Abstract
Understanding the mechanisms of host–pathogen interaction can provide crucial information for successfully manipulating their relationships. Because of its genetic background and practical advantages over vertebrate model systems, the nematode Caenorhabditis elegans model has become an attractive host for studying microbial pathogenesis. Here we report a “Trojan horse” mechanism of bacterial pathogenesis against nematodes. We show that the bacterium Bacillus nematocida B16 lures nematodes by emitting potent volatile organic compounds that are much more attractive to worms than those from ordinary dietary bacteria. Seventeen B. nematocida-attractant volatile organic compounds are identified, and seven are individually confirmed to lure nematodes. Once the bacteria enter the intestine of nematodes, they secrete two proteases with broad substrate ranges but preferentially target essential intestinal proteins, leading to nematode death. This Trojan horse pattern of bacterium–nematode interaction enriches our understanding of microbial pathogenesis.
Keywords: Bacillus nematocida, Caenorhabditis elegans, chemotaxis, pathogen–host interaction, virulence protease
Most model organisms, such as the yeast Saccharomyces cerevisiae, the slime mold Dictyostelium discoideum, the mouse-ear cress plant Arabidopsis thaliana, the common fruit fly Drosophila melanogaster, and the nematode Caenorhabditis elegans, can be infected by microbes, including certain human-pathogenic bacteria (1). For several reasons, C. elegans is an attractive model organism to study host–pathogen interactions: It has simple growth requirements, a short generation time, a well-defined developmental process with invariant cell lineage sorting, a fully sequenced genome, and a suite of well-established genetic tools (2). Using C. elegans as a model, scientists in the last few years have identified a diversity of physical, chemical, and biochemical features involved in microbial pathogenesis (3). For example, Brevibacillus laterosporus secretes extracellular proteases that damage nematode cuticle, and Bacillus thuringiensis produces toxic crystal proteins that disrupt host cellular functions (4, 5). The common human-pathogenic bacterium Pseudomonas aeruginosa kills C. elegans with quorum-sensing controlled-virulence factors (6) and cyanide (7). Several other human pathogens such as the Gram-negative bacteria Burkholderia pseudomallei and Serratia marcescens and the Gram-positive bacteria Enterococcus faecalis, Streptococcus pyogenes, and Staphylococcus aureus also are reported to have nematoxic activities via a neuromuscular endotoxin, a cytolysin, two extracellular proteases (gelatinase and serine protease), and several other toxins (8–12). In S. aureus, several virulence determinants known to be important in mammalian pathogenesis, including the quorum-sensing global virulence regulatory system agr and the global virulence regulator sarA, the alternative sigma factor B, α-hemolysin, and the V8 serine protease, are all required for full pathogenicity against nematodes (13).
Olfactory chemotaxis toward food-associated odors is one of the most robust behaviors of nematodes (14). These odorants are believed to be involved in a variety of nematode behaviors such as aversive olfactory learning (15), the choice of feeding or leaving (16), and the detection and avoidance of pathogens (17). It has been well established that pathogenic microbes can use this olfactory chemotaxis to attract nematodes. However, the chemical nature of these volatile organic compounds (VOCs) remains largely unknown (15, 17). Recently, it was found that nematoxic bacterium P. aeruginosa can produce acylated homoserine lactones, the signal molecules in quorum sensing, as attractants for nematodes (7).
A common group of virulence factors shared among bacterial pathogens are the proteases, and protease inhibitors have proven effective therapeutic agents in treating infectious diseases in vertebrates (18, 19). For example, in the pathogenic bacterium E. faecalis both gelatinase and serine protease are required for systemic infections in mammalian hosts (15, 20, 21). Similarly, the extracellular alkaline serine protease produced by the nosocomial pathogen Stenotrophomonas maltophilia is an important pathogenicity factor and has been recognized as a potential therapeutic target (22). The primary function of proteases in the bacterial kingdom is to provide a source of free amino acids for bacterial survival and growth, but there is accumulating evidence that proteases also play a role in bacterial pathogenesis during the invasion and destruction of host tissues (e.g., by evading host defenses and/or modulating host immune system) (23). The prevalent view regarding the mode of action of the extracellular proteases during nematode infection is that these proteases participate in cuticle penetration (5, 24–29). However, despite frequent observations of the correlation between proteases and the pathogenesis of microbial pathogens, little is known about the direct role that proteases play during host invasion and pathogenesis.
In this study, we investigated the molecular and cellular mechanisms of interaction between a nematocidal bacterium (Bacillus nematocida strain B16) isolated from soil and the model nematode C. elegans. We here report that this bacterium uses a mixture of VOCs as the “lure” in a kill-from-within nematocidal strategy. Once inside the worm, the bacteria secrete two extracellular proteases that kill the nematodes primarily through damage to the intestine, not the cuticle, of its host.
Results
Pathogenic Bacterium Produces VOCs to Attract Nematodes.
