Abstract
During viral infection, effector CD8 T cells contract to form a population of protective memory cells that is maintained by IL-7 and IL-15. The mechanisms that control effector cell death during infection are poorly understood. We investigated how short- and long-lived antiviral CD8 T cells differentially used the survival and cell growth pathways PI3K/AKT and JAK/STAT5. In response to IL-15, long-lived memory precursor cells activated AKT significantly better than short-lived effector cells. However, constitutive AKT activation did not enhance memory CD8 T-cell survival but rather repressed IL-7 and IL-15 receptor expression, STAT5 phosphorylation, and BCL2 expression. Conversely, constitutive STAT5 activation profoundly enhanced effector and memory CD8 T-cell survival and augmented homeostatic proliferation, AKT activation, and BCL2 expression. Taken together, these data illustrate that effector and memory cell viability depends on properly balanced PI3K/AKT signaling and the maintenance of STAT5 signaling.
Keywords: apoptosis, cytokine signaling, interleukin 15, interleukin 7, lymphocytic choriomeningitis virus
Memory CD8 T cells form from a much larger effector population and provide long-term immunity against subsequent infections via rapid reactivation. The exact biochemical mechanism governing the survival of memory cells and apoptosis of the majority of effector cells is not well understood (1). As naïve T cells differentiate into effector and memory T cells, cytokines play a key role in their differentiation and survival. IL-2, IL-7, and IL-15 promote the expansion and survival of activated T cells (2). As memory CD8 T cells form following acute viral infection, two of these cytokines, IL-15 and IL-7, direct memory T cell survival and homeostasis (3, 4). Downstream of their specific receptors, IL-7 and IL-15 activate two primary pathways: janus kinase/signal transducer and activator of transcription 5 (JAK/STAT5) and phosphoinositide 3-kinase (PI3K)/AK-transforming (AKT). Activation of these two pathways results in decreased levels of proapoptotic proteins, increased expression of antiapoptotic genes such as BCL2, and activation of cellular growth and proliferation pathways regulated by mTOR and cyclins (5). The balance between these pro- and antiapoptotic factors controls the survival of CD8 T cells after immunization (6).
Early models predicted that effector CD8 T-cell contraction was a result of deprivation of T-cell growth factor cytokines that wane during the later stages of infection and that survival was determined via the stochastic interaction of effector T cells with these cytokines. However, effector CD8 T cells are a heterogeneous population composed of cells with varying intrinsic potential for survival and differentiation into memory cells (3, 7, 8). During several types of infections [such as lymphocytic choriomeningitis virus (LCMV), vesicular stomatitis virus (VSV), Listeria monocytogenes, and Toxoplasma gondii], most of the effector CD8 T cells terminally differentiate and become short-lived, but a minority maintain memory potential and are capable of self-renewal (9–14). Separation of these subsets is possible because most memory precursor cells express higher amounts of IL-7Rα, CD27, and CXCR3 and lower amounts of KLRG1, whereas shorter-lived effector cells express these receptors in a reciprocal pattern (3, 10, 13, 15). These findings indicate that the process of contraction is not stochastic and that effector CD8 T cells have acquired distinct cell fates; however, it does not discard the possible role of cytokine withdrawal in the contraction of effector cells. IL-7Rα is functionally required for memory precursor survival and optimal memory CD8 T cell generation (3, 4, 16). However, forced IL-7Rα expression does not enhance memory CD8 T cell formation (17–19). IL-15 also helps to sustain memory CD8 T cells and KLRG1hi IL-7Rαlo effector CD8 T cells are acutely dependent on it (2, 15). Furthermore, the common γ-chain cytokine-induced proliferative responses of short-lived (KLRG1hi) effector T cells are subdued in comparison with memory precursor cells, suggesting that intrinsic differences exist between these two cell populations that modulate their cytokine responsiveness and survival (20).
To better understand the role of cytokine-derived signals in the process of memory cell development and effector cell contraction, we interrogated the regulation of JAK/STAT5 and PI3K/AKT signaling pathways in viral-specific CD8+ T cells in vivo. We discovered that terminally differentiated effector CD8 T cells are impaired in the activation of the PI3K/AKT pathway but, surprisingly, constitutive AKT activation did not increase their survival. Rather, it caused feedback inhibition on the expression of IL-7Rα and IL-2/15Rβ chains, which in turn reduced STAT5 signaling, BCL2 expression, and memory cell formation. Conversely, constitutive STAT5 activation dramatically enhanced effector CD8 T cell survival and memory CD8 T cell formation after infection. These data highlight that key survival and mitogenic pathways are modulated in activated T cells by their differentiation state, and effector and memory T-cell longevity is regulated by an optimal balance of STAT5 and AKT activity.
Results
KLRG1lo Effector CD8 T Cells Activate the PI3K/AKT Pathway in Response to IL-15 More Robustly than KLRG1hi Effector Cells.
