Abstract
Anterior cingulate cortex (ACC) hypoactivations during cognitive demand are a hallmark deficit in drug addiction. Methylphenidate (MPH) normalizes cortical function, enhancing task salience and improving associated cognitive abilities, in other frontal lobe pathologies; however, in clinical trials, MPH did not improve treatment outcome in cocaine addiction. We hypothesized that oral MPH will attenuate ACC hypoactivations and improve associated performance during a salient cognitive task in individuals with cocaine-use disorders (CUD). In the current functional MRI study, we used a rewarded drug cue-reactivity task previously shown to be associated with hypoactivations in both major ACC subdivisions (implicated in default brain function) in CUD compared with healthy controls. The task was performed by 13 CUD and 14 matched healthy controls on 2 d: after ingesting a single dose of oral MPH (20 mg) or placebo (lactose) in a counterbalanced fashion. Results show that oral MPH increased responses to this salient cognitive task in both major ACC subdivisions (including the caudal-dorsal ACC and rostroventromedial ACC extending to the medial orbitofrontal cortex) in the CUD. These functional MRI results were associated with reduced errors of commission (a common impulsivity measure) and improved task accuracy, especially during the drug (vs. neutral) cue-reactivity condition in all subjects. The clinical application of such MPH-induced brain-behavior enhancements remains to be tested.
Keywords: prefrontal cortex, behavioral intervention, dopamine agonist, functional MRI blood oxygen level-dependent, emotional Stroop
Drug addiction is a chronically relapsing disorder associated with dysregulated dopaminergic neurotransmission as well as functional impairments in the brain regions innervated by dopamine [e.g., the prefrontal cortex (PFC)] (1, 2). Psychostimulants such as cocaine have high abuse and dependence potential because of their ability to increase dopamine in limbic brain regions. Similarly to cocaine, methylphenidate (MPH; e.g., Ritalin) blocks the dopamine transporter increasing extracellular dopamine. However, although speed of uptake of both drugs is similar, rate of clearance of MPH from the brain is substantially slower than that for cocaine (90- vs. 20-min half-life), and these slower pharmacokinetic properties may contribute to the lower abuse potential for MPH (1). Both these neuropharmacological mechanisms, interference with the binding of the drug to its target and different (i.e., slower) pharmacokinetics, proved valuable in the management of heroin (e.g., with methadone and buprenorphine) and nicotine addiction (e.g., with nicotine patch and nicotine gum) (3). In contrast, adapting a similar pharmacological strategy (use of stimulant medications such as MPH) in individuals with cocaine-use disorders (CUD) did not decrease cocaine use or prevent relapse (1).
Nevertheless, oral MPH decreases abnormal risk taking in patients with frontotemporal dementia (4) and children with attention deficit hyperactivity disorder (ADHD) (5). Furthermore, when on stimulant medication (including MPH), youth with ADHD showed a trend to improved inhibitory control and a normalized (vs. a healthy control group) PFC response to a classical self-regulatory task (6). MPH also improves performance on sustained attention and working memory tasks in ADHD (7, 8), showing promise in normalizing such task-related behavior and brain response in patients with traumatic brain injury (9, 10) and major depression after stroke (11). Common to these studies is the use of cognitive tasks that engage executive functions dependent on the PFC (12) in psychopathologies that, similarly to drug addiction, impact PFC integrity and function. We, therefore, tested whether a beneficial response to oral MPH during performance of a similar cognitive task will also be documented in CUD. We chose a task that, as previously reported, engaged the PFC in CUD; here, subjects are monetarily remunerated for correct pressing for color of drug-related and matched neutral words that they have just read (13).
Importantly, using this task, despite lack of group differences in self-reported engagement or objective behavioral performance, the CUD participants compared with the healthy controls showed robust anterior cingulate cortex (ACC) hypoactivations (14), encompassing the rostroventral ACC [rvACC; extending to the medial orbitofrontal cortex (mOFC)] and the caudal-dorsal ACC (cdACC). The rvACC/mOFC is based on a functional network known as the orbital and medial PFC (15, 16) or the ventromedial PFC (which does not include the central and lateral regions of the OFC) and includes Brodmann Area (BA) 10 (and BAs 11, 13, and 14 but not 47/12 or 45) (17). The cdACC is located within the confines of the posterior medial frontal cortex and includes BA 24 as previously described (18) (BA 32 occupies territories within both ACC subdivisions). Similarly to our previous study (14), we were interested in these two major subdivisions of the ACC that are differentially recruited for the regulation of emotion, cognition, and behavior in response to salient stimuli (19); the rvACC/mOFC has been implicated in maintaining a default brain function that needs to be suspended during goal-oriented tasks, including the regulation of autonomic functions (20), and in the adaptive suppression of emotion (21), and the cdACC has been implicated in performance monitoring (22) and cognitive control (18). Functional neuroimaging studies in posttraumatic stress disorder (23, 24) and depression (25) have indeed implicated the cdACC in emotional conflict monitoring (26), whereas the rvACC/mOFC has been implicated in emotional conflict resolution (27).
