Abstract
It is well established that acute administration of adrenocortical hormones enhances the consolidation of memories of emotional experiences and, concurrently, impairs working memory. These different glucocorticoid effects on these two memory functions have generally been considered to be independently regulated processes. Here we report that a glucocorticoid receptor agonist administered into the medial prefrontal cortex (mPFC) of male Sprague-Dawley rats both enhances memory consolidation and impairs working memory. Both memory effects are mediated by activation of a membrane-bound steroid receptor and depend on noradrenergic activity within the mPFC to increase levels of cAMP-dependent protein kinase. These findings provide direct evidence that glucocorticoid effects on both memory consolidation and working memory share a common neural influence within the mPFC.
Keywords: prelimbic cortex, basolateral amygdala, corticosterone, norepinephrine, protein kinase A
Acute exposure to glucocorticoid hormones has different effects on memory consolidation and working memory: memory consolidation is typically enhanced, whereas working memory is impaired (1–4). Investigations of glucocorticoid effects on memory consolidation and working memory have generally assumed these memory processes to be independently regulated processes by different neuroanatomical systems. Considerable evidence indicates that glucocorticoid-induced enhancement of memory consolidation of emotionally arousing experiences involves a neural circuitry of interacting brain regions that include the basolateral complex of the amygdala (BLA) and its projections to efferent brain regions (5–7), whereas glucocorticoid impairment of working memory depends primarily on influences within the medial prefrontal cortex (mPFC) (3), a brain region involved in higher-order cognitive and affective processing, as well as executive function (8, 9). However, there is also extensive evidence that the BLA and mPFC are anatomically and functionally interconnected (10–12). Maintenance of mPFC activity is known to constrain BLA activity, whereas stress and glucocorticoids alter mPFC functioning, thereby increasing BLA responses to emotionally arousing stimuli (13). We recently reported that stress-level glucocorticoid effects on both memory consolidation and working memory depend critically on interactions between the BLA and mPFC (3, 14). Moreover, the evidence that these two brain regions are inversely coupled (15, 16; but see ref. 12 for an alternative view) suggests that interactions between these brain regions may underlie the different influences of glucocorticoids on memory consolidation and working memory. Thus, it seems possible that stress exposure or glucocorticoid administration alters mPFC activity in such a way that it impairs working memory and concurrently enhances memory consolidation. The present experiments investigated the hypothesis that these opposing glucocorticoid effects on working memory and memory consolidation are intrinsically linked and mediated by a common neural mechanism within the mPFC.
Results
Glucocorticoid Administration into the mPFC Impairs Working Memory and Enhances Memory Consolidation.
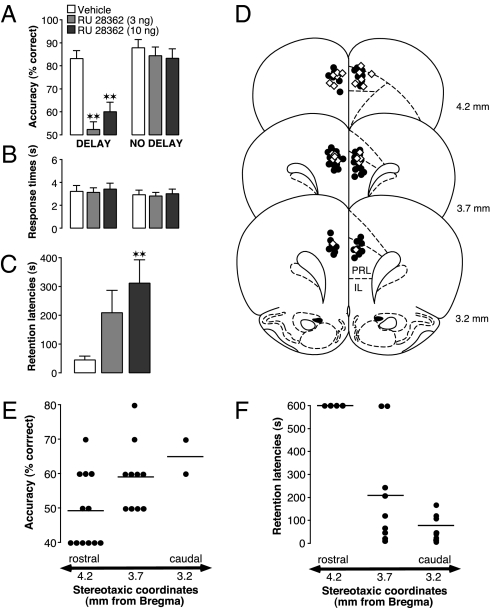
We first investigated whether bilateral infusions of the specific glucocorticoid receptor (GR) agonist RU 28362 administered into the mPFC of male Sprague-Dawley rats produce opposite effects on working memory and memory consolidation. Working memory was assessed in a delayed alternation paradigm in a T-maze. Successful performance of this task requires memory of spatial information during a delay period between trials, together with sustained attention and inhibition of inappropriate behavioral responses (3, 17). As is shown in Fig. 1A, both doses of the GR agonist (3 or 10 ng in 0.5 μL), administered into the mPFC 60 min before cognitive testing, significantly impaired working memory performance, as assessed by reduced task accuracy (F2,22 = 24.95; P < 0.0001; repeated-measures ANOVA). Importantly, the GR agonist infusions did not impair rats’ performance on a control task without delays between trials (F2,16 = 0.38; P = 0.69). Additionally, the GR agonist infusions did not affect motivational or locomotor aspects of the task, as indicated by unaltered response times to reach the choice point in the maze (F2,22 = 0.15; P = 0.86; Fig. 1B). Memory consolidation was investigated, in separate groups of rats, in a single-trial inhibitory avoidance task in which rats display long-term memory of the place in an apparatus where they received footshock (18). As is shown in Fig. 1C, RU 28362 (3 or 10 ng in 0.5 μL) administered into the mPFC immediately after inhibitory avoidance training dose-dependently enhanced long-term memory, as indicated by longer entrance latencies on a 48-h retention-test trial (F2,29 = 4.33; P = 0.02; one-way ANOVA). Histological examination of the injection sites of animals used in these experiments revealed that cannula placement was distributed along the rostro-caudal axis of the prelimbic region of the mPFC (Fig. 1D). However, the GR agonist infusions most effectively modulated both memory functions when administered into the rostral pole of the mPFC, whereas more caudal infusions were less effective (Fig. 1 E and F).