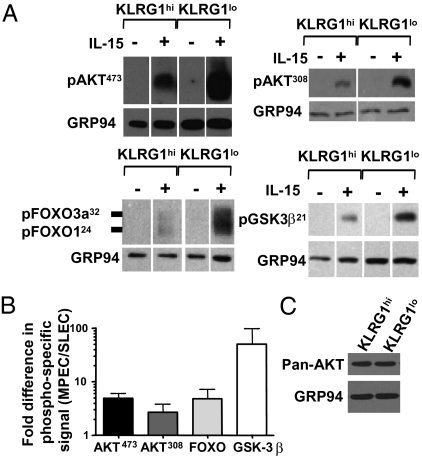
Given that IL-15 is an important cytokine for memory CD8 T-cell homeostasis and survival, we compared the ability of KLRG1hi IL-7Rαlo short-lived effector cells (SLECs) and KLRG1lo IL-7Rαhi memory precursor effector cells (MPECs) to respond to IL-15. IL-15 signaling, as opposed to IL-7 and IL-2, was examined because IL-7Rαhi and IL-7Rαlo effector cells express similar levels of IL-15Rα and IL-2/15Rβ chains, whereas neither subset expresses the high-affinity IL-2Rα chain (Fig. S1A). Both KLRG1hi and KLRG1lo CD8 T cells rapidly phosphorylated STAT5 and ERK1/2 in response to IL-15, indicating that the receptor is functional on both subsets (Figs. S1 B and C and S2 A and B) (17). To examine the ability of effector CD8 T cells to activate the PI3K/AKT pathway, antigen-specific KLRG1lo MPECs and KLRG1hi SLECs were purified from mice infected 8–10 d earlier with LCMV and stimulated with IL-15. In response to IL-15, the KLRG1lo effector CD8 T cells had significantly higher amounts of phosphorylated AKT at both the serine 473 and threonine 308 residues in comparison with the KLRG1hi effector T cells (Fig. 1 A and B). This difference in AKT473 phosphorylation was observed as early as 40 min post–IL-15 stimulation, but the signal increased substantially after overnight stimulation (Fig. 1; Fig. S2 A and B). The difference in phosphorylation could not be attributed to differences in the levels of total AKT protein in the two cell populations (Fig. 1C). Concomitant with increased AKT phosphorylation, the KLRG1lo effector cells also demonstrated a greater ability to phosphorylate downstream AKT targets GSK-3β, FOXO1, and FOXO3a (Fig. 1 A and B). Additionally, AKT phosphorylation could be inhibited by the PI3K inhibitor Ly294002, indicating that cytokine-induced AKT phosphorylation was PI3K-dependent (Fig. S2C). This reduction in AKT phosphorylation in SLECs was not limited to IL-15, as similar results were obtained following both IL-2 and IL-7 stimulation (in which IL-7Rα expression was made equivalent via transgenic IL-7Rα expression) (Fig. S2D). Furthermore, this difference was not mediated by the binding and activation of the inhibitory receptor KLRG1 (21) during sorting because similar results were observed when the cells were separated based on CD27, which inversely correlates with KLRG1 expression (Fig. S2C). Last, the impaired AKT phosphorylation was not specific to the P14 T cell receptor (TCR) transgenic T cells analyzed above because similar results were observed in subsets of KLRG1hi and KLRG1lo cells isolated from polyclonal populations of CD44hi effector cells after LCMV infection (Fig. 2A, compare Wt KLRG1hi and KLRG1lo lanes).
Fig. 1.
KLRG1lo effector CD8 T cells preferentially maintain the ability to activate the PI3K/AKT pathway in response to IL-15. Small numbers (∼2,500) of P14 Thy1.1+ CD8 T cells were transferred into congenic C57BL/6 mice that were subsequently infected with LCMV. (A) KLRG1hi or KLRG1lo CD8+ Thy1.1+ P14 CD8 T cells were isolated by FACS from mice 8–10 d p.i. and ∼1 × 106 cells were cultured overnight with or without IL-15 and then assayed for phosphorylation of AKT, FOXO1/3a, or GSK-3β (A) or total AKT (C) via Western blot. Data shown are representative examples of two to six experiments, and densitometric quantification of phosphorylation experiments is shown in B.
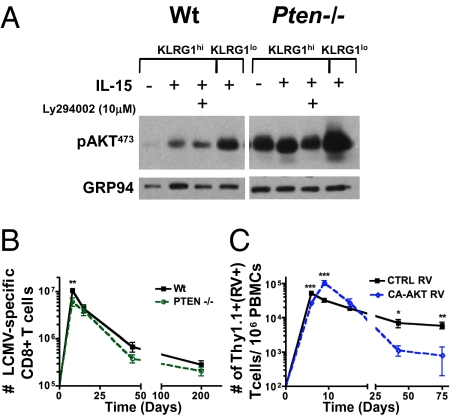
Fig. 2.
Constitutive AKT activation is not sufficient for memory CD8 T-cell formation. (A) Ptenfl/fl Gzb-Cre+ (Pten−/−) or Ptenfl/fl Gzb-Cre− (Wt) were infected with LCMV, and at day 8 p.i., CD44hi CD8 T cells from Pten−/− or Wt controls were isolated by FACS based on KLRG1hi or KLRG1lo expression and cultured overnight with or without IL-15. Approximately 1 × 106 cells per sample were assayed via Western blot for phosphorylated AKT. (B) Line graph shows the number of LCMV-specific (DbNP396–404 and DbGP33–41 tetramer+) CD8 T cells in the spleens of Pten−/− (green line) and Wt (black line) mice at the indicated times p.i. (C) Small numbers (∼2,500 cells) of P14 Ly5.1+ T cells transduced with either CA-AKT or CTRL RV that expresses Thy1.1 were transferred into congenic Ly5.2+ C57BL/6 mice that were infected with LCMV. Line graph shows the number of CA-AKT (blue line) and CTRL RV+ (Thy1.1+; black line) P14 CD8 T cells per 106 peripheral blood mononuclear cells (PBMCs) at the indicated times p.i. All data points show the mean (±SEM) of more than five mice over separate experiments. All statistics were calculated by Student's t test. ***P < 0.0005, **P < 0.005, *P < 0.05.
Increased PI3K/AKT Signaling Does Not Enhance Memory CD8 T-Cell Development.
