locations of the target selection stimuli for smooth pursuit, saccades, and button press responses; the stimulus on the right was placed within the part of the visual field affected by the muscimol injection.
The sample muscimol injection caused a clear deficit in pursuit target selection, indicated by the shift in directions of the initial smooth eye movement. Prior to muscimol injection, when the target appeared on the right side, the monkey correctly pursued the target (in the leftward direction) 66% of the time (Fig. 2C, preinjection). Following injection, the monkey correctly pursued the target in the affected field only 15% of the time (Fig. 2C, postinjection). Before injection, the monkey correctly pursued targets on the left side (in the rightward direction) 71% of the time (Fig. 2D, preinjection). After injection, the monkey correctly pursued targets out of the affected field 96% of the time (Fig. 2D, postinjection).
The sample injection caused a similar deficit in saccade target selection indicated by the change in the endpoints of initial saccades. Prior to injection, when the target appeared on the right side, the monkey made a saccade to the target 88% of the time (Fig. 2E, preinjection), but following injection, a saccade was made to the target in the affected field only 32% of the time (Fig. 2E, postinjection). Before injection, the monkey selected targets on the left side 74% of the time (Fig. 2F, preinjection). After injection, the monkey selected targets out of the affected field 98% of the time (Fig. 2F, postinjection).
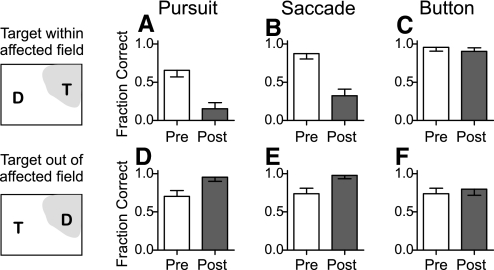
In contrast to smooth pursuit and saccades, the sample muscimol injection caused a small, and not significant, deficit in target choice for button presses. When the target appeared on the right side, injection caused button press performance to drop from 96% correct selections to 91% (Fig. 3C). When the target appeared on the left side, injection caused performance to improve from 74% correct selections to 80% (Fig. 3F). This differed from the larger, statistically significant, changes in pursuit (Fig. 3, A and D) and saccade (B and E) target selection.
Fig. 3.
Changes in target selection from a sample SC inactivation (20). A–C: bar plots of the fraction of correct choices before (pre) and after (post) muscimol injection into the SC when the target appeared within the affected visual field, as indicated by the schematic on the left, separately for smooth pursuit (A), saccade (B), and button press (C) response trials. Error bars are 1-tailed 95% confidence intervals. D–F: bar plots of the fraction of correct choices when the target appeared out of the affected visual field separately for smooth pursuit (D), saccade (E), and button press (F) response trials using the same conventions as A–C.
Summary of changes in target choice
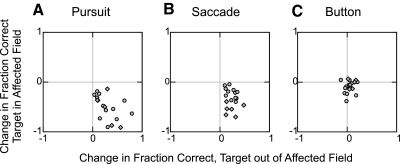
We found similar effects on target choice across the set of 18 SC inactivation experiments. The results for each experiment are summarized in Fig. 4, A–F, by plotting the fraction of correct choices after SC inactivation against the fraction of correct choices prior to SC inactivation, separately for each type of response and by target location.
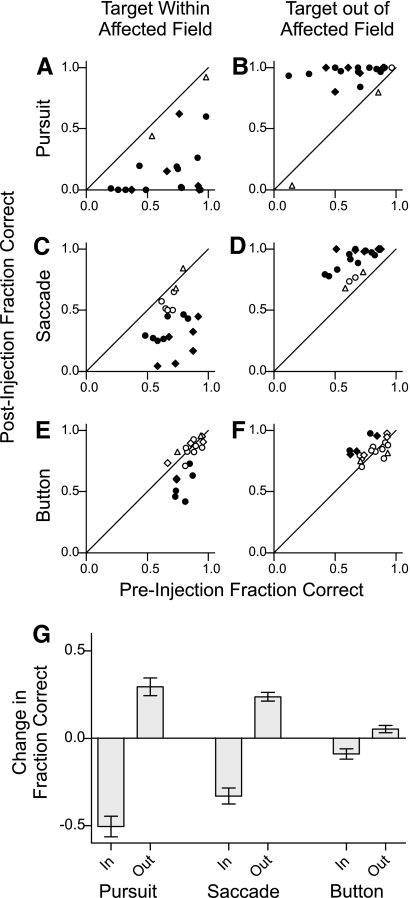
Fig. 4.