In the current functional MRI (fMRI) study, 13 CUD matched on education and intellectual functioning with 14 healthy controls (Table S1 shows demographics) performed this rewarded drug cue-reactivity task on 2 d (mean ± SD for test-day interval; 13.5 ± 11.3 d): after ingesting a single dose of oral MPH (20 mg) or placebo (lactose) in a counterbalanced fashion (Fig. S1 shows study procedures). We hypothesized that, compared with placebo, oral MPH will increase function of these two ACC subregions in the CUD, evidenced by reduced group differences (i.e., normalization in CUD) during MPH use.
Results
Whole-Brain Task-Related Followed by Region of Interest Analyses.
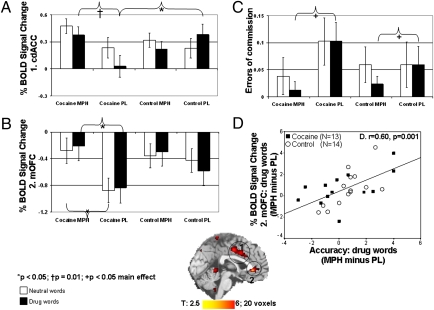
For all subjects compared with a fixation baseline, the fMRI task produced brain-activation and hypoactivation patterns similar to those we previously reported (14) with similar group differences (Table S2). Importantly, an MPH main effect was observed in both our a priori regions of interest (ROIs) as driven by the CUD (Table 1). In a 2 (medication) × 2 (word) × 2 (group) follow-up ROI ANOVA in SPSS (regions in Table 1), there were medication × group (F1,25 = 4.9, P < 0.05) and medication × group × word (F1,25 = 7.7, P = 0.01) interactions in the left cdACC (x = −3, y = 9, z = 36) explained by hypoactivations in CUD compared with controls only during placebo (for drug words, t25 = −2.1, P < 0.05) but not MPH (t25 < 1.4, P > 0.2) and by enhanced MPH vs. placebo signal only in the CUD (for drug words, t12 = 3.1, P = 0.01; mean percent increase in cdACC signal = 9.5% ± 3.2%) (Fig. 1A). Thus, consistent with our previous results in a larger independent cohort that did not receive any pharmacological intervention (14), during placebo, the cdACC was hypoactive in the CUD. Here, MPH bolstered the cdACC signal in the CUD to a level where there were no longer significant differences between the study groups (note specificity of results in the drug-related context). In a more posterior coordinate (x = 6, y = −9, z = 45), this region showed a medication main effect (F1,25 = 14.2, P = 0.001) (Fig. S2). The rvACC, which extended to the mOFC (x = 0, y = 36, z = −3), similarly showed a medication main effect (F1,25 = 12.7, P = 0.001) and a trend to a medication × group interaction (F1,25 = 3.8, P = 0.063), explained by MPH vs. placebo decreased hypoactivations in the CUD (for drug and neutral words, t12 > 2.6, P < 0.05; mean percent increase in mOFC signal = 28.9% ± 11.7%) (Fig. 1B). There were no significant changes between MPH and placebo in our main ROIs for the control subjects (t13 < 1.8, P > 0.1).
Table 1.
MPH effect: drug-word fMRI task in 13 CUD and 14 healthy controls
| BA | Side | Number of voxels | Z | P cluster-level corrected | x | y | z | |
| MPH > PL | ||||||||
| rvACC/mOFC | 32 | M | 59 | 4.5 | 0.003* | 0 | 36 | −3 |
| cdACC | 24 | L | 74 | 5.4 | 0.038 | 6 | −9 | 42 |
| MPH < PL | ||||||||
| Posterior cingulate cortex | 23, 31 | L | 481 | 5.2 | 0.000 | −9 | −30 | 30 |
| CUD: MPH > PL | ||||||||
| rvACC/mOFC | 10, 32 | M | 46 | 5.0 | 0.006* | 0 | 36 | −3 |
| cdACC | 24, 32 | R | 101 | 4.2 | 0.012 | 6 | −9 | 45 |
| 24, 32 | L | 101 | 4.2 | 0.012 | −3 | 9 | 36 | |
| Superior occipital gyrus | 19 | R | 163 | 5.3 | 0.001 | 27 | −78 | 27 |
| CUD: MPH < PL | ||||||||
| Superior frontal gyrus | 8 | L | 131 | 5.2 | 0.004 | −12 | 42 | 42 |
| Posterior cingulate cortex | 23, 31 | L | 687 | 5.5 | 0.000 | −12 | −27 | 33 |
| 23, 31 | R | 687 | 5.5 | 0.000 | 9 | −27 | 30 | |
| Group by medication interaction | ||||||||
| Medial/superior frontal gyrus | 8, 9 | M | 140 | 4.6 | 0.003 | 0 | 42 | 39 |
| Precentral gyrus | 4 | L | 72 | 4.2 | 0.041 | −42 | −6 | 42 |
| Fusiform gyrus | 19 | L | 68 | 4.3 | 0.049 | −27 | −69 | 3 |
| Correlations with behavior (as seed variable): MPH > PL across all subjects | ||||||||
| Drug words | ||||||||
| Accuracy | ||||||||
| mOFC | 10, 32 | L | 27 | +3.5 | 0.024† | −9 | 42 | −6 |
| Errors of omission | ||||||||
| mOFC | 10, 32 | L | 27 | −3.4 | 0.024† | −9 | 42 | −6 |
| Errors of commission | ||||||||
| mOFC, medial frontal gyrus | 10, 32 | R | 35 | −3.2 | 0.016† | 3 | 51 | 9 |
| Inferior temporal gyrus | 37 | L | 87 | −3.9 | 0.009 | −42 | −57 | 0 |
| Middle temporal gyrus | 21 | R | 148 | +3.8 | 0.001 | 48 | −39 | 3 |
| Neutral words | ||||||||
| Errors of commission | ||||||||
| cdACC | 24 | R | 68 | −4.6 | 0.017 | 3 | −3 | 45 |
| Cerebellum | L | 85 | +4.7 | 0.006 | −12 | −39 | −30 | |
| Correlations with drug use (as seed variable): CUD only | ||||||||