B. nematocida strain B16 has a simple but effective strategy for attracting nematodes. The phenomenon was observed in an assay using two Petri plates of identical size. Briefly, a lawn of bacteria was grown on a Petri plate, and this plate then was inverted over a plate containng C. elegans nematodes. Within 8 h, 56% (280 ± 7.6/500) of C. elegans migrated upwards toward the B. nematocida lawn in the upper plate by arduously climbing the bare walls of the Petri dishes. When the bacterial lawn consisted of a similar density of E. coli cells, one of the food items for nematodes, only 12% (60 ± 2.6/500) of the tested worms moved upwards to the top. Controls with uninoculated medium yielded 1.8% (9 ± 1/500) worm migration (Fig. S1). Based on these results, we propose that strain B16 emits volatile compounds that attract nematodes.
Using the uninoculated medium as the negative control, we identified 17 distinct VOCs from cultures of strain B16 based on the gas chromatography (GC)/MS system data banks (NIST05, NIST98, and Wiley 275, Qual > 85) (Fig. S2). These VOCs included aldehydes, ketones, alcohols, alkenes, esters, acids, ethers, heterocyclics, and phenolic compounds. Of the 17 VOCs detected in the B16 culture, 11 were absent from the E. coli culture. Six of these 11 B16-specific VOCs were tested individually using commercially available standards. Of these six VOCs, benzyl benzoate, benzaldehyde, 2-heptanone, and acetophenone each showed potent nematode-attracting abilities (AAs) with AC50 (the concentration of the pure tested compound at which the AA reached 50% within 30 min) ranging from 25 to 123.3 ppm (Table 1). Four of the six VOCs made by both E. coli and B. nematocida B16 also were tested individually for their AAs. Of these, indole, naphthalene, and 2, 5-Dimethy-lanisole showed modest to low AAs with AC50 of 1 mM, 1.5 mM, and 323.3 ppm, respectively (Table 1).
Table 1.
Candidate attractants among the VOCs emitted by bacteria and their attracting ability toward C. elegans
| Unique candidate attractants in B. nematocida |
Shared candidate attractants |
|||||
| VOC (no.) | % Relative content (SD) | AC50 (SD) | VOC (No.) | % Relative content (SD) in B. nematocida | % Relative content (SD) in E. coli | AC50 (SD) |
| Benzaldehyde (1) | 16.7 (0.9) | 46.7 ppm (15.3) | Indole (14) | 5.5 (0.6) | 2.0 (0.3) | 1 mM (0.5) |
| Chloromethyl 4-Chloroheptanoate (3) | 10.7 (0.7) | — | Naphthalene (17) | 2.3 (0.3) | 1.8 (0.2) | 1.5 mM (0.5) |
| 2-Pentanone (2) | 4.1 (0.4) | n.d. | 2-Butanone (12) | 1.3 (0.3) | 0.9 (0.4) | — |
| 2-Heptanone (6) | 3.4 (0.3) | 123.3 ppm (25.2) | Pyrazine, 2,6-dimethyl- (15) | 0.9 (0.3) | 0.2 (0.1) | — |
| 2-Heptanone, 6-methyl- (7) | 2.1 (0.4) | — | 2,5-Dimethy- lanisole (16) | 0.4 (0.1) | 0.1 (0.02) | 323.3 ppm (20.5) |
| 1-Hexanol, 2-ethyl- (4) | 0.7 (0.2) | — | Acetone (13) | 0.1 (0.03) | 0.2 (0.03) | n.d. |
| Acetophenone (5) | 0.6 (0.2) | 93.3 ppm (11.5) | ||||
| 2-Tetradecanone (9) | 0.5 (0.1) | — | ||||
| 2-Nonanone (8) | 0.4 (0.2) | n.d. | ||||
| Benzyl benzoate (11) | 0.3 (0.1) | 25 ppm (5) | ||||
| 1,3,5-Cycloheptatriene (10) | 0.2 (0.1) | — | ||||
Response level of nematodes to different compounds. The numbers in parentheses are those indicated by arrows in Fig. S2. AC50, the concentration of the pure tested compound at which the attractive ability achieved 50% within 30 min; n.d., none detected; SD, SD of three replicates; —, not tested because of lack of pure compounds.
After C. elegans Swallows B. nematocida, the Bacteria Colonizes the Worm's Intestinal Tract.
To study the events after C. elegans is attracted by B. nematocida, a chloramphenicol-resistant bacterial mutant was constructed. Worms were attracted to these mutants and fed on them. Worms exposed to the wild-type B16 strain of B. nematocida, the food bacterium E. coli cells, and blank medium were used as controls. In each case, selected worms were surface sterilized thoroughly and then ground, and the worm macerate was inoculated onto LB plates containing 5 μg/mL chloramphenicol. Chloramphenicol-resistant bacterial colonies were observed from dead worms infected by the mutant strain, but no colonies were recovered from the controls. Furthermore, worms that swallowed wild-type strain B16 and the mutant showed extensive pharyngeal and intestinal damage. In contrast, worms that swallowed E. coli cells had no damage to their intestinal tracts (Fig. S3).
Deletion of Two Proteases Resulted in Significantly Reduced Nematocidal Activity.