Summary of the change in the fraction of correct trials for all injection experiments. A and B: for each experiment, the fraction of correct selections before injection is plotted against the fraction of correct selections after injection for smooth pursuit trials with the target in the affected visual field (A) and smooth pursuit trials with the target out of the affected visual field (B). Muscimol injections into the intermediate SC are indicated subject P (◊) and subject V (○); ▵, saline injections. ♦, ●, significant difference at the 0.05 level. C–F: same format as A and B for saccade (C and D) and button press (E and F) responses. G: bar plot of the mean changes in fraction correct over all injections into the intermediate SC, separated by trial type and target location. Error bars are SE.
For smooth pursuit, SC inactivation significantly decreased the fraction of correct choices for targets placed within the affected visual field in all 18 experiments (Fig. 4A). Conversely, SC inactivation significantly increased the fraction of correct choices for targets placed out of the affected visual field in 17 of the experiments (Fig. 4B). Note that in the case of pursuit, the initial location of the target was in the opposite direction of the eye movement required to select it. Thus for pursuit we observed a dissociation between target location and eye movement direction: the selection deficits occurred when the target was located in the affected field even though these movements were directed toward the unaffected side.
The same pattern of results was found for saccades in which SC inactivation significantly decreased the fraction of correct choices for targets placed within the affected visual field in 13 of 18 experiments (Fig. 4C) and increased the fraction of correct choices for targets placed out of the affected visual field in 16 of the experiments (Fig. 4D).
SC inactivation caused smaller and less consistent deficits in target choice for button press. The fraction of correct choices for targets placed within the affected visual field significantly decreased in 7 of the 18 experiments (Fig. 4E), and the fraction of correct choices for targets placed out of the affected visual field significantly increased in 5 experiments (Fig. 4F).
A four-way ANOVA confirmed that the changes in the fraction of correct selections depended on target location (with respect to the affected field, P < 0.0001) and type of selection response (pursuit/saccade/button press, P = 0.007), and there was a significant interaction between these factors (P < 0.0001). However, changes in fraction correct did not depend on target color (green/blue, P = 0.25) or subject (P/V, P = 0.16), so comparisons were pooled across these factors. Figure 4G plots the mean changes in fraction correct across the 18 inactivations separated by type of response and target location. For targets within the affected visual field, the fraction of correct selections significantly decreased for saccades and smooth pursuit (P < 0.001), and for targets out of the affected visual field, the fraction of correct selections for saccades and pursuit significantly increased (P < 0.001). The results for button press responses followed the same trend as saccades and smooth pursuit, but the changes in fraction correct did not reach significance after corrections for multiple comparisons (P = 0.05).
For most of the experiments, the deficit in selection of targets within the affected field was accompanied by improvement in selection of targets out of the affected field, as was the case for the sample inactivation (Fig. 3). To illustrate the collective effect on target selection across both locations, we plotted the change in fraction correct for targets within the affected visual field against the change for targets out of the affected visual field for each experiment, separated by type of response (Fig. 5). A decrease in fraction correct within the affected field was always coupled with an increase in fraction correct out of the affected field for smooth pursuit (Fig. 5A) and saccades (B); thus SC inactivation did not cause a general increase or decrease in task performance but instead caused subjects to choose the stimulus that was out of the affected visual field more often, increasing performance when that stimulus was the target, and decreasing performance when that stimulus was the distracter.
Fig. 5.
Summary of the overall shift in behavior caused by each SC inactivation. A: the overall change in behavior for smooth pursuit is shown by plotting the change in fraction correct following muscimol injection for trials with targets that started out of the affected visual field against the change in fraction correct for targets that started in the affected visual field. Points lying in the lower right quadrant indicate an overall shift of choice away from the affected visual field. B and C: same format as A for saccade (B) and button press (C) responses.