| Marijuana use: lifetime (MPH, neutral words) | ||||||||
| Medial frontal gyrus/DLPFC | 9 | L | 36 | −4.1 | 0.039 | −42 | 42 | 30 |
| Alcohol use: lifetime (placebo, neutral words) | ||||||||
| Posterior caudate | L | 43 | +4.0 | 0.028 | −27 | −36 | 9 | |
| cdACC | 32 | M | 124 | −5.0 | 0.000 | 0 | 12 | 45 |
| Cocaine use: last 30 d (placebo, neutral words) | ||||||||
| Medial frontal gyrus/DLPFC | 6, 8, 9 | L | 64 | +3.9 | 0.004 | −39 | 21 | 30 |
| Inferior parietal lobule | 40 | R | 47 | +3.9 | 0.020 | 33 | −51 | 30 |
All results were P < 0.05 cluster-level corrected and P < 0.001 voxel-level uncorrected, 20 voxels minimum. Drug-word fMRI task had no significant results for control subjects and all word-related comparisons. MPH, methylphenidate; CUD, individuals with cocaine-use disorders; BA, Brodmann Area; PL, placebo; L, left; R, right; M, middle; rvACC/mOFC, rostroventral anterior cingulate cortex/medial orbitofrontal cortex; cdACC, caudal-dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; Z (+) value, positive correlation; Z (−) value, negative correlation.
*Small-volume correction (SVC).
†P < 0.05 cluster-level corrected and P < 0.005 voxel-level uncorrected, 20 voxels minimum, with an SVC (at 10 voxels radius).
Fig. 1.
MPH enhances fMRI anterior cingulate cortex activations and reduces commission errors on a cue-reactivity fMRI task. Variables are mean percent BOLD signal change from a fixation baseline as a function of drug vs. neutral words on the fMRI task in the (A) cdACC (x = −3, y = 9, z = 36) that showed medication × group (F1,25 = 4.9, P < 0.05) and medication × group × word (F1,25 = 7.7, P = 0.01) interactions, explained by hypoactivations in CUD compared with controls only during placebo (for drug words, t25 = −2.1, P < 0.05) but not MPH (t25 < 1.4, P > 0.2) and MPH- vs. placebo-enhanced signal in the CUD (for drug words, t12 = 3.1, P = 0.01; mean percent increase in cdACC signal = 9.5 ± 3.2). (B) rvACC/mOFC (x = 0, y = 36, z = −3) showed a medication main effect (F1,25 = 12.7, P = 0.001) and a trend to a medication × group interaction (F1,25 = 3.8, P = 0.063), explained by MPH- vs. placebo-decreased hypoactivations in the CUD (for drug and neutral words, t12 > 2.6, P < 0.05; mean percent increase in mOFC signal = 28.9 ± 11.7). (C) Errors of commission showed a medication main effect (F1,25 = 5.6, P < 0.05; driven by MPH vs. placebo difference for drug words: Z = −2.3, P = 0.021; percent decrease = 4.7% ± 1.9%; driven by the CUD = 6.7 ± 3.2). (D) Correlation is between percent BOLD signal change (MPH − placebo) in the rvACC/mOFC (x = −9, y = 42, z = −6) with the respective change for accuracy during drug words. Error bars represent SEM. Midsagittal map on the bottom of the figure shows the cdACC and rvACC/mOFC ROIs. Subjects are 13 individuals with CUD and 14 healthy control. MPH, methylphenidate; PL, placebo.
In addition to our two ROIs, an MPH effect was evident in the superior occipital gyrus (activations with MPH > deactivations with placebo), posterior cingulate cortex, and superior frontal gyrus (deactivations with MPH < activations with placebo; all driven by CUD). A group by medication interaction was evident in the dorsolateral PFC (medial/superior frontal gyrus and the precentral gyrus) and the fusiform gyrus such that CUD showed deactivations during MPH and activations during placebo, whereas controls showed the opposite pattern (Table 1 shows BAs). Given that these results were not a priori hypothesized, their discussion is deferred while awaiting replication in a larger sample size.
Behavior.
Task performance.
Errors of commission showed a medication main effect (F1,25 = 5.6, P < 0.05) driven by MPH vs. placebo differences for drug words (Z = −2.3, P = 0.021; percent decrease = 4.7% ± 1.9%; driven by the CUD = 6.7% ± 3.2%) (Fig. 1C). Thus, MPH decreased task-related errors of commission (a common measure of response impulsivity) (28) in all study subjects, most notably during the drug-related context. Accuracy (number of correct responses per task epoch) and errors of omission showed a trend to a medication × word × group interaction (F1,25 > 3.8, P = 0.064) (Fig. S3). Inspecting the means, these three-way interactions were driven by MPH-enhanced accuracy and lower errors of omission (this time during the neutral-word condition for the CUD and the drug-word condition for the controls). No other effect (e.g., group differences) for these or other behavioral (e.g., reaction time) variables reached significance.