Fig. 1.
Glucocorticoid administration into the mPFC impairs working memory and enhances memory consolidation. (A) The GR agonist RU 28362 (3 or 10 ng) infused bilaterally into the mPFC 60 min before working memory testing impaired delayed alternation performance (n = 12) but not performance on a control task without delays between trials (n = 9). Results represent mean ± SEM percentage correct choices. **P < 0.01 vs. vehicle. (B) RU 28362 did not affect response times on either task. (C) RU 28362 (3 or 10 ng) infused into the mPFC immediately after inhibitory avoidance training enhanced 48-h retention latencies (n = 10–11). Data represent 48-h retention latencies (mean ± SEM) in seconds. **P < 0.01 vs. vehicle. (D) Infusions were distributed along the rostro-caudal axis of the prelimbic region of the mPFC (delayed alternation: open diamonds, n = 12; inhibitory avoidance: black circles, n = 32). PRL, prelimbic; IL, infralimbic. (E) Correlation between cannula location within the mPFC and response accuracy on the delayed alternation task after infusions of RU 28362 (3 or 10 ng). Circles represent individual cases. Lines show the mean accuracy per location. (F) Correlation between cannula placement within the mPFC and 48-h inhibitory avoidance retention latencies of animals given posttraining infusions of RU 28362 (3 or 10 ng).
Unilateral Glucocorticoid Administration into the mPFC Does Not Impair Working Memory but Enhances Memory Consolidation.
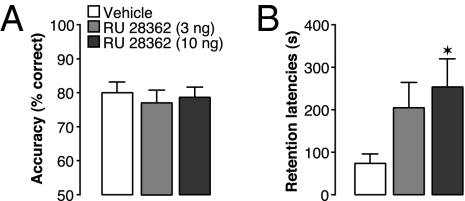
To determine whether GR agonist effects in the mPFC on both memory functions are functionally linked, we investigated whether impaired working memory is essential for enhancement of long-term memory. A temporary decrement of working memory capacity might inhibit interference, thus protecting newly encoded information from distraction (19, 20), and thereby enable enhancement of memory of emotionally significant experiences. If this were the case, then GR agonist administration into the mPFC should enhance memory consolidation only under conditions that impair working memory. To investigate this, we administered the GR agonist unilaterally into the left mPFC either 60 min before delayed alternation testing or, in other animals, immediately after inhibitory avoidance training. As is shown in Fig. 2, a unilateral infusion of RU 28362 (3 or 10 ng in 0.5 μL) did not impair working memory (F2,14 = 0.16; P = 0.86; repeated-measures ANOVA; Fig. 2A), most likely because working memory could be maintained by the untreated mPFC. However, and most importantly, a unilateral GR agonist infusion into the left mPFC was sufficient to enhance long-term memory of inhibitory avoidance training (F2,33 = 3.74; P = 0.03; one-way ANOVA; Fig. 2B). Thus, these findings indicate that GR agonist-induced strengthening of memory consolidation is produced under experimental conditions (i.e., unilateral stimulation of the mPFC) that do not impair working memory.
Fig. 2.
Unilateral glucocorticoid administration into the mPFC does not impair working memory but enhances memory consolidation. (A) The GR agonist RU 28362 (3 or 10 ng) infused unilaterally into the left mPFC 60 min before working memory testing did not impair delayed alternation performance (n = 8). Results represent mean ± SEM percentage correct choices. (B) RU 28362 (3 or 10 ng) infused unilaterally into the left mPFC immediately after inhibitory avoidance training enhanced 48-h retention latencies (n = 10–14). Data represent 48-h retention latencies (mean ± SEM) in seconds. *P < 0.05 vs. vehicle.