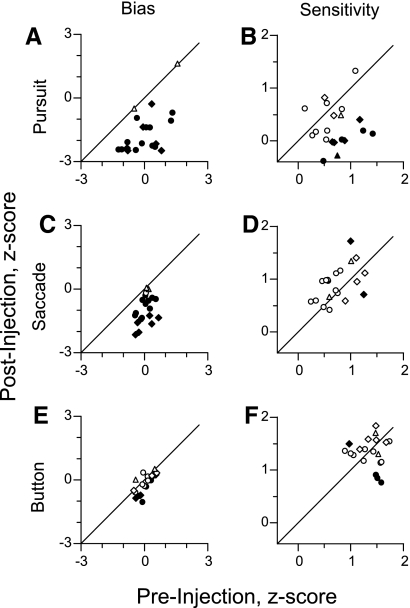
The spatial bias in target choice can be quantified more compactly with signal detection theory by comparing the rates of “hits” and “false alarms” at a particular location (MacMillan and Creelman 2005). This measurement of bias depends on the fact that as the rates of hits and false alarms to one location increase, the rates of misses and correct rejections to the other location decrease. A similar analysis can also be used to quantify the monkeys' ability to discriminate the two stimuli (sensitivity, measured as d′). Measurement of bias and sensitivity indicates the overall effect of each experiment without having to compare data from two separate locations. We used the constant criterion method for calculating bias (see methods), and bias away from the affected visual field was arbitrarily defined as a positive value.
SC inactivation biased choices away from stimuli in the affected visual field, significantly, in all 18 experiments for smooth pursuit and 17 of the experiments for saccades (Fig. 6, A and C, respectively), whereas for button presses, choice was significantly biased away from the affected visual field in 7 of the 18 experiments (Fig. 6E). Unlike bias, SC inactivation did not cause consistent changes in sensitivity for saccades and button press responses (Fig. 6, D and F) but did tend to decrease sensitivity for smooth pursuit, with a significant decrease in sensitivity in 8 of the 18 experiments (Fig. 5B). ANOVAs for the changes in bias and sensitivity found that the type of response (P < 0.001) was a significant factor. Significant changes in spatial bias were found for all three response types (P < 0.02), although the shift in bias for smooth pursuit and saccades were significantly larger than for button press (P < 0.01). The change in bias for smooth pursuit was, on average, larger than the change in bias for saccades, but this result was not significant (P = 0.05). SC inactivation did not significantly change sensitivity for any of the response types; however, there was a trend toward a reduction in sensitivity for smooth pursuit (P = 0.05); this was a result of several inactivations causing a near-complete bias to pursue the stimulus out of the affected region, which due to the balanced trial design forced sensitivity toward zero.
Fig. 6.
Summary of the shifts in bias and sensitivity caused by each SC injection. A and B: for the pursuit trials from each experiment, preinjection bias (A) and sensitivity (B) are plotted against the postinjection values. Positive bias values indicate a bias toward the affected visual field, and symbol conventions are the same as Fig. 4, A–F. C–F: same format as A and B for saccade (C and D) and button press (E and F) responses.
We calculated the correlations among change in bias for the three response types. The correlation coefficient was 0.37 between saccades and pursuit and 0.22 between saccades and button presses, but neither was correlated significantly (P = 0.13 and P = 0.37, respectively). A somewhat stronger correlation coefficient of 0.55 was found between pursuit and button presses, but it was not significant after correction for multiple comparisons (P = 0.05).