To determine whether the reduced PI3K/AKT signaling in the KLRG1hi effector CD8 T-cell subsets contributed to their shortened lifespan, we tried to “rescue” this defect in the effector CD8 T cells using two different approaches—phosphatase and tensin homolog (PTEN) deficiency and overexpression of constitutively active AKT by retroviral transduction. We first examined PTEN protein levels by Western blotting, but found no significant differences between the KLRG1hi and KLRG1lo effector cell subsets, implying that relative PTEN levels are not responsible for the differences in AKT phosphorylation (Fig. S3A). To analyze the effects of PTEN deficiency in virus-specific effector and memory CD8 T cells, we crossed mice carrying a floxed allele of Pten onto mice that express Cre recombinase under control of the Granzyme B promoter (Ptenf/f; gzb-Cre+; referred to as Pten−/− mice) and then infected Pten−/− mice with LCMV (22, 23). Ptenf/f; gzb-Cre− littermate animals (referred to as Wt) were used as controls. In this system, Pten is expressed normally during thymic development and in naïve T cells, but upon T-cell activation Cre recombinase is expressed and thus the Pten locus is deleted from >90% of the activated CD8 T cells during LCMV infection (23). We confirmed by flow cytometry that PTEN levels were reduced in Ptenf/f; Gzb-Cre+ LCMV tetramer-specific T cells and that Pten−/− T cells from LCMV-infected mice had constitutive AKT phosphorylation (Fig. 2A and Fig. S3B). Notably, the Pten−/− KLRG1lo effector cells still retained a better ability to phosphorylate AKT than the Pten−/− KLRG1hi cells (Fig. 2A), which implied that PTEN-independent mechanisms were involved in the differential activation of AKT in effector CD8 T-cell subsets. PTEN deficiency induced better survival of effector CD8 T cells under serum-starvation conditions in vitro as previously described (Fig. S4A) (24) but, surprisingly, there were only modest effects on the expansion and contraction of effector CD8 T cells in response to LCMV infection (Fig. 2B). In general, the clonal burst of Pten−/− effector CD8 T cells was approximately one half that of the Wt littermate controls, and there was a slight, but not significant, reduction in the number of memory Pten−/− T cells that formed in lymphoid and nonlymphoid tissues compared with WT controls (Fig. 2B). These results demonstrated that increased activation of AKT induced by PTEN deficiency was insufficient to prevent apoptosis of effector CD8 T cells or increase memory CD8 T cell formation following viral infection.
Because PTEN deficiency affects other PI3K-regulated signaling pathways and the Granzyme B Cre conditional knockout system does not limit gene excision to only antigen-specific CD8 T cells, we also examined the role of AKT signaling in memory cell development by expressing a constitutively active form of murine AKT (referred to as CA-AKT) that is myristoylated and constitutively phosphorylated at Ser473 and Thr308 (25). In these experiments, P14 TCR transgenic T cells (Ly5.1+) were transduced with a retrovirus (RV) expressing CA-AKT and the marker Thy1.1, and then transferred to congenic (Ly5.2+) LCMV-infected animals (26). P14 CD8 T cells transduced with empty control (CTRL) RV were used for comparison. Similar to Pten−/− cells, CA-AKT RV effector CD8 T cells harvested from day 8 LCMV-infected mice survived better under serum starvation in vitro compared with CTRL RV cells (Fig. S4B). When analyzed in vivo during LCMV infection, the CA-AKT P14 CD8 T-cell expansion was slightly increased, but the death of the CA-AKT RV P14 CD8 T cells during the contraction phase, between days 8 and 75 postinfection (p.i.), was considerably greater than CTRL RV cells (Fig. 2C). These results indicate that increased and prolonged AKT activation does not improve the viability of effector CD8 T cells and can actually be detrimental to their subsequent survival and memory CD8 T-cell generation.
Impairment of Memory CD8 T-Cell Survival and Function by Constitutive AKT Signaling Is Associated with Down-Regulation of Cytokine Receptors and STAT5 Signaling.
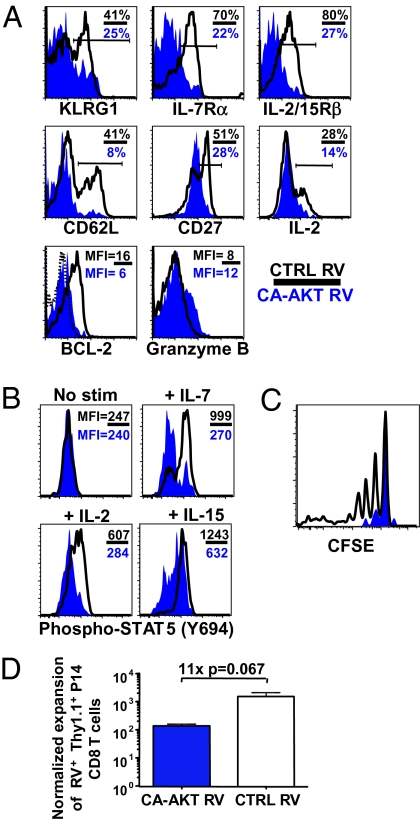
Although constitutive activation of PI3K/AKT had insignificant (Pten−/−) or detrimental (CA-AKT) effects on the formation of CD8 memory T cells, it was important to test whether increasing AKT activity modified the composition and function of the effector and memory CD8 T-cell populations. First, the subsets of surviving CD8 T cells that formed with constitutive AKT activity were examined. The percentage of KLRG1hi LCMV-specific CD8 T cells was not increased by constitutive AKT activity and, in fact, declined more than control cells over the weeks following infection (Fig. 3A and Fig. S5). This indicated that increased AKT activity alone could not rescue the death of the KLRG1hi effector cell subset, even though these cells typically exhibit reduced AKT activity. Additionally, the expression of both IL-7Rα and CD62L were considerably down-regulated in PTEN-deficient and CA-AKT–expressing LCMV-specific memory CD8 T cells, consistent with previous findings (Fig. 3A and Fig. S5) (27, 28). In addition, CA-AKT also caused the down-regulation of the costimulatory receptor CD27 and IL-2/15Rβ chain, a hitherto undescribed effect of hyperactive AKT that may also explain the more rapid loss of the IL-15–dependent KLRG1hi effector cells in the CA-AKT cell population (Fig. 3A) (10, 29). This effect on IL-2/15Rβ expression was not observed in Pten−/− CD8 T cells (Fig. S5), perhaps because the steady-state levels of AKT activity were lower in these cells compared with CA-AKT cells based on ribosomal protein S6 phosphorylation (Fig. S6). Maintenance of IL-2/15Rβ expression may allow for better survival of Pten−/− CD8 T cells following infection compared with CA-AKT-expressing cells (Fig. 2 B and C).