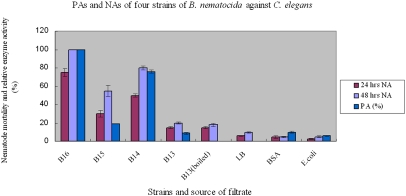
In previous studies, we showed that two proteases, Bace16 and Bae16, in B. nematocida are putative virulence factors (30, 31). To examine the relative contributions of these two proteases to B. nematocida virulence, we constructed three knockout strains (the bace16-knockout strain B15, the bae16-knockout strain B14, and the bace16/bae16 double-knockout strain B13) (Fig. S4) and compared their proteolytic activities (PAs) with the wild-type progenitor strain B16. The PAs of culture filtrate of strain B16 using 0.2 M casein as substrate was 7.9 U/mL (SD 0.3), and this value was defined as 100% PA. Strains B14 and B15 showed 76% and 18.9% PAs, respectively. Strain B13 showed almost no PA (Fig. 1). In nematocidal activity (NA) assays, more than 90% of C. elegans were alive after 5 d in the negative controls, including LB containing 5 μg/mL chloramphenicol, BSA (0.5 mg/mL) or the filtrates from E. coli cultures. In contrast, only about 5% of the nematodes were alive after 5-d exposure to filtrates from the strain B16 culture. Among the three knockout mutants, nematode viabilities within 48 h were 20% and 45%, respectively, when treated with filtrates from strains B14 and B15. Filtrates from B13 showed 80% nematode viability (Fig. 1). These results demonstrated a strong correlation between NA and PA. Moreover, the two extracellular proteases contributed the majority of the NA, with Bace16 contributing more than Bae16.
Fig. 1.
PA and NA of four strains of B. nematocida against C. elegans. PA of culture filtrate of strain B16 using 0.2 M casein as the substrate was 7.9 U/mL (SD 0.3) = 100%. Means ± SD of three replicates were plotted.
Intestinal Damage from Virulence Proteases Causes Nematode Death.
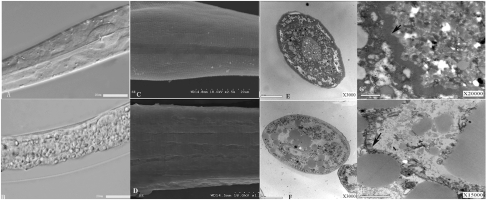
To study the function and site of action of the two proteases during infection, we constructed three additional strains, B17, B18, and B19, expressing different fluorescence-tagged proteases (Fig. S5). Our localization experiments demonstrated that the two proteases were localized mainly in the intestine of nematodes, with minor localization on the cuticle (Fig. S6). Observations of infected and dead nematodes under light and SEM showed severe intestinal damage but little damage to the cuticle (Fig. 2 B–D). Examination of transverse sections of the dead nematodes under transmission electron microscopy (TEM) revealed disordered and loose intestinal walls (Fig. 2F). In comparison with the brush border within the midgut of healthy nematodes (Fig. 2 E and G), the microvilli along the brush border of the dead nematodes was destabilized, with a lack of conglutination, defective membrane tethering, microvilli vesiculation, and membrane shedding (Fig. 2H). In the negative control treatment, worms fed on E. coli had intact gut, pharynx, and cuticles (Fig. 2A).
Fig. 2.
Microscopic examination of B. nematocida strain B16 target sites. (A) Both the intestine and cuticle of nematodes were intact when treated with E. coli. (B) Structures of pharynx, muscle, and intestine were disorganized when treated with B. nematocida strain B16. (C) Nematodes in the E. coli-treated control group had smooth undisturbed surfaces with a healthy cuticle structure that included the regular striae and lateral lines. (D) Nematodes infected with B. nematocida strain B16 showed a lightly exfoliated cuticle. (E) The cross-section of an untreated, healthy nematode showed a highly ordered and compact intestinal structure. (F) The cross-section of a nematode infected with B. nematocida strain B16 showed numerous defects including fusion, vesiculation, and loosening of various organs. (G) Low-magnification TEM of the midgut of the control nematode showed ordered, densely arrayed, and normal-looking microvilli. (H) Microvilli in strain B16-infected nematodes appeared destroyed with significant membrane-tethering defects. Arrows indicate healthy (G) and damaged (H) and microvilli.
Next, crude protease extracts from strain B16 were applied externally to the cuticle of adult C. elegans, were microinjected into their intestines, or were applied externally and microinjected simultaneously. In comparison with the worms treated with PBS buffer, C. elegans treated externally with proteases yielded little change in mortality (P > 0.05), but C. elegans that received intestinal microinjections (P < 0.001) or simultaneous treatments of both the intestine and the cuticle (P < 0.001) suffered significant mortality. In separate experiments with the three knockout strains, a crude protease extract was prepared from each strain and was microinjected into the intestines of adult C. elegans. After 8 h, strain B14 yielded 69.6% mortality; strain B15 yielded 19.8% mortality; and strain B13 yielded only 5.2% mortality (Table 2). These mortality rates were similar to those observed when C. elegans was infected with live B. nematocida strains of corresponding genetic backgrounds.