Glucocorticoid Effects in the mPFC on Both Working Memory and Memory Consolidation Require β-Adrenoceptor-cAMP/Protein Kinase A Activity.
To determine whether working memory impairment and memory consolidation enhancement produced by bilateral glucocorticoid infusions are linked because of the recruitment of a common intracellular mechanism, we examined whether GR agonist effects on these two memory functions both depend on noradrenergic activity within the mPFC. We focused here on the noradrenergic system because prior evidence from our laboratory indicates that glucocorticoids crucially depend on training-induced noradrenergic activity within the BLA to enhance memory consolidation of emotionally arousing experiences (18, 21–23). Moreover, stress-induced activation of the noradrenergic system within the mPFC is known to induce working memory impairment (9), whereas a systemically administered β-adrenoceptor antagonist blocks corticosterone-induced working memory impairment (3).
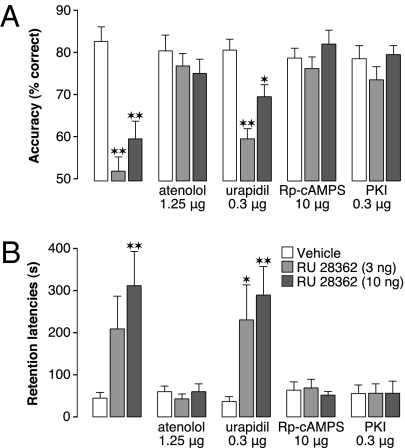
Blockers of different components of the noradrenergic system were administered into the mPFC together with the GR agonist (3 or 10 ng in 0.5 μL) either 60 min before working memory testing or immediately after inhibitory avoidance training. Two-way ANOVA for accuracy on the delayed alternation task revealed significant GR agonist (F2,100 = 21.85; P < 0.0001), noradrenergic inhibitor (F4,100 = 9.34; P < 0.0001), and interaction effects (F8,100 = 5.87; P < 0.0001). Highly comparably, two-way ANOVA for 48-h inhibitory avoidance retention latencies revealed significant GR agonist (F2,136 = 6.76; P = 0.002), noradrenergic inhibitor (F4,136 = 7.84; P < 0.0001), and interaction effects (F8,136 = 2.86; P = 0.006). As is shown in Fig. 3 A and B, a low dose of the β-adrenoceptor antagonist atenolol (1.25 μg) administered into the mPFC did not affect working memory or memory consolidation when administered alone but completely blocked the GR agonist-induced working memory impairment and memory consolidation enhancement. In contrast, the α1-adrenoceptor antagonist urapidil (0.3 μg) did not block either the GR agonist-induced working memory impairment or the memory consolidation enhancement. Unlike the α1-adrenoceptor, the β-adrenoceptor is coupled directly to adenylate cyclase to stimulate the intracellular cAMP/protein kinase A (PKA) signaling pathway. Previous findings indicated that, when administered into the BLA, a β-adrenoceptor, but not α1-adrenoceptor, antagonist blocks glucocorticoid effects on memory consolidation (21). In further support of the view that β-adrenoceptor activation stimulates the cAMP/PKA signaling pathway, we found that the cAMP inhibitor Rp-cAMPS (10 μg) as well as the selective PKA inhibitor PKI 14-22 amide (0.3 μg) also blocked the GR agonist effects on both working memory and memory consolidation (Fig. 3 A and B).
Fig. 3.
Glucocorticoid effects in the mPFC on both working memory and memory consolidation require β-adrenoceptor–cAMP/PKA activity. (A) The β-adrenoceptor antagonist atenolol (1.25 μg), cAMP inhibitor Rp-cAMPS (10 μg), or PKA inhibitor PKI 14-22 amide (0.3 μg), but not α1-adrenoceptor antagonist urapidil (0.3 μg), infused bilaterally into the mPFC 60 min before working memory testing blocked the impairing effects induced by concurrent infusions of the GR agonist RU 28362 (3 or 10 ng) on delayed alternation performance. Results represent mean ± SEM percentage correct choices. *P < 0.05; **P < 0.01 vs. vehicle (n = 9–13). (B) The β-adrenoceptor antagonist atenolol (1.25 μg), cAMP inhibitor Rp-cAMPS (10 μg), or PKA inhibitor PKI 14-22 amide (0.3 μg), but not α1-adrenoceptor antagonist urapidil (0.3 μg), infused into the mPFC immediately after inhibitory avoidance training blocked the enhancing effects induced by concurrent infusions of RU 28362 (3 or 10 ng) on 48-h retention performance. Data represent 48-h retention latencies (mean ± SEM) in seconds. *P < 0.05; **P < 0.01 vs. vehicle (n = 9–11).