Posttask word-value ratings.
Although the word main effect was significant (drug < neutral, F1,25 = 42.3, P < 0.0001), all other effects were not (F < 1.9, P > 0.2). Thus, all subjects rated the drug words as more negative than the neutral words (−0.96 ± 0.22 vs. 0.92 ± 0.18, respectively), and this effect was not modulated by group or MPH.
Craving ratings.
A 2 (medication) × 3 (repetition: baseline, 55 and 75 min after MPH or placebo) × 2 (group) ANOVA showed a significant group main effect such that compared with controls, CUD reported higher craving at all three assessments (F1,24 = 21.8, P < 0.0001); however, these craving reports were not modulated by MPH (all other effects: F < 2.0, P > 0.2) (Fig. S4). SI Text describes MPH effects on other task ratings (motivation to gain money, sleepiness, interest in task, and confidence in performance), profile of mood states, and cardiovascular measures (including heart rate).
To summarize, although, in the current sample, the task was not associated with increased accuracy to monetary reward as we previously reported in an independent larger sample (14), this task was emotionally salient as evidenced by subjective ratings (negative value attributed to the drug vs. neutral words) across all subjects. Furthermore, although CUD reported more craving throughout the task, there were no group differences in task performance.
Brain–Behavior Correlations.
To inspect whether the placebo-controlled MPH enhancements of both fMRI activations and behavior were intercorrelated, we examined their respective change scores (MPH − placebo separately for drug and neutral words for both fMRI activations and performance variables). SI Text shows results of first-order effects (i.e., correlations between our a priori ROIs and the task-performance measures for each of the study conditions separately). Using the change scores, correlations were significant for both a priori ROIs (the cdACC with errors of commission during neutral words and the mOFC with accuracy, errors of omission, and errors of commission during drug words) as confirmed with whole-brain analyses. These brain–behavior correlations show that the higher that the MPH enhanced regional activations (directly compared with placebo), the better the respective task performance across all study subjects (Table 1, Fig. 1D, and Fig. S5). In addition to these ROIs, correlations were also observed for the temporal gyrus and anterior cerebellum (Table 1).
Effect of Potential Covariates on Main Results.
The two study groups differed on age, depression, and cigarette smoking (Fig. 1 and Table S1). These variables were entered as separate covariates in the relevant ANOVAs if significantly (P < 0.05) associated with our selected dependent variables across all study subjects (29) (the same procedures were applied to control for the effect of other potentially contributing variables, such as heart rate, as further described in Discussion and SI Text).
ROIs.
The cdACC was negatively associated with age and depression (for both, drug words during placebo: r = −0.46, P < 0.05). Entering age as covariate, the three-way interaction was still discernible (F1,24 = 3.4, P = 0.076), although it did not survive correction for depression (F1,24 = 1.8, P > 0.2); nevertheless, inspection of means revealed the same pattern of results as in Fig. 1A. The rvACC/mOFC was not associated with age or depression (r < −0.38, P > 0.054). There were no differences in these two ROIs between cigarette smokers and nonsmokers (t25 < |1.8|, P > 0.08). For the main scatterplot (Fig. 1D), the ROI (and behavioral) difference scores were not associated with any of these covariates (r < |0.35|, P > 0.07; t < 1.7, P > 0.1).
Task performance.
Age, depression, and cigarette smoking were not associated with errors of commission (Spearman r = rS < |0.37|, P > 0.054; t25 < |1.8|, P > 0.09).
Correlations with Drug Use in CUD.
Whole-brain analyses, which followed significant ROI analyses (SI Text), showed a significant correlation between the cdACC and lifetime alcohol use (Table 1 and Fig. S6) (correlation survived corrections for age, depression, and cigarette smoking; rS > −0.94, P < 0.0001). These whole-brain analyses also revealed correlations between drug use and the dorsolateral PFC, caudate, and inferior parietal lobule (Table 1). Notably, a significant ROI correlation between the rvACC/mOFC and recent cocaine use (SI Text) did not survive whole-brain corrections, and this effect, although echoing our prior correlation between this region with craving (14), needs to be studied further in larger sample sizes. There were also no significant correlations between the selected drug-use variables (Table S1) and any of the task-performance variables (rS < |0.67|, P > 0.01).
Discussion
Our current results show that, compared with placebo, oral MPH (i) enhanced the activation of the cdACC and rvACC/mOFC in response to a cognitive task in CUD and (ii) decreased response impulsivity (errors of commission) to the task both in controls and CUD. Results further showed a brain-behavior MPH-specific correlation such that (iii) the greater the improvement in task accuracy with MPH compared with placebo, the larger the respective increase in the rvACC/mOFC fMRI signal (Fig. 1). These results are consistent with the benefit conferred by MPH during performance of other PFC-mediated cognitive tasks in various psychopathologies [e.g., frontotemporal dementia (4), ADHD (5–8), traumatic brain injury (9, 10), and depression after stroke (11)]. These results are also consistent with two recent studies in CUD: an fMRI study showed that i.v. MPH compared with placebo improved inhibitory control on a neutral cognitive task (30), and a positron emission tomography study showed that oral MPH (20 mg) attenuated the inhibition of metabolism in limbic brain regions that followed cocaine-cues exposure (31). Our fMRI study shows that a therapeutic dose of the feasibly administered oral MPH (20 mg) improved cognitive performance (decreased errors of commission, a measure of impulsivity) both in CUD and control participants during performance of a salient cognitive task as associated with enhanced activation of the ACC in the CUD. In the CUD, we speculate that these beneficial effects are contingent on an enhancement of dopamine neurotransmission by MPH (which blocks the dopamine transporter) in these dopamine-deficient individuals. However, the direct or downstream effects of other neurotransmitters such as norepinephrine (32), also disrupted with chronic cocaine exposure (33), remain to be empirically tested.