Table 2.
Mortality of C. elegans worms exposed to or injected with different strains of B. nematocida
| Strains and treatment methods | Incubation time | No. nematodes in treatment |
% Immobilized nematodes | No. nematodes in PBS buffer control |
Statistics* |
|||
| Immobile | Mobile | Immobile | Mobile | χ2 | P | |||
| Whole nematode treated with crude protease from B19 | 30 min, 24 h in water | 58 | 4 | 93.5 | 2 | 48 | 89.236 | <0.001 |
| Intestine injected with crude protease from B19 | 30 min, 24 h in water | 60 | 5 | 92.3 | 3 | 49 | 87.054 | <0.001 |
| Cuticle treated with crude protease from B19 | 30 min, 24 h in water | 5 | 54 | 8.5 | 3 | 46 | 0.216 | 0.642 |
| Intestine injected with crude protease from B19 | 8 h | 89 | 7 | 92.7 | 4 | 53 | 110.172 | <0.001 |
| Intestine injected with crude protease from B19 + PMSF | 8 h | 24 | 76 | 24 | 2 | 48 | 9.305 | 0.002 |
| Intestine injected with crude protease from B13 | 8 h | 5 | 92 | 5.2 | 2 | 42 | 0.024 | 0.877 |
| Intestine injected with crude protease from B14 | 8 h | 64 | 28 | 69.6 | 1 | 40 | 51.143 | <0.001 |
| Intestine injected with crude protease from B15 | 8 h | 16 | 65 | 19.8 | 2 | 49 | 6.660 | <0.05 |
*χ2 tests (df = 1) compared the numbers of dead and live nematodes in treated versus control samples.
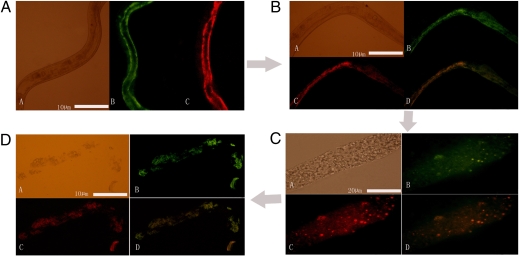
Worms injected with crude protease extracts of the dual-labeled strain B19 were examined 2, 5, 8, 12, and 24 h after microinjection. Little difference was observed between the negative controls and the protease-treated worms at 2 h. However, 5 h after treatment, the worms treated with extracts from strain B19 were immobilized with disorganized intestines (Fig. 3B)., and 8 h after treatment more than 90% of the protease-treated worms were dead, with severely damaged intestines and relatively intact cuticles (Fig. 3C). Worms showed some degree of decomposition by 12 h, and most were completely disintegrated at 48 h (Fig. 3D). Breaking down the host cuticle has been regarded as the dominant protease-mediated pathogenesis mechanism of microorganisms that kill insects and nematodes (5, 24–29, 32); the extracellular proteases of B. nematocida that act on intestines represent another mechanism of bacterial pathogenesis.
Fig. 3.
Intestinal damage in C. elegans by the two proteases when infected by strain B19. In each panel, subpanels A, B, C, and D represent, respectively, nematodes observed under visible light, Bace16-GFP fluorescence, Bae16-DsRed fluorescence, and an overlay of the two fluorescent signals. (A) Live C. elegans 2 h after microinjection. (B) Dying C. elegans 5 h after microinjection. (C) Dead C. elegans 8 h after microinjection. (D) Decomposed C. elegans 24 h after microinjection.
To understand better the role of these two proteases in pathogenesis, we compared treatments with and without phenylmethanesulfonyl fluoride (PMSF), a common inhibitor of protease hydrolytic activity. PMSF reduced the mortality of nematodes injected with strain B19 from more than 90% to 24% (Table 2), indicating that the proteases likely killed nematodes through protein hydrolysis, not via the typical ligand–receptor interaction mechanism as exemplified by the Bt toxin produced by Bacillus thuringiensis (6).
To study the potential molecular targets of the two proteases, we used high-resolution 2D gel electrophoresis (2DE) and identified 12 preferentially targeted proteins from epithelial tissues of the nematode intestine (Fig. S7 and Table 3). Each of the 12 proteins decreased by more than 3-fold within 1 h of treatment: six proteins targeted by Bace16, two proteins targeted by Bae16, and four proteins targeted by both Bace16 and Bae16. The six proteins significantly hydrolyzed by protease Bace16 included two myosin-associated proteins, two ATPase-associated proteins, one filament-formation protein, and one unknown protein with a significant homology to a RNA-binding domain vigilin-like protein. Hegan et al. (33) reported that myosin 1B (Myo1B) is essential for the maintenance of the enterocyte brush border structure and is important for the resistance of D. melanogaster against the bacterial pathogen Pseudomonas entomophila. Fly larvae without Myo1B are hypersensitive to oral infection by P. entomophila (34). Myo1B also plays an important role in the local innate immune response by midgut enterocytes (35). For the ATPase subunit E-associated proteins, Ji et al. (36) recently found that the protein VHA-8 is essential for proper intestinal function, and the null mutants of vha-8 showed necrotic cell death in both the hypodermis and the intestine of the arrested fly larvae.