Fig. 3.
Increased PI3K/AKT signaling in virus-specific memory CD8 T cells leads to diminished cytokine receptor expression, STAT5 signaling, homeostatic turnover, and recall responses. As in Fig. 2, P14 CD8 T cells were transduced with CA-AKT or CTRL RVs, and ∼2,500 cells were transferred into C57BL/6 mice that were infected with LCMV. (A) Histogram plots show expression of indicated proteins in CA-AKT (blue) and CTRL (black line) RV+ (Thy1.1+) P14 memory CD8 T cells from mice infected 60 d previously with LCMV. In the top two rows, the percentage of cells expressing “high” levels of each protein and in the bottom row the mean fluorescence intensity (MFI) of the protein are shown. The dotted line indicates staining of BCL2 isotype control antibody. (B) Day 30 CA-AKT (blue) and CTRL (black line) RV+ (Thy1.1+) P14 effector CD8 T cells were stimulated with or without the cytokines IL-2, IL-7, or IL-15 for 30 min, fixed, permeabilized, and stained for intracellular STAT5Tyr694 phosphorylation. Overlapping histograms show levels of phosphorylated STAT5 based on flow cytometry. (C) CA-AKT (blue) and CTRL (black line) RV+ (Thy1.1+) memory CD8 T cells were isolated 30 d p.i., labeled with carboxyfluoroscein succinimidyl ester (CFSE), and transferred into naïve (Ly5.2+) C57BL/6 hosts. Approximately 45 d later the donor cells were assayed for cell division by flow cytometry, and relative levels of CFSE are shown. (D) CA-AKT (blue bar) or CTRL (white bar) RV+ (Thy1.1+) P14 CD8 T cells from day 15 post-LCMV infection was transferred into naïve C57BL/6 mice that were subsequently infected with Listeria-GP33 and analyzed 7 d later. The fold expansion (normalized to the number of donor RV+ CD8 T cells transferred) is shown in the bar graph. All data shown are representative of two or three experiments with more than two mice per experimental group.
We tested whether reduced expression of cytokine receptors in CA-AKT–expressing CD8 T cells prevented the phosphorylation of STAT5 in response IL-2, IL-7, and IL-15 in vitro, and found that STAT5 phosphorylation was inhibited ∼2- to 3-fold (Fig. 3B). Likewise, the key antiapoptotic protein BCL2, which is also a STAT5 target gene, was almost undetectable in both the Pten−/− and CA-AKT RV memory T cells (Fig. 3A and Fig. S5). This effect on BCL2 expression likely contributed to the inability of increased AKT activity to enhance effector CD8 T cell survival and memory development (Fig. 2 B and C).
Last, we examined how constitutive AKT activity affected several important attributes of functional, long-lived memory CD8 T cells. The Pten−/− and CA-AKT memory CD8 T cells were able to produce IFNγ, TNFα, and Granzyme B, but the production of IL-2 was diminished (Fig. 3A and Figs. S5 and S7). Thus, increased AKT activity impaired formation of “polyfunctional” memory CD8 T cells (30). The reduced responsiveness of the CA-AKT memory CD8 T cells to IL-15 also greatly reduced their ability to self-renew, as they proliferated significantly less after transfer into congenic lymphoreplete hosts (Fig. 3C) (31). Likewise, when challenged with recombinant Listeria expressing the LCMV peptide GP33–41 (Listeria-GP33), CA-AKT effector CD8 T cells had weakened secondary responses relative to the control T-cell populations (Fig. 3D). Together, these findings suggest that defective AKT signaling alone is not the primary cause of terminal effector cell apoptosis following infection, because constitutive AKT activation did not hinder cell death. Instead, it induced a negative feedback loop that repressed the expression of IL-7 and IL-15 cytokine receptors and impaired STAT5 signaling and BCL2 expression (6). These results do not necessarily rule out that the basal levels of AKT activity are important for memory T-cell survival but rather underscore that the proper balance of AKT signaling is critical for optimal memory CD8 T-cell formation and function.
STAT5 Signals Are Sufficient for the Survival of CD8 Effector and Memory T Cells.