We also analyzed changes in how often the monkeys failed to make responses following muscimol injection. The monkeys failed to make a response on <1% of saccade choice trials before and after injection with no failures for most experiments. The median rate of failure to respond was not >0 (P = 0.25). The failure rate for pursuit choice trials increased from 3.2% before injection to 3.5% after injection; this was not significant (P = 0.57). However, there was a significant increase in response failure from 2.8 to 5.2% when the target was within the affected visual field (P = 0.045), and decrease when the target was out of the affected visual field (P = 0.063). Thus these changes in failure to respond follow the same pattern as the observed bias in target choice in that the monkeys were less likely to respond when the target was in the affected visual field and more likely to respond when the stimulus was out of the affected visual field. The rate of failure for button presses also increased from 2.1 to 5.4% of choice trials (P = 0.002); however, there was no difference in the rate of failure to respond based on target location relative to the affected visual field (P = 0.48), and so the failures seem to be unrelated to effects on target choice.
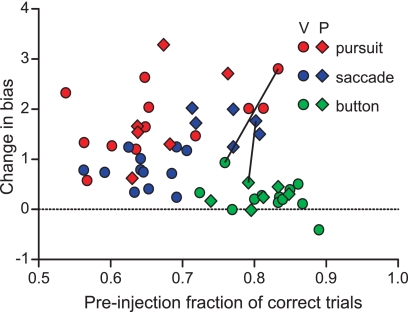
Influence of task difficulty on changes in choice bias
Performance on the button press task (0.81 mean fraction correct) was typically greater than performance on the saccade and pursuit tasks (0.69 and 0.67 mean fraction correct, respectively). However, there was some variance in performance from week to week. Figure 7 compares the difficulty of each task, measured prior to muscimol injection, to the effect of the SC inactivation on target selection. Even when performance on the saccade and pursuit tasks was comparable to performance on the button press task, the shift in bias was much larger for saccades and pursuit. This was also apparent in the rare cases when saccade or pursuit performance was better than button press performance in the same experimental session (Fig. 7, connected symbols).
Fig. 7.
Changes in bias given task performance. The change in bias caused by SC inactivation for the pursuit (red), saccade (blue), and button press (green) tasks is plotted against the monkey's performance, indicated by the preinjection fraction of correct trials. Circles indicate experiments with subject V, diamonds with subject P. Connected symbols are results from the same SC inactivation experiment.
Control experiments
One possible explanation for the effects on target selection is that on some trials the monkeys were not able to execute a response to the stimulus within the affected visual field. To test this explanation, we interleaved trials that showed only the target stimulus in each of the 18 SC inactivation experiments. Even under baseline conditions, the monkeys would occasionally fail to select the target on these trials (<3% of trials for any of the sessions), either by waiting too long to respond, breaking fixation, or in the case of button press trials, pressing the wrong button. Following SC inactivation, we found no changes in the rate of error for selecting a single stimulus placed within the affected visual field for any of the response types (P > 0.12).
To test that the changes in target selection were caused by the action of muscimol, and not simply the injection of fluid, each monkey received an injection of saline at the same site and depth of a successful inactivation experiment. Neither saline injection caused a significant change in the fraction of correct selections for any of the response types, regardless of the target location (Fig. 4, A–F, ▵). Consequently, virtually no changes in spatial bias were observed for either injection (Fig. 6, A, C, and E).
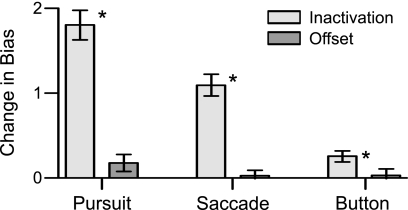
SC inactivation is known to shift the position of central gaze a small amount away from the affected visual field when tracking a visual target (Hafed et al. 2008). If offsets in central gaze occurred during fixation in our target selection tasks, they would shift stimuli placed outside the affected field closer to central gaze, which could influence the stimulus choice. We measured the shifts in central gaze caused by muscimol injection (see methods, Table 1), which were typically small (mean 0.08° in V, 0.32° in P). To test whether offsets in the position of gaze contributed to our results, we performed a series of yoked control experiments. For each SC inactivation, we conducted a control session in which we collected target selection data before and after shifting the fixation spot by the measured offset in central gaze during that inactivation experiment. The changes in bias for pursuit, saccades, and button press caused by matched offsets in central gaze were significantly smaller than the changes in bias caused by SC inactivation (Fig. 8, P < 0.05). Therefore the behavioral changes caused by SC inactivation cannot be explained by the resulting shifts in central gaze. However, if the offsets in central gaze caused some nonzero bias in target choice, then they still may have contributed to the total changes in bias caused by SC inactivation. This was only the case for smooth pursuit (Fig. 8, P = 0.032), which resulted in a change in bias ∼10% as large as the change in bias caused by SC inactivations.