Table 3.
Intestinal proteins in C. elegans preferentially hydrolyzed by pathogenic proteases of B. nematocida B16
| Protein name in C. elegans database | Accession no. | Protein MW | Protein PI | Hydrolyzed by | Putative function |
| 1. Hypothetical protein F07A5.7 | gi|3875472 | 100573.4 | 5.35 | Bace16 | Myosin tail |
| 2. Paramyosin | gi|6896 | 101888.2 | 5.28 | Bace16 | Myosin tail |
| 3. Hypothetical protein C08H9.2 | gi|5824378 | 134261.9 | 6.36 | Bace16 | Homologous to vigilin-like RNA-binding domain |
| 4. Vacuolar h ATPase protein 8 | gi|14550335 | 25570.46 | 6.78 | Bace16 | Vacuolar-type H+-ATPase subunit E |
| 5. Hypothetical protein F44B9.8 | gi|52839833 | 41149 | 5.71 | Bace16 | AAA-superfamily of ATPases associated with a wide variety of cellular activities |
| 6. Actin | gi|6628 | 41681.6992 | 5.3 | Bace16 | Filament formation |
| 7. Phosphoenolpyruvate carboxykinase | gi|159183 | 69611.01 | 6.86 | Bae16 | Phosphoenolpyruvate carboxykinase (PEPCK) |
| 8. Hypothetical protein R11A5.4a | gi|3879140 | 73384.69 | 5.79 | Bae16 | Associated with phosphoenolpyruvate carboxykinase (PEPCK) |
| 9. Hypothetical protein F49H6.5 | gi|3947550 | 43979.1016 | 9.35 | Bace16&Bae16 | Molybdenum cofactor synthesis protein A |
| 10. Temporarily assigned gene name protein 300 | gi|7331730 | 55998.8789 | 6.14 | Bace16&Bae16 | Homologous to Pfam domains |
| 11. Actin protein 4, isoform c | gi|51011295 | 40400.2188 | 5.56 | Bace16&Bae16 | Filament formation and component of cytoskeleton |
| 12. Hypothetical protein T04C12.4 | gi|3879475 | 41768.7305 | 5.3 | Bace16&Bae16 | Filament formation |
Numbers 1–12 in column 1 correspond to those on 2DE in Fig. S7. MW, molecular weight; PI, isoelectric point.
The two proteins targeted by Bae16 were PEPCK and a PEPCK-associated protein that are involved in gluconeogenesis in the small intestine, liver, and kidney of a diverse group of organisms ranging from fish to rodents to humans (37, 38). The four proteins hydrolyzed by both proteases were either filament-associated proteins or molybdenum cofactor biosynthesis proteins. It has been reported that the invasion of human intestinal Caco-2 epithelial cells by Enterobacter sakazakii is enhanced significantly by the disruption of the tight junctions that contained actin filaments and microtubule structures within the host tissues (39). The significant sequence identity and similar distribution pattern of these proteins in intestinal epithelia of diverse groups of organisms suggest that they likely play roles similar to those in nematodes.
B. nematocida Attracts and Kills Nematodes in Soil.
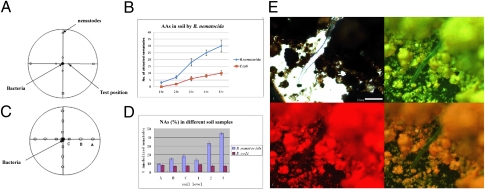
Experiments using natural soil were conducted to demonstrate that the AA and NA of B. nematocida are not just laboratory phenomena. Our results show that nematodes cultivated in soil also are more attracted to the pathogenic bacterium B. nematocida strain B16 than to the nonpathogenic E. coli strain DH5α (Fig. 4 A and B). The worms attracted to strain B16 subsequently were infected and killed. Both the number of bacteria and their distance from the nematodes affected AA and NA. When 50 μL, 100 μL, and 150 μL of bacterial inocula (inocula 1, 2, and 3, respectively) were compared, the soil samples with the two higher levels of inocula had higher NAs. Similarly, when the bacterial inocula were placed 3 cm (point A), 2 cm (point B), and 1 cm (point C) from the center (Fig. 4C), the soil samples taken closest to the B. nematocida inoculum (soil from point C) had the highest NA (Fig. 4D). The differences between the B. nematocida-treated samples and the controls inoculated with E. coli strain DH5α were all highly significant (P < 0.005).
Fig. 4.
Assays of AA and NA in soil. (A) The scheme for AA assays in soil (details are given in SI Materials and Methods). (B) Results of assays of AA in soil. (C) Activity levels in soil at points A, B, and C as determined by distances from the bacterial inoculum. (D) Assays for different NA levels in soil. (E) Assay of the nematode-immobilizing ability of B. nematocida strain B19 in natural soil.