Although the task in this study was instrumental in documenting ACC hypoactivations in CUD and their reversal by MPH, future studies are needed to evaluate the generalizability of our findings to other tasks or activities that involve the ACC. Particularly relevant will be explorations of the potential benefits of MPH in an emotionally charged context, because our findings (except for the rvACC/mOFC main effect) were most robust during the negatively valenced drug-related context.
Using different PFC-mediated cognitive tasks, other fMRI studies similarly reported cdACC hypoactivations in addicted individuals (review in ref. 34). Our current results suggest that these hypoactivations may be associated with the cumulative effect of lifetime drug use, which is most clearly evident for alcohol (Fig. S6). The specificity of results to alcohol may be statistically driven (that is, we noted similar correlations with other drugs that did not survive the high-significance thresholds and whole-brain corrections), which remains to be preclinically tested. Nevertheless, this association with the cdACC was not explained by age, depression, or cigarette smoking and could potentially reflect a premorbid factor predisposing individuals to drug use or a marker for a more severe level of addiction. Importantly, such PFC hypoactivations (including in the cdACC) predispose treatment-seeking drug-addicted individuals to relapse (35), whereas cognitive impairment is associated with lower treatment retention (36). Our results, therefore, suggest that, by enhancing PFC function and associated cognitive performance (decreasing commission errors/impulsivity), clinical outcome may be improved.
In our previous study, we reported that the more the rvACC/mOFC deactivation (from baseline) in the CUD, the better was their ability to suppress task-induced craving (14). In our current smaller sample, craving was not enhanced by the task, and current ROI correlation results suggested an opposite direction of effect (the more the deactivations, the more the recent cocaine use; SI Text). A similar direction of effect was recently suggested by a correlation between rvACC/mOFC response (to fictive errors) and subjective craving in unsated cigarette smokers (37); nevertheless, because direction of this response remains to be specified and given the trend level of our current ROI correlation results, the role of the rvACC/mOFC in craving and drug-seeking remains to be fully explored. Importantly, although our task used drug-related words, the low oral dose of MPH (20 mg) did not modulate craving (Fig. S4). These results are consistent with prior studies where the drug cues were video scenes of people self-administering cocaine (31, 38). In contrast, i.v. MPH administration (0.5 mg/kg) increases cocaine craving in CUD (39). Therefore, in designing future studies and potential neurocognitive interventions, route of MPH administration (and dose) have to be closely monitored.
Similarly to the effect in CUD as mentioned above (31), in healthy participants, MPH attenuates the brain-metabolic or blood-flow increases induced by a cognitive task (e.g., numerical calculations or spatial working memory), especially in regions activated by the task (including the PFC) (40, 41). Such attenuation possibly reflects better signal-to-noise ratio and optimized activity (e.g., processing efficiency) in the brain regions supporting accurate task performance (40, 41); indeed, the MPH-induced improvements were greatest in subjects with lower baseline capacity (41). The relative increases in signal in our study can probably be attributed to the imaging modality used and remain to be tested in a separate comparative study. Other pharmacological fMRI studies using oral MPH generally show enhanced cortical and subcortical blood oxygen level-dependent (BOLD) responses in healthy controls or individuals with ADHD (reviewed in ref. 42), which are most consistently shown in the PFC (including the ACC and OFC) and dorsal striatum (43). Nevertheless, disparity in direction of results may also be attributed to other factors [e.g., type of task (it would be important to use a sensorimotor task as an active control task) and severity of impairment (baseline capacity needs to be tested in studies with larger sample sizes)] and remains to be tested.
There were limitations to this study. (i) Results need to be replicated in a larger and more heterogeneous sample (e.g., one that includes a balanced gender distribution) and study groups that are matched on age, depression, cigarette smoking, and other drug use (e.g., alcohol and marijuana). (ii) Absence of an overt behavioral conflict when directly comparing the drug with neutral words prevents attribution of results to a specific drug-related response. In future studies, an event-related task design could help measure drug-related conflict separately from error, further permitting exploration of trial-by-trial brain-behavioral dynamic changes (synchronization). (iii) One could question whether the impact of MPH on the cdACC and rvACC/mOFC activations reflects its vasoactive and stimulant properties; however, controlling for heart rate, blood pressure, and sleepiness (if significantly correlated with our dependent variables) did not change results, and the groups did not significantly differ in plasma levels of MPH (SI Text, Table S1, and Fig. S7). (iv) Using this cross-sectional design, it is not possible to attribute results to the direct effects of chronic drug use or factors predisposing to drug use and addiction. This issue remains to be resolved in longitudinal studies or research that targets relevant populations (e.g., drug-naïve offspring of addicted individuals, in utero exposed individuals, or those who are stratified by selected genes).