Fig. 8.
Mean change in bias for SC inactivation and fixation offset control experiments. A bar plot of the mean changes in bias over all 18 SC inactivation experiments, light gray, and the yoked offset control experiments, dark gray, separately for each type of response. Error bars are SE; *, the difference in bias is significant at the 0.05 level.
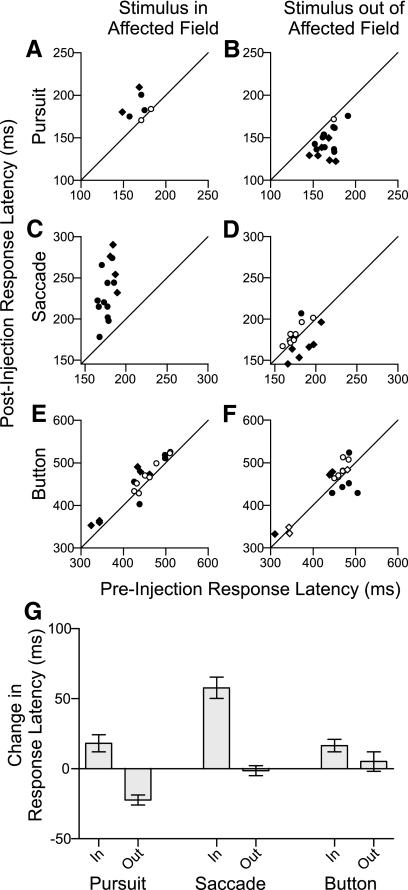
Effects of SC inactivation on choice latency
In addition to biasing the distribution of target choices, SC inactivation also changed the latency of choices, depending on the stimulus location relative to the affected visual field. The effects of SC inactivation on response latency for individual experiments is shown in Fig. 9, A–F; for statistical reasons, we only analyzed response latency for conditions with ≥10 trials, which unfortunately excluded some data from the experiments with the strongest effects on target choice. As expected from role of the SC in saccade generation, inactivation significantly increased the mean latency of saccades to stimuli in the affected visual field for all 15 experiments analyzed (Fig. 9C). This trend was also apparent for smooth pursuit in which 5 of the 7 experiments analyzed showed a significant increase in latency (Fig. 9A), and 10 of 18 experiments analyzed showed a significant increase in the latency of button press for stimuli in the affected field (Fig. 9E). For selections of stimuli out of the affected field, SC inactivation decreased the latency of smooth pursuit significantly in 16 of 17 experiments analyzed (Fig. 9B). SC inactivation also significantly decreased the latency of saccades in 6 of 18 experiments analyzed, all from monkey P (Fig. 9D), and there was no consistent change for button presses, with a significant decrease in latency for 4 of 18 experiments and a significant increase in latency for another 4 of the experiments (Fig. 9F).
Fig. 9.
Summary of the changes in latency for all injection experiments. A and B: for each experiment, the mean latency of selections before injection is plotted against the mean latency of selections after injection for smooth pursuit of the stimulus within the affected visual field (A) and smooth pursuit of the stimulus out of the affected visual field (B). The symbol conventions are the same as Fig. 4, A–F. C–F: same format as A and B for saccade (B and C) and button press (E and F) responses. G: bar plot of the mean change in latency in ms for all injections into the intermediate SC layers, separated by trial type and target location. Error bars are SE.