Genetically marked strain B19 also was examined for the expression and potential roles of the two proteases during nematode infection in soil. Strain B19 successfully infected C. elegans in the soil environment, and abundant green and red fluorescence were observed (Fig. 4E). From 8 to 96 h after treatment, we selectively analyzed newly immobilized or recently dead nematodes. These worms were washed with sterile PBS buffer to remove soil particles, and then the patterns of fluorescence and the extent of intestinal damage of these immobilized nematodes were observed, similar to methods used for C. elegans on solid agar media (Figs. S3 and S6).
Discussion
Studies of bacterial pathogenesis in invertebrate hosts during the past decade have resulted in important insights into the molecular mechanisms of bacterial pathogenesis and host defense. Here, we show that the bacterium B. nematocida lures nematodes to their death by a Trojan horse mechanism. The bacterium first produces a mixture of VOCs that C. elegans nematodes sense as a delectable food source. Two of the VOCs identified in this study, benzaldehyde and 2-heptanone, were mentioned previously as attractants for C. elegans at low concentrations (14). Altogether, our current study confirmed seven VOCs as capable of acting individually as potent attractants. Six of these attractants contain benzene rings and emit fragrance at low concentrations. The seventh, 2-heptanone, lacks a benzene ring but has a penetrating fruity odor. The majority of the seven attractants are potential products derived from the shikimate pathway where commonly found carbohydrates serve as substrates for the synthesis of aromatic compounds (40), suggesting that bacteria have adapted a low-cost strategy to generate attractants. The bacterium is capable of releasing a variety of food-like odors and has subverted the nematode olfactory chemotaxis system successfully to attain access to the nematodes. Once inside the nematode, the bacteria secrete extracellular proteases that primarily target intestinal proteins, colonizing and eventually killing the hosts.
Extracellular proteases produced by microbial pathogens of nematodes and insects previously were thought to be involved mainly in breaking down host external physical barriers and releasing nutrients for their growth (e.g., ref. 25). The results in this study indicate that the alimentary tract, not the external physical barrier (i.e., the cuticle), of the nematodes is the primary target of the B. nematocida strain B16 proteases. Our study reveals that these proteases play important roles in nematode pathogenesis by helping the bacteria invade and destroy host internal tissues, thereby modulating the host immune system and evading host defenses.
As described in the Introduction, a variety of virulence factors in bacteria participate in their pathogenesis to nematodes. Our identification of two proteases, Bace16 and Bae16, as virulence factors is largely consistent with the importance of extracellular proteases in bacterial pathogenesis found in previous studies. However, our study indicates that other factors probably contribute to the virulence of B. nematocida as well. Specifically, filtrates from the double-knockout strain B13 still caused more than 20% nematode mortality, about four times the mortality (∼5%) in negative control treatments. In addition, heat treatment (i.e, boiling to denature all proteins) did not reduce the nematocidal activity of the filtrate of strain B13 (Fig. 1), suggesting that some heat-resistant component(s) in the cell filtrate also contribute to NA in B. nematocida strain B16.
Nematotoxic bacteria exhibit diverse models of action. However, there have been no reports of pathogenic bacteria actively attracting and killing nematodes. Our study suggests that in natural environments the interactions between pathogenic bacteria and their nematode hosts are not random but involve a series of active events. The Trojan horse mechanism of B. nematocida pathogenesis adds to our understanding of the diverse repertoire of pathogenic mechanisms used by bacteria. The discovery of this pattern of nematode–bacterium interaction could help in the development of new and efficient biocontrol strategies to facilitate ecologically sound and more sustainable management of nematode pests.
Materials and Methods
Attraction Assays.
The nematode AAs of bacteria and their products were assayed using a modified Petri dish. Briefly, fresh water agar and LB agar plates were prepared in Petri dish lids. Lids containing the LB agar medium were inoculated with a bacterial lawn (5 × 5 cm) and incubated for 2 d at 37 °C. The inoculated lid was inverted on top of another lid without medium but containing a drop of nematode suspension in the center (∼500 nematodes). The two lids were sealed with air-permeable adhesive tape and were incubated in a dark chamber at 26 °C. Within 8 h, the movement of nematodes on the plates was assessed by counting the number of nematodes on the upper lid using a stereomicroscope. The same diameter of E. coli lawn and blank medium were used as negative controls.
The AA assays of specific compounds were similar to the above setup and were carried out using a previously described method (41).
Solid-Phase Microextraction/GC-MS Analysis.
VOCs from bacteria were analyzed using solid-phase microextraction (SPME) in combination with GC-MS. Extracts from three cultures were analyzed: the virulent strain B16, the harmless nematode food E. coli, and the negative control of sterile, uninoculated LB medium.
The VOCs were collected by 75-μm fibers (Supelco), and the SPME fibers were inserted into the front inlet of an HP 6890A gas chromatograph connected to an HP 5973 mass spectrometer (Agilent Technologies) and desorbed at 250 °C for 2 min. VOCs were identified by comparing the mass spectrum of the substance with GC/MS system data banks [Wiley InterScience 138 and NBS software (Agilent) 75k library].