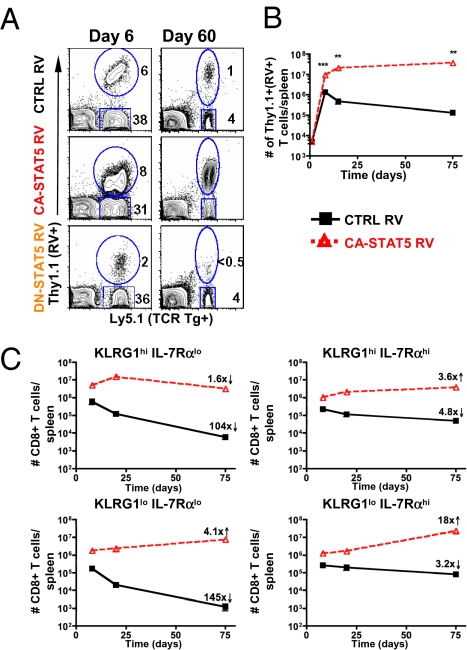
Next, we investigated the role of STAT5 signaling in effector CD8 T-cell survival because it is the other primary signaling pathway downstream of IL-7 and IL-15. To do so, P14 CD8 T cells were transduced with constitutively active (CA-STAT5) or dominant-negative (DN-STAT5) RVs and transferred into LCMV-infected mice. The percent and number of RV+ (Thy1.1+) effector CD8 T cells were measured over the course of LCMV infection, and we found that the different forms of STAT5 had striking effects on effector cell expansion and memory cell formation (Fig. 4A). By day 8 p.i., the CA-STAT5 effector P14 CD8 T cells expanded ∼2- to 3-fold more than CTRL RV cells. CA-STAT5 transduced T cells continued to expand until day 15 p.i. and did not contract thereafter, leading to the formation of ≈30–40 × 106 memory CD8 T cells in the spleen of each animal (Fig. 4B). The CTRL RV population declined normally, and therefore the expression of CA-STAT5 resulted in an ∼280-fold increase in the number of memory CD8 T cells that formed 75 d p.i. (Fig. 4B). The preferential survival of the CA-STAT5 CD8 T cells was also evident in that they rose from ∼20% to ∼75–90% of the transferred P14 CD8 T-cell population (Fig. 4A). Conversely, DN-STAT5 expression substantially reduced expansion and memory cell formation compared with the CTRL RV P14 CD8 T cells (Fig. 4A). Thus, constitutive STAT5 activity profoundly enhances virus-specific effector and memory T-cell survival after infection.
Fig. 4.
STAT5 signals profoundly augment survival of all LCMV-specific CD8 T cells during and after infection. Activated P14 Ly5.1+ CD8 T cells were transduced with CA-STAT5, DN-STAT5, or CTRL RVs that express Thy1.1. Small numbers (∼2,500) of RV+ (Thy1.1+) P14 CD8 T cells were transferred into congenic Ly5.2+ C57BL/6 mice that were infected with LCMV. (A) Contour plots showing the percent RV+ (Thy1.1+) and RV− (Thy1.1−) P14 CD8 T cells in PBMCs at days 6 and 30 p.i. (B) Line graphs show the number of CA-STAT5 (red) and CTRL RV+ (black) (Thy1.1+) P14 CD8 T cells recovered from the spleen at different times postinfection. (C) As in B, total numbers of splenic KLRG1hi IL-7Rαlo, KLRG1lo IL-7Rαhi, KLRG1hi IL-7Rαhi, and KLRG1lo IL-7Rαlo CA-STAT5 (red) and CTRL RV+ (black) (Thy1.1+) CD8 T cells after LCMV infection are shown in the line graphs. Data shown are representative of the mean (±SEM) of 5–20 animals from five separate experiments. All statistics were calculated with Student's t test. ***P < 0.0005, **P < 0.005, *P < 0.05.
We next inspected how CA-STAT5 affected the survival of the different effector and memory CD8 T cell subsets, based on KLRG1 and IL-7Rα expression, following LCMV infection. As expected, the KLRG1hi IL-7Rαlo CTRL RV population contracted nearly 100-fold between days 8 and 75 p.i. (Fig. 4C). In contrast, this same cell population expressing CA-STAT5 only declined ∼2-fold during this time period. Thus, unlike CA-AKT, the constitutive activation of STAT5 could rescue the survival of terminal effector cells considerably. Rather than contracting, CA-STAT5 actually caused the other three subsets of P14 CD8 T cells (KLRG1hi IL-7Rαhi, KLRG1lo IL-7Rαlo, and KLRG1lo IL-7Rαhi) to increase in cell number between the effector and memory stages. The effect of CA-STAT5 was greatest on the KLRG1lo IL-7Rhi LCMV-specific CD8 T cells, as this population increased in number ∼18-fold during this time period and was the most abundant in the memory CD8 T-cell population (Fig. 4C).
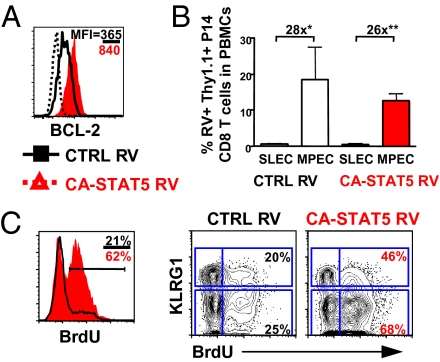
To determine whether CA-STAT5 affected other phenotypes and functions of the memory CD8 T cells, we performed a similar analysis as that described above for CA-AKT transduced cells. Unlike the effects of CA-AKT, we found less profound alterations in the expression of proteins such as IL-7Rα, IL-2/15Rβ, CD27, and CD62L (Fig. S8). In contrast to CA-AKT transduced T cells, IL-2/15Rβ and CD27 were slightly up-regulated in the CA-STAT5 memory CD8 T-cell population. As expected, because it is a target of STAT5, BCL2 was substantially increased in the CA-STAT5 effector and memory CD8 T cells (Fig. 5A and Fig. S8) (32). Next, we examined the recall responses of CA-STAT5 CD8 T cells to secondary LM-GP33 infection, but did not see any substantial beneficial effect of increased STAT5 activity on either subset of effector CD8 T cells (Fig. 5B).