A four-way ANOVA indicated that type of response (P < 0.0001) and stimulus location (P < 0.0001) were significant factors influencing the changes in choice latency caused by SC inactivation, and these factors had a significant interaction (P < 0.0001), whereas subject (P = 0.1) and stimulus color (P = 0.9) were not significant factors. Figure 9G plots the mean change in latency by type of response and stimulus location. For smooth pursuit and saccades, the latency of selecting stimuli within the affected field significantly increased compared with the latency of selecting stimuli out of the affected field (P < 0.001). This trend was also apparent for the button press responses but was not significant (P = 0.12). The mean latency of smooth pursuit not only increased for stimuli within the affected visual field but also significantly decreased for stimuli out of the affected visual field (P < 0.05), and during some experiments, the pursuit latency was comparable to the latency of pursuit to single stimuli.
DISCUSSION
In addition to its well-known role in the control of saccades, the primate SC has been implicated in the processes of target choice for overt orienting movements and the allocation of covert spatial attention. The results from our present study demonstrate that the SC is crucial for selecting the target of eye movements in addition to its role in the selection, preparation, and execution of saccades. The smaller effects of SC inactivation on the button press task could be a result of the role of the SC in visual processing, the allocation of attention, or evidence for an additional role of the SC in selecting visual objects that guide arm and hand movements.
SC is crucial for target choice of orienting movements
Our primary result is that SC inactivation biases the target choice away from the affected visual field for smooth pursuit and saccades. These effects on target choice are consistent with studies that have identified SC activity that is predictive of the target choice for saccades (Glimcher and Sparks 1992; Horwitz and Newsome 1999, 2001a,b; McPeek and Keller 2002) or otherwise related to target probability (Basso and Wurtz 1998). Previous inactivation experiments have shown that SC activity plays a causal role in selecting the upcoming saccade (McPeek and Keller 2004); however, because saccades are directed to the target location, this effect could be limited to saccade selection rather than a general effect on target selection.
The effects of inactivation on smooth pursuit are important for concluding that the SC is crucial for target choice because smooth pursuit permits a dissociation of the target location from the direction of the eye movement. To exploit this, we used step-ramp stimuli (Rashbass 1961) that appeared in one visual hemifield and moved smoothly into the opposite visual hemifield. If the SC was only important for choosing an oculomotor plan, then inactivation should cause more eye movements directed away from the affected visual field. Instead SC inactivation resulted in more eye movements directed toward the affected visual field due to increased selection of stimuli placed outside the affected visual field. These results on target selection are consistent with our earlier findings that some SC neurons show an elevated level of activity when the target is located in their response field for both smooth pursuit and saccades (Krauzlis and Dill 2002) and that microstimulation biases target choice for smooth pursuit to the stimulus at the corresponding location even though the pursuit was directed away from the site of stimulation (Carello and Krauzlis 2004). Whereas single unit recording and microstimulation demonstrated that the SC is connected to the circuits responsible for selecting the targets of eye movements, our inactivation results demonstrate that activity from the SC is crucial for normal target selection.
The effects of SC inactivation on target selection are not a result of impairments in motor execution to, or the inability to see, the visual stimulus within the affected region because the ability to select stimuli presented alone, without a distracter, was unimpaired.
SC inactivation also affected the latency of saccades and smooth pursuit, compatible with an effect on target choice. Models of response latency generally assume that the response is triggered when some decision-related signal reaches a threshold (Carpenter and Williams 1995; Ratcliff and McKoon 2008; Ratcliff et al. 1999; Reddi and Carpenter 2000). If the SC is part of the circuit that produces such a decision-related signal, then we would expect changes in choice latency in addition to the distribution of choices. Specifically the removal of SC activity supporting selection of a stimulus within the affected visual field should also increase the latency of those selections. This is precisely what was observed for smooth pursuit and saccades. The mean latency of button presses also increased following SC inactivation, but for both stimulus locations (Fig. 9G) and so may have been due to fatigue of the arm and hand. Nevertheless, there was a trend for the increase in button press latency to be larger when selecting the stimulus in the affected field (P = 0.12).
Why does SC inactivation affect target choice for the button press?
In addition to affecting target choice for eye movement, SC inactivation also significantly biased target choices for button press responses away from stimuli in the affected visual field.