To summarize, our fMRI results show that a low oral dose of MPH improved response of the cingulate cortex and associated task performance in CUD. These results tentatively suggest that, by enhancing midline PFC function in CUD, MPH may have therapeutic benefits that remain to be fully investigated. Thus, although clinical trials with MPH for the treatment of cocaine addiction have not been effective in decreasing drug use, it remains to be tested whether MPH, when combined with specific cognitive interventions, can be used for behavioral modification (e.g., impulse control) to facilitate recovery in addicted individuals.
Materials and Methods
Subjects.
Subjects were recruited using advertisements in local newspapers and by word-of mouth. Twenty-eight right-handed native English-speaking subjects (13 CUD and 15 controls) underwent a full physical and neuropsychiatric examination by a neurologist and a diagnostic interview by a clinical psychologist. This interview included the Structured Clinical Interview for Diagnostic and Statistical Manual of mental disorders (DSM-IV) Axis I Disorders (research version in refs. 44 and 45), the Addiction Severity Index (46), the Cocaine Selective Severity Assessment Scale (47), and the Cocaine Craving Questionnaire (48). Subjects were excluded for (i) history of head trauma, loss of consciousness (>30 min), or other neurological disease of central origin (including seizures), (ii) abnormal vital signs at time of screening and history of major medical conditions, encompassing cardiovascular (including high blood pressure, cardiac arrhythmias apart from sinus bradycardia, or an abnormal electrocardiography at time of screening), endocrinological (including metabolic), oncological, or autoimmune diseases, (iii) history of major psychiatric disorder (other than substance abuse or dependence for the CUD and/or nicotine dependence for both study groups), (iv) except for cocaine in the CUD, positive urine screens for other psychoactive drugs or their metabolites (phencyclidine, benzodiazepines, cannabis, opiates, barbiturates, and inhalants), (v) pregnancy as inspected with a urine test in all females, (vi) contraindications to the MRI study, (vii) history of glaucoma, and (viii) because of the verbal nature of the task, more than 2 SDs below the norm on a verbal intelligence measure. Of the 28 subjects, 27 subjects (13 CUD and 14 controls) completed the fMRI task without loss of data because of motion or technical difficulties (see thresholds below). All subjects were healthy individuals, not taking any medications, able to understand, and able to give informed consent (which was obtained after the nature and possible consequences of the studies were explained in accordance with Stony Brook University’s Committee on Research Involving Human Subjects).
All CUD used crack/cocaine (mostly by smoked route) in the past 30 d and met DSM-IV criteria for current cocaine dependence (which encompass loss of control over excessive use, despite multiple attempts to curtail or stop drug use, even when faced with dire consequences). Urine was positive for cocaine in five CUD on both study days, whereas three CUD were positive for cocaine on the MPH but not placebo day; urine was negative for all drugs in all other subjects on both study days. Self-reported time since last drug use (number of days; mean ± SD) was 4.9 7.3 d and 6.3 ± 9.7 d for the MPH and placebo days, respectively (with no significant difference between the study d; Z = −1.5, P > 0.1). One CUD also met criteria for current heroin dependence. Current use of alcohol was reported by 12 CUD, whereas current use of marijuana was endorsed by 1 CUD; however, 0 of these 13 CUD met criteria for current dependence on alcohol or marijuana. Use of or dependence on other drugs was denied and corroborated by the prescan urine tests in all subjects (drug-use measures shown in Table S1).
The CUD and control groups did not differ in gender, race, education, general intellectual functioning, socio-economic status, and baseline cardiovascular measures (Table S1 shows demographics). Nevertheless, there were significant differences between the groups in age, depression, cigarette smoking, and as expected, most drug-use variables (Table S1).
Task.
The fMRI task (developed in E-prime; Psychology Software Tools) uses 40 regular drug words; non-English or slang drug words were not used (because they may not have been recognized by the control subjects) (13). Forty household words were matched to the drug words on length, frequency in the English language (49), and part of speech (noun, adjective, adverb, and verb) (13). Similar to other fMRI tasks of emotion, the two word types were presented in a blocked on-off or off-on order (i.e., drug-neutral or neutral-drug) (50) and counterbalanced between subjects. Subjects had to press one of four buttons (yellow, blue, red, or green) on a commercially available response pad (Lumina model LP-400; Cedrus), matching the color of the word they had just read; word color order was pseudorandomized across all task runs. There were six 3.4-min (206 s) task repetitions, each containing two task epochs of 20 drug or neutral words (preceded by a 3-s instruction slide) interleaved between three 20-s baseline periods (a fixation cross) (figure S3 in ref. 14). Task onset was 60 min after MPH (or placebo) administration, and its duration (20.6 min or 1,236 s) was entirely contained within peak MPH effects (start at 60 min postadministration and last for at least another 60 min) (51). The task was presented through MRI-compatible goggles. Additional task details are given in SI Text.
Behavioral Measures.