2D Gel Electrophoresis.
To prepare protein samples for 2DE, C. elegans worms were washed five times using sterile ice-cold PBS (pH 7.5). Intestinal proteins from nematodes were extracted with a buffer consisting of 5 M carbamide, 2 M isothiourea, and 5% DTT. The optimal conditions were established based on pilot 1D SDS/PAGE gels to check the distribution of intestinal proteomes. Then 350 μg extracted intestinal proteins were mixed with 1 μg of the Bace16 protease and/or 10 μg of the Bae16 protease. For the negative control, no proteases were added. All samples were incubated for 1 h at 4 °C and then subjected to 2DE.
2DE was carried out according to the GE Healthcare 2DE protocol using pH 3–11 nonlinear immobilized pH gradient (IPG) strips (18 cm long) (GE Healthcare) for the 1D separation and 11% polyacrylamide gel in a Laemmli buffer system for the 2D separation (42). After the completion of electrophoresis, Colloidal Coomassie Brilliant Blue G250 staining was carried out. Proteins that were affected by the various protease treatments were identified using PDQuest software (Bio-Rad). These proteins then were extracted from the gel, and their sequences were determined using MALDI-TOF MS spectra and Biotools software (Bruker Daltonics). The amino acid sequences were identified using a locally installed Mascot search engine (Matrix Science) by searching all databases comprising all ORFs of C. elegans.
Further details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Dr. Wen Wang at the Kunming Institute of Zoology, Chinese Academy of Sciences, for his help in the microinjection experiments. We thank Dr. Nathan Schroeder for his expert review of the manuscript. The research described here is supported by Grants 2007CB411600 and 2010CB134503 from the National Basic Research Program of China, 30630003 and 30770070 from the National Natural Science Foundation Program of China, and 2007C0007Z and 2006PY01-27 from the Department of Science and Technology of Yunnan Province, China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007276107/-/DCSupplemental.
See Commentary on page 16411.
References
- 1.Strauss EM. Simple hosts may help reveal how bacteria infect cells. Science. 2000;290:2245–2247. doi: 10.1126/science.290.5500.2245. [DOI] [PubMed] [Google Scholar]
- 2.Plasterk RH. The year of the worm. Bioessays. 1999;21:105–109. doi: 10.1002/(SICI)1521-1878(199902)21:2<105::AID-BIES4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Rahme LG, et al. Plants and animals share functionally common bacterial virulence factors. Proc Natl Acad Sci USA. 2000;97:8815–8821. doi: 10.1073/pnas.97.16.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian BY, Yang JK, Zhang KQ. Bacteria used in the biological control of plant-parasitic nematodes: Populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol. 2007;61:197–213. doi: 10.1111/j.1574-6941.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 5.Griffitts JS, Whitacre JL, Stevens DE, Aroian RV. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science. 2001;293:860–864. doi: 10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- 6.Beale E, Li G, Tan MW, Rumbaugh KP. Caenorhabditis elegans senses bacterial autoinducers. Appl Environ Microbiol. 2006;72:5135–5137. doi: 10.1128/AEM.00611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darby C, Cosma CL, Thomas JH, Manoil C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:15202–15207. doi: 10.1073/pnas.96.26.15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Quinn AL, Wiegand EM, Jeddeloh JA. Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell Microbiol. 2001;3:381–393. doi: 10.1046/j.1462-5822.2001.00118.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurz CL, Ewbank JJ. Caenorhabditis elegans for the study of host-pathogen interactions. Trends Microbiol. 2000;8:142–144. doi: 10.1016/s0966-842x(99)01691-1. [DOI] [PubMed] [Google Scholar]
- 10.Garsin DA, et al. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci USA. 2001;98:10892–10897. doi: 10.1073/pnas.191378698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin X, Singh KV, Weinstock GM, Murray BE. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000;68:2579–2586. doi: 10.1128/iai.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sifri CD, et al. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun. 2002;70:5647–5650. doi: 10.1128/IAI.70.10.5647-5650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sifri CD, Begun J, Ausubel FM, Calderwood SB. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect Immun. 2003;71:2208–2217. doi: 10.1128/IAI.71.4.2208-2217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 16.Rankin CH. Nematode behavior: The taste of success, the smell of danger. Curr Biol. 2006;16:89–91. doi: 10.1016/j.cub.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Pradel E, et al. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:2295–2300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano-Luna J, Cervantes-Sandoval I, Tsutsumi V, Shibayama M. A biochemical comparison of proteases from pathogenic Naegleria fowleri and non-pathogenic Naegleria gruberi. J Eukaryot Microbiol. 2007;54:411–417. doi: 10.1111/j.1550-7408.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro-Guimarães ML, et al. Expression analysis of proteases of Mycobacterium leprae in human skin lesions. Microb Pathog. 2007;43:249–254. doi: 10.1016/j.micpath.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Singh KV, Qin X, Weinstock GM, Murray BE. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J Infect Dis. 1998;178:1416–1420. doi: 10.1086/314453. [DOI] [PubMed] [Google Scholar]