One explanation for the effect on button press target choice is that the SC is important for selecting the target of the arm and hand, in addition to the eye movement. Some neurons in the SC respond during reaching movements and button press interactions with objects (Nagy et al. 2006; Stuphorn et al. 2000; Werner et al. 1997a,b). These neurons can broadly be divided into two classes based on whether their response fields are gaze-centered or -independent. Neurons with gaze-centered response fields are more heavily distributed in the intermediate and deep layers of the SC and were probably inhibited by the injection of muscimol. However, it is unlikely that inactivation of these reach-related neurons caused the effects on button press target choice because the response fields of these neurons appear to be organized randomly with respect to the retinotopic map for eye movement, such that it would be unlikely to preferentially affect neurons selective for contralateral reaching movements (Stuphorn et al. 2000). Furthermore, even if there were a retinotopic deficit for reach-related neurons, the reach goals (i.e., the buttons) were located far below the portion of the visual field targeted by muscimol injection.
A more likely explanation for the effects on button press target choice is that SC inactivation affected visual processing of the stimuli located within the affected visual field, either by disrupting the monkey's ability to attend to the stimulus or by altering its appearance. Some neurons in the SC show elevated activity when attending to a stimulus within their response fields (Ignashchenkova 2004; Kustov and Robinson 1996), and activation of the SC, by microstimulation, causes behavioral effects that mimic the shifts in attention instructed by visual cues (Cavanaugh and Wurtz 2004; Müller et al. 2005). Furthermore, focal inactivation centered in intermediate SC layers demonstrates that SC activity is crucial to covertly select a cued signal in the presence of a distracting signal (Lovejoy and Krauzlis 2010). Thus it is likely that our muscimol injections caused some deficit in the normal visual processing of stimuli within the affected visual field, biasing the subjects to base their responses on stimuli outside of the affected visual field. From this perspective, the effects of SC inactivation on button press responses could help parcel out the contribution of visual deficits to the observed changes in eye movement target selection. However, because the effects of SC inactivation on button press responses could have been due to several factors, as we discuss in the following text, we do not think that our button press data can be used to make a quantitative estimate of the size of this putative visual component of the SC inactivation effect.
Why are the effects on button press responses smaller?
Although SC inactivation caused significant changes in button press target choice, these effects were also significantly smaller than effects on target choice for eye movements. The smaller effects could be due to differences between the button press and eye movement forms of the choice task. For example, monkeys tended to have better baseline performance on the button press task, and mean latencies were greater for button presses than for saccades or smooth pursuit. The differences in task difficulty are probably not responsible for the differences in effects on target selection because SC inactivation caused larger selection deficits for saccades and pursuit even when performance was comparable to the button press task (Fig. 7). However, it is possible that the greater urgency associated with initiating eye movements made them more susceptible to SC inactivation. Additionally, the eye movement task required the target's precise spatial location to execute a response, whereas the button press task only required knowing the target's side before the response could be converted into coordinates related to the arm and hand for the button press, which could be less dependent on SC activity. It is possible that these differences made the button press task less demanding on visual attention, resulting in smaller effects on target selection.
Another explanation is that the SC is important for target selection of overt orienting in addition, and distinct from, its role in selection for covert visual attention. In this case, the stronger target selection deficits for saccades and pursuit may be due to SC activity with a direct role in selection for overt orienting, perhaps via descending projections to the pontine nuclei or other brain stem regions (Harting 1977). In contrast, the influence of the SC on covert attention is likely mediated via ascending pathways through thalamus, possibly through the mediodorsal nucleus, to the frontal eye fields (Sommer and Wurtz 2004), a structure implicated in the control of visual attention (Moore and Armstrong 2003; Moore and Fallah 2004). Identifying these different processing pathways, and how they interact, could provide a new approach for investigating the relationship between perception and action.
Future directions
The presence of effects on button press choice raises additional questions that could be addressed in future experiments. For example, the arm and hand movements in our task were not directed to the spatial position of the target on the screen; it would be interesting to investigate whether there would be a larger deficit in choice of arm and hand movement if the monkey were required to touch the stimulus on the screen to select it. It also remains unknown how the effects on button press choice depended on the direction of the movement, which in our experiment tended to be correlated with the target. One way of testing this would be to associate the left button with the target on the right and vice versa. Finally, we do not know the spatial specificity of the inactivation effects on target choice. It would be informative to investigate whether deficits still occur if both the target and distracter are located in the same visual hemifield or if the effects on target choice are precisely restricted to just the spatial locations where a deficit in saccade reaction time is observed. Together, these issues highlight the growing recognition that the primate SC is not just a motor map for saccades but plays a broader role in controlling the selection of behavioral responses.