Glucocorticoids Increase PKA Activity in the mPFC in Relation to Working Memory and Memory Consolidation.
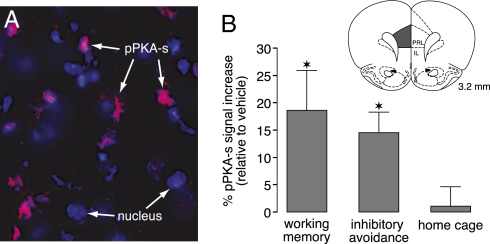
Because previous evidence indicates that an activation of PKA within the mPFC impairs working memory (17), whereas increased cAMP/PKA activity within the BLA and hippocampus seems necessary for influencing long-term neuroplasticity and memory formation (24, 25), we examined next whether glucocorticoid administration influences memory by increasing PKA activity in the mPFC. Corticosterone (1 mg/kg), the rat’s endogenous glucocorticoid, or vehicle was administered s.c. either 30 min before delayed alternation testing or immediately after inhibitory avoidance training, and the rats were killed 30 min after drug administration. To determine PKA activity, we assessed phosphorylated PKA substrate (pPKA-s) levels. Changes in pPKA-s immunoreactivity are reported to be a reliable and sensitive measure of altered PKA activity (26). As is shown in Fig. 4A, pPKA-s immunoreactivity was found in ≈10–20% of mPFC neurons (i.e., within the rostral area of the prelimbic cortex). Corticosterone increased total pPKA-s immunoreactivity levels in the mPFC assessed after working memory testing by 18.6% as compared with that of vehicle-treated rats (P = 0.04; paired t test), whereas a 14.5% increase in pPKA-s immunoreactivity was found in the mPFC of rats administered corticosterone after inhibitory avoidance training (P = 0.01; Fig. 4B). This PKA activity most likely reflects a general arousal effect rather than being a direct measure of task performance. In support of the view that glucocorticoids require arousal-associated noradrenergic activation in both increasing PKA activity and inducing cognitive effects (22), corticosterone administration to home cage control rats did not increase pPKA-s immunoreactivity levels in the mPFC (1.1% ± 3.5%; P = 0.77).
Fig. 4.
Glucocorticoids increase PKA activity in the mPFC in relation to working memory and memory consolidation. (A) Illustration of pPKA-s immunoreactivity (magenta) in a subpopulation of mPFC neurons as visualized by a DAPI nuclear counterstain. (B) Systemic corticosterone (1 mg/kg) increased pPKA-s immunoreactivity levels in the mPFC of animals subjected to either working memory testing (n = 7) or inhibitory avoidance training (n = 6), but not in home cage controls (n = 5), as compared with simultaneously processed vehicle-injected rats. Average pPKA-s immunoreactivity levels in the mPFC were determined in the dark area shown in the diagram. *P < 0.05 vs. vehicle.
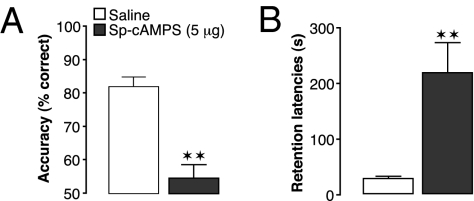
To determine whether this glucocorticoid-induced increase in PKA activity within the mPFC is sufficient to mediate glucocorticoid effects on working memory and memory consolidation, the PKA activator Sp-cAMPS (5 μg in 0.5 μL) or saline was administered into the mPFC either 60 min before delayed alternation testing or immediately after inhibitory avoidance training. Like the GR agonist, Sp-cAMPS infusions impaired working memory (F1,10 = 22.13; P = 0.0008; repeated-measures ANOVA; Fig. 5A) and enhanced memory consolidation (P = 0.002; t test; Fig. 5B).
Fig. 5.