- 21.Dupont H, Montravers P, Mohler J, Carbon C. Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infect Immun. 1998;66:2570–2575. doi: 10.1128/iai.66.6.2570-2575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windhorst S, et al. The major extracellular protease of the nosocomial pathogen Stenotrophomonas maltophilia: Characterization of the protein and molecular cloning of the gene. J Biol Chem. 2002;277:11042–11049. doi: 10.1074/jbc.M109525200. [DOI] [PubMed] [Google Scholar]
- 23.Travis J, Potempa J, Maeda H. Are bacterial proteinases pathogenic factors? Trends Microbiol. 1995;3:405–407. doi: 10.1016/s0966-842x(00)88988-x. [DOI] [PubMed] [Google Scholar]
- 24.Jansson HB, Nordbring-Hertz B. Infection mechanisms in the fungus-nematode system. In: Poinar GO Jr, Jansson HB, editors. Diseases of Nematodes. Vol. 2. Boca Raton, FL: CRC; 1988. pp. 59–72. [Google Scholar]
- 25.Lopez-Llorca LV. Purification and properties of extracellular proteases produced by the nematophagous fungus Verticillium suchlasporium. Can J Microbiol. 1990;36:530–537. [Google Scholar]
- 26.Lopez-Llorca LV, Robertson W. Immunocytochemical localization of a 32 kDa protease from the nematophagous fungus Verticillium suchlasporium in infected nematode eggs. Exp Mycol. 1992;16:261–267. [Google Scholar]
- 27.Tunlid A, Rosén S, Ek B, Rask L. Purification and characterization of an extracellular serine protease from the nematode-trapping fungus Arthrobotrys oligospora. Microbiology. 1994;140:1687–1695. doi: 10.1099/13500872-140-7-1687. [DOI] [PubMed] [Google Scholar]
- 28.Bonants PJ, et al. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiology. 1995;141:775–784. doi: 10.1099/13500872-141-4-775. [DOI] [PubMed] [Google Scholar]
- 29.Zhao ML, Mo MH, Zhang KQ. Characterization of a neutral serine protease and its full-length cDNA from the nematode-trapping fungus Arthrobotrys oligospora. Mycologia. 2004;96:16–22. doi: 10.1080/15572536.2005.11832991. [DOI] [PubMed] [Google Scholar]
- 30.Niu QH, et al. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl Microbiol Biotechnol. 2006;69:722–730. doi: 10.1007/s00253-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 31.Niu QH, et al. A neutral protease from Bacillus nematocida, another potential virulence factor in the infection against nematodes. Arch Microbiol. 2006;185:439–448. doi: 10.1007/s00203-006-0112-x. [DOI] [PubMed] [Google Scholar]
- 32.Smithson SL, et al. Cloning and characterisation of a gene encoding a cuticle-degrading protease from the insect pathogenic fungus Metarhizium anisopliae. Gene. 1995;166:161–165. doi: 10.1016/0378-1119(95)00609-3. [DOI] [PubMed] [Google Scholar]
- 33.Hegan PS, Mermall V, Tilney LG, Mooseker MS. Roles for Drosophila melanogaster myosin IB in maintenance of enterocyte brush-border structure and resistance to the bacterial pathogen Pseudomonas entomophila. Mol Biol Cell. 2007;18:4625–4636. doi: 10.1091/mbc.E07-02-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vodovar N, et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci USA. 2005;102:11414–11419. doi: 10.1073/pnas.0502240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 36.Ji YJ, et al. VHA-8, the E subunit of V-ATPase, is essential for pH homeostasis and larval development in C. elegans. FEBS Lett. 2006;580:3161–3166. doi: 10.1016/j.febslet.2006.04.067. [DOI] [PubMed] [Google Scholar]
- 37.Rajas F, Croset M, Zitoun C, Montano S, Mithieux G. Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes. 2000;49:1165–1168. doi: 10.2337/diabetes.49.7.1165. [DOI] [PubMed] [Google Scholar]
- 38.Rajas F, et al. Immunocytochemical localization of glucose 6-phosphatase and cytosolic phosphoenolpyruvate carboxykinase in gluconeogenic tissues reveals unsuspected metabolic zonation. Histochem Cell Biol. 2007;127:555–565. doi: 10.1007/s00418-006-0263-5. [DOI] [PubMed] [Google Scholar]
- 39.Kim KP, Loessner MJ. Enterobacter sakazakii invasion in human intestinal Caco-2 cells requires the host cell cytoskeleton and is enhanced by disruption of tight junction. Infect Immun. 2008;76:562–570. doi: 10.1128/IAI.00937-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrmann KM, Weaver LM. The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:473–503. doi: 10.1146/annurev.arplant.50.1.473. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa M, Shuto Y, Watanabe H. β-Myrcene, a potent attractant component of pine wood for the Pine Wood Nematode, Bursaphelenchus xylophilus. Agric Biol Chern. 1986;50:1863–1866. [Google Scholar]
- 42.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.