Cocaine ratings (how much do you want cocaine right now from not at all to very much; 0–10) were obtained four times, at baseline (just before baseline cardiovascular monitoring), just before the fMRI drug-word task (around 55 min postmedication), immediately at its conclusion (around 75 min postmedication), and immediately after an additional fMRI task (the color-word Stroop task, results to be reported separately, around 100 min postmedication). Because our current analyses concentrated on the drug-word task, here, we analyzed the first three time points. Immediately after completion of the MRI session (outside the scanner and immediately preceding the last cardiovascular measure), all subjects also rated all task words on valence (how negative or positive a word is from extremely negative to extremely positive; −5 to +5). All ratings were obtained using custom programs written in C++. Inside the scanner, the questions were presented through MRI-compatible goggles.
Study Procedures.
MPH or placebo was administered in a counterbalanced (across all subjects) single-blinded fashion (given the stimulant effects of MPH, including enhancing cardiovascular reactivity and reducing sleepiness, of special concern in populations with select psychopathologies such as drug addiction, study personnel were not blinded to the administered challenge). There were no differences between study days in post-fMRI guesses for the medication received (guess MPH vs. placebo; χ21 = 3.0, P = 0.083), indicating that subjects were not fully aware of the exact type of medication received. Fig. S1 shows study procedures.
MRI Data Acquisition.
MRI scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. The BOLD responses were measured as a function of time using a T2*-weighted single-shot gradient-echo echoplanar imaging sequence [echo time/repetition time (TE/TR) = 20/1,600 ms, 4-mm slice thickness, 1-mm gap, typically 33 coronal slices, 20 cm field of view, 64 × 64 matrix size, 90° flip angle, 200-kHz bandwidth with ramp sampling, 128 time points, and 4 dummy scans to be discarded to avoid nonequilibrium effects in the fMRI signal]. Padding was used to minimize subject motion, which was also monitored immediately after each fMRI run (52). Earplugs (−28-dB sound attenuation, Aearo Ear TaperFit 2; Aearo Company) and headphones (−30-dB sound attenuation, Commander XG MRI Audio System; Resonance Technology Inc.) were used to minimize the interference effect of scanner noise during fMRI (53). Anatomical images were collected using a T1-weighted 3D modified driven equilibrium Fourier transform sequence (54) (TE/TR = 7/15 ms, 0.94 × 0.94 × 1-mm spatial resolution, axial orientation, 256 readout and 192 × 96 phase-encoding steps, and 16-min scan time) and a modified T2-weigthed Hyperecho sequence (55) (TE/TR = 42/10,000 ms, echo train length = 16, 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86-mm in-plane resolution, 5-mm thickness, no gap, and 2-min scan time), both reviewed to rule out gross brain morphological abnormalities that can affect fMRI results.
MRI Data Processing.
Analyses were performed with the statistical parametric mapping package version 2 (SPM2) (Welcome Department of Cognitive Neurology). A general linear model (56) and a box-car design convolved with a canonical hemodynamic response function, and low-pass (hemodynamic response function) and high-pass (cutoff frequency = 1/520 Hz) filters were used to calculate individual BOLD–fMRI maps. A six-parameter rigid body transformation (three rotations and three translations) was used for image realignment and to correct for head motion; 2-mm displacement and 2° rotation were used as criteria for acceptable motion [averaged across all axes and task repetitions separately for translations and rotations; there were no significant differences between the study days, groups, or interactions in these parameters: F < 1.3, P > 0.3, translation = 0.38 ± 0.04, rotation = 0.57 ± 0.04 (mean ± SEM)]. The realigned datasets were spatially normalized to the standard frame (Talairach) with a 12-parameter affine transformation (57) using a voxel size of 3 × 3 × 3 mm3. An 8-mm full-width half-maximum Gaussian kernel was used to smooth the data.
Four contrast maps per subject were calculated, reflecting percent signal change from a fixation baseline for each of the two task (type of word) conditions at each study day (type of medication). These individual contrast maps were included in a second-order (random-effects) repeated measures ANOVA SPM2 model with two within-subjects factors (word is drug or neutral and medication is MPH or placebo) and one between-subjects factor (group is CUD or control). Brain-activation clusters with at least 20 voxels (540 mm3) and P < 0.05 (cluster-level corrected for multiple comparisons using the continuous random-field calculation implemented in SPM2 and P < 0.001 voxel-level uncorrected) were considered significant. Small-volume corrections (58) were only applied to our a priori ROIs (rvACC/mOFC and cdACC). In all SPM analyses, anatomical specificity was corroborated with a coplanar stereotaxic atlas of the human brain (59).
To confirm the voxel-based analyses, functional ROIs with an isotropic volume of 27 voxels (729 mm3) were defined at the selected rvACC/mOFC and cdACC coordinates (Table 1) to extract (with a custom program written in IDL; ITT) the average (and variability) BOLD–fMRI signal amplitudes in these regions. These ROI measures were used in follow-up analyses (e.g., ANOVA with least significant difference pairwise multiple comparison corrections, t tests, and correlations) conducted in SPSS 11.5 (SPSS Inc.). Statistical significance for these ROI analyses was defined at P < 0.05 uncorrected (note that here we only inspected the regions that were significant at P < 0.05 cluster-level corrected at the whole-brain analyses, providing protection against type I error). In all analyses, the appropriate corrections were used in cases of violation of homogeneity of variance (e.g., as tested with Levene's test or Mauchly's test of sphericity).