Activation of PKA in the mPFC is sufficient to impair working memory and enhance memory consolidation. (A) The selective PKA activator Sp-cAMPS (5 μg in 0.5 μL) infused bilaterally into the mPFC 60 min before working memory testing impaired delayed alternation performance (n = 11; repeated-measures ANOVA). Results represent mean ± SEM percentage correct choices. **P < 0.01 vs. saline. (B) Sp-cAMPS (5 μg in 0.5 μL) infused into the mPFC immediately after inhibitory avoidance training enhanced 48-h retention latencies (n = 11–12). Data represent 48-h retention latencies (mean ± SEM) in seconds. **P < 0.01 vs. saline.
Activation of a Membrane Steroid Receptor in the mPFC Impairs Working Memory and Enhances Memory Consolidation.
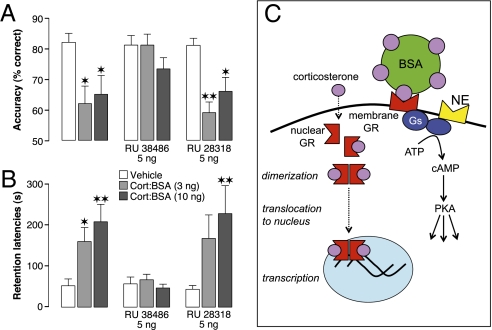
How do glucocorticoids influence PKA activity in regulating working memory and memory consolidation? Although the classically recognized mode of action of glucocorticoids is to alter gene transcription via direct translocation of ligand–receptor dimer complexes into the nucleus (27), such genomic effects do not seem to account for the rapid onset of corticosterone effects on PKA activity (21). Findings of several studies suggest that some behavioral and physiological effects of glucocorticoids occur too rapidly to be mediated by alterations in gene transcription (28). Emerging evidence indicates that these nongenomic effects of glucocorticoids occur through the activation of G protein-coupled receptors or membrane-associated cytosolic steroid receptors (29–31). Our findings suggest that glucocorticoids modulate both working memory and memory consolidation by activating such a putative cell-surface receptor. As is shown in Fig. 6A, corticosterone conjugated to a membrane-impermeable BSA molecule (cort:BSA; 3 or 10 ng in 0.5 μL) infused into the mPFC 60 min before delayed alternation testing significantly impaired working memory performance. Moreover, the same doses of cort:BSA enhanced 48-h inhibitory avoidance retention latencies when administered immediately after the training session (Fig. 6B). Coadministration of the GR antagonist RU 38486 (5 ng), but not the mineralocorticoid receptor antagonist RU 28318 (5 ng), blocked the cort:BSA effects on both working memory and memory consolidation. Two-way ANOVA for accuracy on the delayed alternation task revealed significant cort:BSA (F2,52 = 11.76; P < 0.0001) and steroid receptor antagonist effects (F2,52 = 5.91; P = 0.008), as well as a significant interaction between both factors (F4,52 = 2.63; P = 0.04). Two-way ANOVA for 48-h inhibitory avoidance retention latencies also revealed significant cort:BSA (F2,81 = 10.11; P < 0.0001), steroid receptor antagonist (F2,81 = 7.14; P = 0.001), and interaction effects (F4,81 = 2.77; P = 0.03). These findings suggest that both the glucocorticoid-induced impairment of working memory and enhancement of memory consolidation depend on activation of a putative membrane-associated GR. Fig. 6C summarizes the proposed action of glucocorticoids, via membrane GRs, on the noradrenergic signaling cascade as well as its classic genomic action.
Fig. 6.
Activation of a membrane steroid receptor in the mPFC impairs working memory and enhances memory consolidation. (A) The membrane-impermeable ligand cort:BSA (3 or 10 ng) infused into the mPFC 60 min before working memory testing impaired delayed alternation performance. Coinfusion of the GR antagonist RU 38486 (5 ng), but not mineralocorticoid receptor antagonist RU 28318 (5 ng), blocked the cort:BSA effect. Results represent mean ± SEM percentage correct choices. *P < 0.05; **P < 0.01 vs. vehicle (n = 9–10). (B) Cort:BSA (3 or 10 ng) infused into the mPFC immediately after inhibitory avoidance training enhanced 48-h retention latencies. Coadministration of the GR antagonist RU 38486 (5 ng), but not mineralocorticoid receptor antagonist RU 28318 (5 ng) blocked the cort:BSA effect. Data represent 48-h retention latencies (mean ± SEM) in seconds. *P < 0.05; **P < 0.01 vs. vehicle (n = 8–12). (C) Diagram of the proposed action of glucocorticoids, via membrane GRs, on the noradrenergic system as well as its classic genomic pathway. Gs, stimulatory G protein; NE, norepinephrine.