GRANTS
This research was funded by the National Eye Institute Grant EY-012212 and by the Aginsky Estate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Adler SA, Bala J, Krauzlis RJ. Primacy of spatial information in guiding target selection for pursuit and saccades. J Vision 2: 627–644, 2002 [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron 43: 575–583, 2004 [DOI] [PubMed] [Google Scholar]
- Carpenter RH, Williams ML. Neural computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62, 1995 [DOI] [PubMed] [Google Scholar]
- Case GR, Ferrera VP. Coordination of smooth pursuit and saccade target selection in monkeys. J Neurophysiol 98: 2206–2214, 2007 [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci 24: 11236–11243, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Goffart L, Sparks DL. A simple method for constructing microinjectrodes for reversible inactivation in behaving monkeys. J Neurosci Methods 107: 81–85, 2001 [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. In: Confidence Intervals Based on Bootstrap Percentiles. Boca Raton, FL: CRC LLC, 1998, p. 168–176 [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992 [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28: 8124–8137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta). J Comp Neurol 173: 583–612, 1977 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol 53: 266–291, 1985a [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulate. J Neurophysiol 53: 292–308, 1985b [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science 284: 1158–1161, 1999 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. J Neurophysiol 86: 2527–2542, 2001a [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86: 2543–2558, 2001b [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci 7: 56–64, 2004 [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Neuronal activity in the rostral superior colliculus related to the initiation of pursuit and saccadic eye movements. J Neurosci 23: 4333–4344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. The control of voluntary eye movements: new perspectives. Neuroscientist 11: 124–137, 2005 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Shared motor error for multiple eye movements. Science 276: 1693–1695, 1997 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84: 876–891, 2000 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron 35: 355–363, 2002 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Release of fixation for pursuit and saccades in humans: evidence for shared inputs acting on different neural substrates. J Neurophysiol 76: 2822–2833, 1996 [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature 384: 74–77, 1996 [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357–360, 1988 [DOI] [PubMed] [Google Scholar]
- Liston D, Krauzlis RJ. Shared response preparation for pursuit and saccadic eye movements. J Neurosci 23: 11305–11314, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston D, Krauzlis RJ. Shared decision signal explains performance and timing of pursuit and saccadic eye movements. J Vision 5: 678–689, 2005 [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13: 261–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol 25: 1032–1037, 1998 [DOI] [PubMed] [Google Scholar]
- MacMilllan NA, Creelman CD. Detection Theory: A User's Guide (ed. 2). New York: Lawrence Erlbaum, 2005, p. 3–29 [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature 42: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol 91: 152–162, 2004 [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA 102: 524–529, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Kruse W, Rottmann S, Dannenberg S, Hoffmann KP. Somatosensory-motor neuronal activity in the superior colliculus of the primate. Neuron 52: 525–534, 2006 [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol 159: 326–338, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Van Zandt T, McKoon G. Connectionist and diffusion models of reaction time. Psychol Rev 106: 261–300, 1999 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20: 873–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi BA, Carpenter RH. The influence of urgency on decision time. Nat Neurosci 3: 827–830, 2000 [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808, 1972 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924, 1972 [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol 91: 1381–1402, 2004 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol 83: 1283–1299, 2000 [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res 115: 191–205, 1997a [DOI] [PubMed] [Google Scholar]
- Werner W, Hoffmann KP, Dannenberg S. Anatomical distribution of arm-movement-related neurons in the primate superior colliculus and underlying reticular formation in comparison with visual and saccadic cells. Exp Brain Res 115: 206–216, 1997b [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3: 189–226, 1980 [DOI] [PubMed] [Google Scholar]