We conducted correlations between selected ROIs and respective behavioral measures (e.g., rvACC/mOFC activations during drug words with accuracy during the same task condition, separately during both medication conditions). To inspect the specific effect of MPH on brain-behavior correlations, we calculated a difference score between MPH and placebo for all dependent measures of interest. Correlations were also conducted between the ROIs with six selected drug-use variables (Table S1). These exploratory correlations were conducted to inspect associations between the brain regions that differed as a function of MPH and recent and lifetime drug use in the CUD. Given that multiple correlations were conducted, all correlations in SPSS were inspected with a family-wise corrected threshold (P < 0.01; an exception was the performance-difference scores that were inspected at the more lenient statistical threshold, P < 0.05, for our a priori regions only). As a further protection against type I error, significant ROI correlations were tested with SPM and whole-brain corrections (simple regressions with selected behavioral variables as seed values regressed against selected contrast maps). In all whole-brain correlations, the same threshold was used as for the main whole-brain analyses; an exception was reduction of the significance threshold for correlations between our a priori ROIs with task performance (differential scores) to P < 0.05 cluster-level corrected and P < 0.005 voxel-level uncorrected, with a minimum of 20 contiguous voxels and a small-volume correction.
The behavioral measures were analyzed with the appropriate ANOVAs as described in Results. All continuous and normally distributed variables were inspected with parametric tests, whereas self-reported measures and the nonnormally distributed task accuracy/errors were inspected with the respective nonparametric tests.
Supplementary Material
Acknowledgments
This study was supported by National Institute on Drug Abuse Grants R01DA023579 (to R.Z.G.), R21DA02062 (to R.Z.G.), and R01DA020949 (to D.S.), General Clinical Research Center Grant 5-MO1-RR-10710, and the Office of Biological and Environmental Research, Department of Energy (for infrastructure support).
Footnotes
Conflict of interest statement: R.Z.G. received a consultation fee from Medical Directions, Inc., for design of educational material and an honoraria fee from the Federal Judicial Center and the Gruter Institute for Law and Behavioral Research for lectures, both about neuroimaging in drug addiction.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011455107/-/DCSupplemental.
References
- 1.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 4.Rahman S, et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVito EE, et al. The effects of methylphenidate on decision making in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64:636–639. doi: 10.1016/j.biopsych.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson BS, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain SR, Robbins TW, Sahakian BJ. The neurobiology of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1317–1319. doi: 10.1016/j.biopsych.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain SR, Müller U, Robbins TW, Sahakian BJ. Neuropharmacological modulation of cognition. Curr Opin Neurol. 2006;19:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Ko MH, Na SY, Park SH, Kim KW. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: A double-blind placebo-controlled study. Clin Rehabil. 2006;20:24–30. doi: 10.1191/0269215506cr927oa. [DOI] [PubMed] [Google Scholar]
- 10.Chen AJ, Abrams GM, D'Esposito M. Functional reintegration of prefrontal neural networks for enhancing recovery after brain injury. J Head Trauma Rehabil. 2006;21:107–118. doi: 10.1097/00001199-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ramasubbu R, Goodyear BG. Methylphenidate modulates activity within cognitive neural networks of patients with post-stroke major depression: A placebo-controlled fMRI study. Neuropsychiatr Dis Treat. 2008;4:1251–1266. doi: 10.2147/ndt.s4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein RZ, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein RZ, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci USA. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 16.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 17.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 18.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 19.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 20.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 21.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex. II. During anticipatory anxiety. Proc Natl Acad Sci USA. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Shin LM, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 25.Mitterschiffthaler MT, et al. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38:247–256. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- 26.Haas BW, Omura K, Constable RT, Canli T. Interference produced by emotional con-flict associated with anterior cingulate activation. Cogn Affect Behav Neurosci. 2006;6:152–156. doi: 10.3758/cabn.6.2.152. [DOI] [PubMed] [Google Scholar]
- 27.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 28.Lezak MD. Neuropsychological Assessment. 3rd Ed. New York: Oxford University Press; 1995. [Google Scholar]
- 29.Stevens J. Applied Multivariate Statistics for the Social Sciences. 2nd Ed. Hillside, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 30.Li CS, et al. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci USA. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkow ND, et al. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS ONE. 2010;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 33.Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 36.Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu PH, Lohrenz TM, Montague PR. Smokers’ brains compute, but ignore, a fictive error signal in a sequential investment task. Nat Neurosci. 2008;11:514–520. doi: 10.1038/nn2067. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkow ND, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: Relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta MA, et al. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65:1–6. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller U, et al. Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacology (Berl) 2005;180:624–633. doi: 10.1007/s00213-005-2264-9. [DOI] [PubMed] [Google Scholar]
- 43.Bullmore E, et al. Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cereb Cortex. 2003;13:144–154. doi: 10.1093/cercor/13.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First MB, Spitzer RL, Gibbon M, Williams J. Williams J. Structured Clinical Interview for DSM-IV Axis I disorders—Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 45.Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 46.McLellan AT, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 47.Kampman KM, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 48.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 49.Francis NW, Kučera H. Frequency Analysis of English Usage. Boston: Houghton Mifflin; 1982. [Google Scholar]
- 50.Whalen PJ, et al. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 52.Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- 53.Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JH, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 55.Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- 56.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 57.Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6:344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- 58.Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 59.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.