During the past decade, the roundworm Caenorhabditis elegans has become a popular model for the study of host/pathogen relationships, leading to a wealth of information about microbial virulence factors and host defense pathways (1). Although the complicated interactions between C. elegans and the many pathogens that it encounters in the soil have become clearer in recent years, there is still much to learn. What are the cues that worms use to detect food sources? How do worms choose which bacterial species to eat and which to leave alone? Once a pathogen is encountered, what are the microbial killing mechanisms and the nematode’s survival mechanisms? The paper by Niu et al. (2) in PNAS provides a rare 360° view of one C. elegans/pathogen relationship. It describes the Bacillus nematocida signals that attract C. elegans, the virulence factors it uses to kill worms from within, and the specific host proteins targeted.
Smells Good Enough to Eat
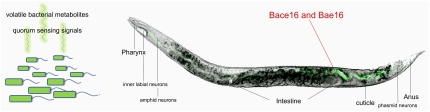
Roundworms burrowing through the soil encounter thousands of species of bacteria, but how do they find safe food choices on this vast buffet? A major part of the nematode’s ability to distinguish between food sources relies on a sophisticated chemosensory system that enables it to sense and respond to a wide range of volatile and water-soluble chemicals (3). This network of 32 chemosensory neurons, located at both the anterior and the posterior ends of the nematode (amphid, phasmid, and inner labial neurons in Fig. 1), facilitates olfactory chemotaxis, allowing worms to move toward or away from odors associated with food. More than 5% of C. elegans genes and ≈1,000 G-protein–coupled chemoreceptors mediate odor perception, hinting at the magnitude of its importance (4). Bargmann’s group and others have identified a wide array of chemicals that either attract or repel C. elegans (3), and it has long been observed that C. elegans displays distinct preferences in food choice (5–7); however, the specific identity of the attractants and repellants responsible for chemotaxis in the worm’s natural environment has remained largely unknown.
Fig. 1.
C. elegans uses a sophisticated chemosensory system to sense and move toward microbial by-products in its environment. Once a pathogen is consumed, it may kill worms slowly by dividing within the worm’s intestine and causing a prolonged, possibly disseminated infection or kill them quickly by releasing diffusible toxins. However, in PNAS, Niu et al. (2) describe a virulence strategy by which B. nematocida attracts C. elegans with a variety of volatile organic compounds and, once consumed, kills the worm by excreting two proteases, Bace16 and Bae16, which target host proteins essential for intestinal homeostasis.
C. elegans is attracted and repelled by some amino acids and bacterial metabolites, which in theory could serve as food choice odorants (3), and a few recent studies have matched specific attractants and/or repellants to the microbial species producing them. For example, after observing that quorum sensing (QS) strains of Pseudomonas aeruginosa were more attractive to C. elegans than QS-negative strains, Beale et al. (8) demonstrated that worms were attracted to the acylated homoserine lactones that serve as interbacterial chemical signals that facilitate QS in Pseudomonas and other Gram-negative bacteria. Pradel et al. (9) later reported that the cyclic lipodepsipentapeptide, serrawettin W2, was a C. elegans repellant produced by Serratia marcescens. In PNAS, Niu et al. (2) identify several volatile organic compounds (VOCs) as attractants for B. nematocida and Escherichia coli.
Niu et al. (2) use a simple migration assay to demonstrate the intense attraction of C. elegans to B. nematocida. When a lawn of B. nematocida was placed on an inverted agar layer in the upper lid of a petri plate, 56% of the nematodes migrated toward the bacterial lawn by laboriously climbing up the walls of the petri dishes. Because only 1.8% and 12% of worms migrated to agar alone or to a lawn of E. coli (the worm’s normal dietary species in the laboratory), respectively, the authors speculated that B. nematocida produced one or more volatile compounds that attracted C. elegans. Using solid-phase microextraction–gas chromatographic–mass spectrometric analysis, Niu et al. (2) identify 17 distinct VOCs from cultures of B. nematocida, and of the several compounds that were tested individually as odorants, benzyl benzoate, benzaldehyde, 2-heptanone, and acetophenone, each showed potent nematode-attracting abilities. Although benzaldehyde and 2-heptanone have previously been reported as attractants for C. elegans (3), this report identifies them as attractants produced by a specific bacterial species and provides more evidence that pathogenic microbes can use this olfactory chemotaxis to attract nematodes. Interestingly, benzyl benzoate was previously reported to repel C. elegans (3). Given that many chemicals can serve as odorants at one concentration and repellants at others, it will be important to ascertain the concentration of odorants expected to be produced in situ.