Fig. 5.
STAT5 increases the survival, proliferation, and AKT activation of both KLRG1hi IL-7Rαlo SLECs and KLRG1lo IL-7Rαhi MPECs. As in Fig. 4, P14 CD8 T cells were transduced with CA-STAT5 or CTRL RVs and ∼2,500 cells were transferred into C57BL/6 mice that were infected with LCMV. (A) Histogram plots show expression of BCL2 in CA-STAT5 (red) and CTRL (black line) RV+ (Thy1.1+) P14 CD8 T cells 15 d post-LCMV infection. The MFIs of the proteins are indicated and the dotted line indicates staining of BCL2 isotype control antibody. Data are representative of five separate experiments. (B) Approximately 5,000 P14 TCR transgenic T cells containing CA-STAT5 or CTRL RV+ (Thy1.1+) cells were isolated from mice infected 15 d previously with LCMV and transferred into naïve C57BL/6 mice that were subsequently infected with Listeria-GP33. Seven days later, the expansion of the donor CA-STAT5 or CTRL RV+ (Thy1.1+) P14 CD8 T cells was analyzed in the peripheral blood. Data are representative of three to five mice. (C) Mice containing CA-STAT5 (red) or CTRL (black line) RV (Thy1.1+) P14 CD8 T cells were treated with BrdU in their drinking water from day 65 to 75 p.i. and then stained intracellularly for BrdU. Contour plots on the right show the percent of BrdU+ cells within the KLRG1hi or KLRG1lo memory CD8 T cells. Data shown are representative of two experiments.
Continued proliferation could contribute to the increase in memory CD8 T-cell numbers by constitutive STAT5 activation. To examine this point, we treated animals containing CA-STAT5 or CTRL RV P14 memory CD8 T cells with BrdU from day 65 to 75 p.i. and found CA-STAT5 expression induced a significant increase in proliferation compared with controls (Fig. 5C). In addition, the KLRG1lo cells incorporated BrdU to a greater degree than the KLRG1hi cells, indicating that sustained proliferation contributes to the profound rise in the number CA-STAT5–expressing KLRG1lo memory CD8 T cells (Fig. 5C).
Finally, in CD4 T cells and myeloid leukemias, it has been shown that STAT5 signaling increases cell viability by potentiating PI3K/AKT signaling (33, 34). To test whether a similar mechanism was occurring in the CA-STAT5 RV-expressing T cells, we examined the ability of these cells to phosphorylate AKT in response to IL-15. We found that CA-STAT5 expression increased the ability of both KLRG1hi and KLRG1lo T cells to induce pAKT473 both unstimulated and in response to IL-15 (Fig. S9). Thus, the profound increase in effector and memory CD8 T-cell survival and proliferation by CA-STAT5 may not solely stem from increased expression of STAT5 target genes, but may partially rely on augmented AKT signaling.
Discussion
Terminally differentiated effector and memory CD8 T cells, which can be distinguished by a KLRG1hi CD27lo IL-7Rαlo phenotype, generally have a shorter lifespan and reduced proliferative responses compared with KLRG1lo CD27hi IL-7Rαhi cells (10, 13, 20, 35). The reduced proliferative capacity in the KLRG1hi CD27lo IL-7Rαlo cells has been attributed to increased expression of the cell cycle inhibitor p27kip and decreased expression of BMI1 relative to the IL-7Rαhi subset (17, 36). In addition, the terminally differentiated KLRG1hi CD27lo IL-7Rαlo T cells show poor AKT activation in response to both cytokine and TCR stimulation (21, 37, 38). Given that AKT activity is necessary for cell survival in a variety of cell types, we hypothesized that the reduced AKT activity in KLRG1hi IL-7Rαlo terminal effector cells was the basis of their shortened lifespan (39). However, our results showed that elevating AKT activity in virus-specific CD8 T cells could increase effector T-cell survival under cytokine deprivation conditions in vitro, but could not reduce effector CD8 T cell death during the contraction phase in vivo and, in the case of CA-AKT expression, even exacerbated their contraction. Persistent AKT activity in the virus-specific CD8 T cells induced a negative feedback mechanism that repressed expression of IL-7Rα and IL-2/15Rβ chains and the signals they transduce, such as STAT5. This effect correlated with impaired homeostatic turnover of memory CD8 T cells and secondary responses to reinfection. Both approaches we took to activating AKT (Pten−/− and CA-AKT) produced chronic and strong AKT signaling. Presumably, more subtle increases in AKT activation that do not affect cytokine receptor expression and downstream STAT5 activity could enhance the survival of effector CD8 T cells. Although it is likely that defective AKT signaling in the KLRG1hi effector CD8 T cells contributes to their reduced fitness and longevity, our data suggest that activating AKT alone is not sufficient to “save” effector CD8 T cells and that proper regulation of this molecule is needed for optimal effector cell viability and memory CD8 T-cell function and homeostasis.
In contrast, our data clearly show that constitutive STAT5 signaling is sufficient for survival of effector CD8 T cells and formation of larger numbers of memory CD8 T cells. Expression of CA-STAT5 had a profound ability to block contraction of the KLRG1hi IL-7Rαlo SLECs and inflate the other subsets of memory T cells. This inflation was associated with a greater amount of “homeostatic” proliferation in the CA-STAT5 memory CD8 T cells, especially in the KLRG1lo cells, which naturally have a higher proliferative capacity relative to the KLRG1hi cells (10, 17, 20). It was surprising that CA-STAT5 did not augment the proliferative capacity of CD8 T cells to secondary infection, suggesting that increasing STAT5 activity does not boost secondary antigen-driven proliferation. Unlike CA-AKT, CA-STAT5 augmented the expression of CD27, IL-2/15Rβ chains, and BCL2. Thus, elevated STAT5 activity appears to greatly enhance the survival and homeostasis of all antigen-specific effector and memory CD8 T cells, which is consistent with the observation that mice made transgenic for STAT5 have significantly increased numbers of memory cells (40–42).
The implications of these results are that STAT5 activity is somehow limiting in KLRG1hi effector cells after infection and that this contributes to their death. In support of this idea, bona fide antiapoptotic STAT5 target genes, such as Bcl2, Pim2, and Serpina3g (Spi2a), are up-regulated in KLRG1lo IL-7Rhi effector CD8 T cells compared with KLRG1hi IL-7Rlo cells (3, 10, 43). Additionally, an increased ability to activate STAT5 has been reported in human CD27hi CCR7hi “central memory” cells (TCM) compared with CD27lo CCR7lo “effector memory” T cells (TEM) (38). Although KLRG1hi and KLRG1lo effector CD8 T cells can phosphorylate STAT5 similarly in response to cytokine signaling in vitro (17, 18), it remains to be determined whether STAT5 signals are differently regulated in vivo between the two subsets and contribute to their relative short-term and long-term fates. It is possible that the KLRG1lo IL-7Rhi MPECs contain more STAT5 activity at steady state in vivo simply because they can see more IL-7 and IL-15. For example, KLRG1hi IL-7Rαlo cells have slightly reduced expression of IL-15Rα and IL-2/15Rβ compared with KLRG1lo IL-7Rαhi cells, which may become important at physiological IL-15 levels in vivo. Conversely, KLRG1hi IL-7Rαlo and KLRG1lo IL-7Rαhi CD8 T cells may occupy distinct niches in tissues and interact differentially with IL-15- and IL-7-producing cell types. In support of this idea, exogenous administration of IL-2 and IL-15 can keep KLRG1hi effector CD8 T cells alive after infection, but this effect is dependent on continuous cytokine treatment (20, 44). Furthermore, under steady-state conditions in vivo, the KLRG1hi IL-7Rαlo cells contain considerably less BCL2 than the KLRG1lo IL-7Rαhi cells, but the KLRG1hi IL-7Rαlo cells up-regulate BCL2 when exposed to IL-2 and IL-15 (17). Taken together, these findings suggest that exposure of KLRG1hi terminal effector CD8 T cells to survival cytokines is limited in vivo. It is also possible that STAT5 activation is relatively similar between KLRG1hi and KLRG1lo effector CD8 T cells during the contraction phase in vivo but that differential expression of other transcription factors or microRNAs modulates STAT5-dependent gene expression, leading to differential gene expression and cell survival. Finally, in a T-cell line, it has been shown that STAT5 cannot act alone, and requires PI3K/AKT signaling, to activate ccnd2 (CYCLIN D2) and bcl2l1 (BCLXL) transcription (45). Thus, KLRG1hi cells would be unable to activate genes requiring both STAT5 and PI3K/AKT activity. Our studies with CA-AKT were unable to test this possibility because of the down-regulation of the cytokine receptors and STAT5 signaling.
The cause of the defect in AKT phosphorylation in terminally differentiated KLRG1hi CD27lo IL-7Rαlo effector and memory CD8 T cells is not known. Our data showed that CA-STAT5 could potentiate AKT signaling and complement AKT activation in KLRG1hi cells, indicating cross-talk between the two pathways. Perhaps a paucity of STAT5 activity in the KLRG1hi effector CD8 T cells in vivo leads to the observed defect in AKT activity, which, in turn, reduces the long-term fitness and survival of this cell population. Expression of KLRG1 may also contribute to reduced AKT activation. A recent report showed that blocking the inhibitory receptor KLRG1 from engaging its ligands, E- and N-cadherins, on dendritic cells augmented TCR-driven AKT473 phosphorylation and proliferation of human KLRG1hi CD27lo CD8 T cells (21). All of our biochemical studies were performed on FACS-purified CD8 T cells, which do not express high levels of E-cadherin, and therefore the role of KLRG1 was not directly investigated here (21).
As effector CD8 T cells transition to memory cells, they switch from glycolysis to fatty acid oxidation due to the down-regulation of TORC1, a downstream target of AKT (42, 46). Indeed, blocking TORC1 in T cells during viral infection increases formation of memory CD8 T cells. Thus, the poor memory CD8 T cell development and function observed with CA-AKT in our experiments may be linked to this mechanism as well. PI3K/AKT/TORC1 signaling can also regulate the expression of L-selectin (CD62L), which distinguishes the TEM and TCM memory T-cell subsets (28). KLRG1lo IL-7Rαhi MPECs give rise to both TEM and TCM populations, whereas the fraction of terminally differentiated KLRG1hi IL-7Rlo cells that persists into the memory stage is overwhelmingly composed of CD62Llo TEM cells (10, 13, 47). However, terminally differentiated TEM (KLRG1hi CD27lo CD62Llo) display impaired AKT phosphorylation compared with their KLRG1lo CD27hi counterparts, which suggests that an additional AKT-independent mechanism for CD62L repression exists in CD8 T cells (21, 37). We would postulate that PI3K/AKT-dependent regulation of CD62L expression predominantly occurs in the TCM subset, which has increased AKT signaling.
Multiple reports now show that a CD8 T cell's differentiation state influences its ability to survive and proliferate by a variety of mechanisms. Our work shows that sustained STAT5 activity is a very powerful signal for enhancing memory CD8 T-cell survival, regardless of differentiation state, and suggests that this signal may be lacking in terminally differentiated cells. Furthermore, STAT5 activation increased the production of memory T cells without any obvious oncogenic effects, and therefore modulation of this molecule may be beneficial for vaccine development and treatments of chronic infections or tumors.