Bacteria Bite Back
One could reason that producing a nematode repellant would be advantageous to bacteria in the soil, but could the chemoattractive nature of these microbial products actually benefit bacteria as well? There are dozens of bacterial species pathogenic to C. elegans that kill worms by a variety of mechanisms. Typically, pathogens that are consumed by worms accumulate in the intestine and kill either by a persistent infection, which causes significant intestinal distension and death over a period of days, or by a diffusible toxin-mediated “fast-killing” (reviewed in ref. 10). Less frequently, some pathogens kill by direct invasion from the outside. For example, Brevibacillus laterosporus and some pathogenic fungi secrete proteases that degrade the worm’s cuticle and cause an invasive, disseminated lethal infection (10, 11). Previously, there had been no reports of ingested pathogens that invade intestinal cells from the digestive tract, which suggested that C. elegans intestinal cells were remarkably resistant to microbial invasion. However, in PNAS, Niu et al. (2) describe two proteases produced by B. nematocida that target the intestine from within.
Niu et al. (2) find that the culture filtrates from B. nematocida strains incapable of producing the serine and neutral proteases, Bace16 and Bae16, respectively, displayed significantly lower levels of proteolytic and nematocidal activities. Whereas 90% of worms were alive after a 5-d exposure to E. coli culture filtrates, only 5% were alive after a 5-d exposure to culture filtrate from wild-type B. nematocida. However, 80% of nematodes were still viable after exposure to the filtrates of a double bace16/bae16 knockout strain. Localization experiments with fluorescence-tagged proteins demonstrated that the two proteases localized mainly to the intestine of nematodes and correlated with severe intestinal damage, including disordered and loose intestinal walls and destabilized microvilli along the brush border.
Previously, the major proposed mechanism for protease-mediated microbial pathogenesis has been via the breakdown of the nematode cuticle. Thus, Niu et al. (2) tested whether crude protease extracts from wild-type B. nematocida or bace16/bae16 knockouts would cause nematode mortality either when microinjected into the intestine or when applied to their cuticle. In support of their localization experiments, Niu et al. (2) observe that C. elegans receiving intestinal microinjections of protease extracts from wild type, but not from the mutant strains, displayed significantly higher levels of mortality than those treated externally with proteases. Niu et al. (2) next use high-resolution, 2D gel electrophoresis to identify the host proteins
Niu et al. propose that B. nematocida actively lures in C. elegans by producing odorants.
within the nematode epithelia targeted by B. nematocida proteases. Twelve nematode proteins were deemed as preferential targets because their expression decreased more than threefold after 1 h of treatment with Bace16/Bae16. Several of the identified proteins, including PEPCK and VHA-8, are essential for intestinal function and support the conclusion that B. nematocida excretes proteases that act on the nematode intestine and represents a unique mechanism of pathogenesis.
Finicky Eater
Niu et al. (2) propose that B. nematocida actively lures in C. elegans by producing odorants that serve as attractants and that, once inside the intestine, uses a “Trojan horse” mechanism to kill the worms. This implies that these encounters are not simply random, but that specific predatory mechanisms have evolved in bacteria. This is an interesting concept that undoubtedly needs further investigation because if microbes are the true predators, is C. elegans completely defenseless to their baits and lures? It turns out that the worm is not as dumb as it looks. In fact, C. elegans can use olfactory learning to recognize the odorants produced by some pathogens as cues to avoid them (6, 8). Thus, odors that served as attractants to naive worms may become repellants after the worms are exposed to the pathogens excreting them. Furthermore, because many of the bacterial metabolites that C. elegans finds attractive are produced by friends and foes alike, these olfactory learning mechanisms must be highly specific but adaptable. It remains to be seen whether C. elegans can learn to avoid the VOCs produced by B. nematocida after it has been exposed to the pathogen and, if so, whether this negative conditioning will cause the worm to avoid all microbes secreting similar VOCs.
Acknowledgments
Research in the Rumbaugh Laboratory is supported by the American Diabetes Association.
Footnotes
The author declares no conflict of interest.
See companion article on page 16631.
References
- 1.Kurz CL, Ewbank JJ. Infection in a dish: High-throughput analyses of bacterial pathogenesis. Curr Opin Microbiol. 2007;10:10–16. doi: 10.1016/j.mib.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Niu Q, et al. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc Natl Acad Sci USA. 2010;107:16631–16636. doi: 10.1073/pnas.1007276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 4.Bargmann CI. Chemosensation in C. elegans. In: The C. elegans Research Community, editor. WormBook. 2006. Available at doi/10.1895/wormbook.1.7.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrew PA, WL Effect of bacteria on dispersal of Caenorhabditis elegans (Rhabditidae) Nematologica. 1976;22:451–461. [Google Scholar]
- 6.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 7.Abada EA, et al. C. elegans behavior of preference choice on bacterial food. Mol Cells. 2009;28:209–213. doi: 10.1007/s10059-009-0124-x. [DOI] [PubMed] [Google Scholar]
- 8.Beale ELG, Li G, Tan MW, Rumbaugh KP. Caenorhabditis elegans senses bacterial autoinducers. Appl Environ Microbiol. 2006;72:5135–5137. doi: 10.1128/AEM.00611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradel E, et al. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:2295–2300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sifri CD, Begun J, Ausubel FM. The worm has turned: Microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13:119–127. doi: 10.1016/j.tim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, et al. An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res Microbiol. 2005;156:719–727. doi: 10.1016/j.resmic.2005.02.006. [DOI] [PubMed] [Google Scholar]