Materials and Methods
Mice and Viral/Bacterial Infection.
C57BL/6Ncr mice were purchased from the National Cancer Institute. B6.SJL-Ptprca Pepcb/BoyJ (Ly5.1+) and Pten floxed (Ptenfl/fl) animals were purchased from The Jackson Laboratory. Granzyme B Cre transgenic animals were the kind gift of Joshy Jacob (Emory University, Atlanta, GA). P14 and P14 IL-7Rα transgenic (IL-7Rαtg) mice have been previously described (17). To make P14 chimeric mice, small numbers (∼2,500) of Wt or P14 CD8 T cells were adoptively transferred into C57BL/6 mice that were subsequently infected with 2 × 105 pfu LCMV Armstrong i.p. For secondary infection, C57BL/6 mice containing ∼2,500 P14 CD8 T cells were infected with 2 × 104 cfu recombinant Listeria monocytogenes i.v. that expresses the LCMV GP33–41 epitope (strain XFL203, but referred to as Listeria-GP33) (3). All experiments were done with approved Institutional Animal Care and Use Committee protocols.
Retroviral Transfection.
Activated P14 TCR transgenic T cells were transfected with murine stem cell virus retroviruses containing CA-AKT, CA-STAT5, or DN-STAT5 and coexpressing Thy1.1, as described previously (10, 26). CA-AKT, DN-STAT5, and CA-STAT5 retroviruses have been described previously (25, 48).
Cell Sorting, CFSE Labeling, and Adoptive Transfer.
Splenocytes containing Wt P14 CD8 T cells or CA-AKT/CA-STAT5 retrovirally transfected cells were isolated from LCMV-infected mice, stained with antibodies to CD8, Thy1.1, KLRG1, and/or Ly5.1, and then sorted using the FACSAria (BD Biosciences) instrument. Alternatively, activated Wt or Pten−/− T cells were sorted by expression of CD8, CD44, and KLRG1. In all experiments, splenocytes were depleted of non-CD8 T cells by magnetic separation before sorting/adoptive transfer. In homeostatic turnover experiments, equal numbers (∼1 × 106) of P14 retrovirally transfected memory (d30 p.i.) CD8 T cells were transferred i.v. into naïve C57BL/6 mice. Cells were labeled with CFSE (Invitrogen) per the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank A. Poholek, J. Rathmell, G. Shadel, B. Su, J. Mandl, Y. Belkaid, and the members of the Kaech laboratory for helpful comments and discussions; Z. Zhao and G. Lyon for technical assistance; J. Jacob for the Granzyme B Cre mice; S. Haxhinasto and C. Benoist for the CA-AKT retrovirus; and A. Abbas and H. Dooms for the CA-STAT5 and DN-STAT5 retroviruses. This work was supported by National Institutes of Health Grant R01 AI066232-01 (to S.M.K.) and the Cancer Research Institute (S.M.K.) and a Canadian Institutes of Health Research Doctoral Research Award (MDR 75905) (to T.W.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.R.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003457107/-/DCSupplemental.
References
- 1.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 2.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 4.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Q, et al. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Wojciechowski S, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huster KM, et al. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi NS, Kaech SM. Effector CD8 T cell development: A balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 9.Jordan KA, et al. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infect Immun. 2009;77:3894–3901. doi: 10.1128/IAI.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 12.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-γ production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 15.Hikono H, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborne LC, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R α mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci USA. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haring JS, et al. Constitutive expression of IL-7 receptor α does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 19.Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 22.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 23.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 25.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandele A, et al. Formation of IL-7Rαhigh and IL-7Rαlow CD8 T cells during infection is regulated by the opposing functions of GABPα and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue HH, et al. IL-2 negatively regulates IL-7 receptor α chain expression in activated T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yajima T, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 30.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 31.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord JD, McIntosh BC, Greenberg PD, Nelson BH. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 33.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harir N, et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood. 2007;109:1678–1686. doi: 10.1182/blood-2006-01-029918. [DOI] [PubMed] [Google Scholar]
- 35.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 36.Heffner M, Fearon DT. Loss of T cell receptor-induced Bmi-1 in the KLRG1(+) senescent CD8(+) T lymphocyte. Proc Natl Acad Sci USA. 2007;104:13414–13419. doi: 10.1073/pnas.0706040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HR, Hwang KA, Kang I. Dual roles of IL-15 in maintaining IL-7RαlowCCR7− memory CD8+ T cells in humans via recovering the phosphatidylinositol 3-kinase/AKT pathway. J Immunol. 2007;179:6734–6740. doi: 10.4049/jimmunol.179.10.6734. [DOI] [PubMed] [Google Scholar]
- 38.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly J, et al. A role for Stat5 in CD8+ T cell homeostasis. J Immunol. 2003;170:210–217. doi: 10.4049/jimmunol.170.1.210. [DOI] [PubMed] [Google Scholar]
- 41.Burchill MA, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: Development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 42.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu N, et al. Serine protease inhibitor 2A is a protective factor for memory T cell development. Nat Immunol. 2004;5:919–926. doi: 10.1038/ni1107. [DOI] [PubMed] [Google Scholar]
- 44.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 45.Moon JJ, Rubio ED, Martino A, Krumm A, Nelson BH. A permissive role for phosphatidylinositol 3-kinase in the Stat5-mediated expression of cyclin D2 by the interleukin-2 receptor. J Biol Chem. 2004;279:5520–5527. doi: 10.1074/jbc.M308998200. [DOI] [PubMed] [Google Scholar]
- 46.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Intlekofer AM, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onishi M, et al. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.