Discussion
The present findings significantly extend those of previous experiments investigating the role of the mPFC in mediating GR agonist effects on memory (3, 14). Our results show that GR agonist effects on impairment of working memory as well as enhancement of memory consolidation are mediated by activation of a membrane-bound steroid receptor and depend on noradrenergic activity within the mPFC to increase PKA levels. These findings provide direct evidence that glucocorticoid effects on both memory consolidation and working memory share a common neural influence within the mPFC.
Although treatment with RU 28362 induces a selective activation of GRs (32), there is extensive evidence that exposure to a mild stressor or systemic administration of corticosterone or cortisol induces comparable enhancement of memory consolidation (18, 33, 34) or impairment of working memory in rats, monkeys, and humans (1, 9). However, it should be noted that the influences of stress and glucocorticoids on both working memory and memory consolidation follow an inverted-U-shaped dose-response relationship and vary with the severity of the stressor, drug dosage, or other experimental conditions (9, 33–35). Importantly, stress and glucocorticoids are known to influence working memory especially under emotionally arousing test conditions, whereas the valence of the information acquired in working memory does not seem to be an important variable (36, 37). Similarly, it is well established that glucocorticoids administered shortly before or after training enhance long-term memory of both appetitively and aversively motivated emotionally arousing training experiences (4, 38, 39). Thus, it is highly unlikely that, in the present experiments, GR agonist infusions into the mPFC induced opposite effects on these cognitive tasks simply because the two tasks differed in the type of reinforcement used.
These findings provide strong evidence that acute glucocorticoid administration both enhances memory consolidation and impairs working memory via a membrane GR-induced facilitation of noradrenergic signaling mechanisms within the mPFC. Extensive evidence indicates that excessive levels of norepinephrine, such as those released during periods of stress, induce mPFC dysfunction and working memory impairment, at least in part via an activation of the intracellular cAMP/PKA signal transduction pathway (9). Accordingly, electrophysiological studies have shown that stress-level noradrenergic activation inhibits mPFC neuronal activity normally evoked in the delay period during which the information needs to be retained (8, 40). However, and most importantly, the present findings indicate that such a glucocorticoid/norepinephrine-induced disruption of mPFC functioning induces not only impairment of working memory but also enhancement of long-term memory consolidation. Findings of a recent functional imaging study in human subjects are consistent with this view and indicate that the combined administration of glucocorticoid and noradrenergic agonists during the encoding of emotionally arousing material induces a strong deactivation of the PFC and that the level of deactivation correlates positively with enhanced performance on a retention test given 1 wk later (41). It should be emphasized, however, that the consequences of glucocorticoid (or norepinephrine) administration and PKA activation on cellular physiology within the mPFC, especially during task performance, are far from clear (42). Further, although our findings indicate that both memory effects depend predominantly on changes within the rostral subdivision of the mPFC, it is presently unknown whether PKA activation induces comparable effects in all mPFC neurons within this subdivision and, thus, whether the same or different sets of neurons are responsible for mediating each memory effect. Nevertheless, our findings suggest that a glucocorticoid-induced change in mPFC functioning creates a brain state that favors the long-term storage of emotionally arousing events and, thus, preserve significant information. The temporary impairment of working memory as well as other mPFC-dependent processes (e.g., thought and decision making) observed concurrently by this pattern of brain activity might be a cost incurred to enable such enhanced memory consolidation of emotionally significant information.
Although several previous studies have suggested a role for the mPFC in memory consolidation, including inhibitory avoidance (43, 44), it is unclear whether glucocorticoid administration induces a local memory trace in the mPFC or whether manipulation of the mPFC modulates memory consolidation via interactions with other brain regions. As indicated above, findings of several electrophysiological and behavioral studies indicate that there are reciprocal connections between the mPFC and BLA (10, 12, 16) that regulate affect and memory (3, 11, 14). Basal mPFC activity is known to inhibit BLA activity, whereas stress and glucocorticoids alter mPFC functioning, thereby increasing BLA responses to emotionally arousing stimuli (3, 15). Indeed, we recently found that GR agonist infusion into the mPFC after inhibitory avoidance training induced a rapid, nongenomically mediated increase in BLA levels of phosphorylated extracellular signal-regulated kinase 1/2 (pErk1/2) (14). Blockade of this pErk1/2 activity in the BLA with the mitogen-activated protein kinase kinase inhibitor PD98059 prevented the memory enhancement, suggesting that GR agonist administration into the mPFC strengthens memory consolidation processes via modulation of BLA activity. Such BLA activation may then enhance the consolidation of different kinds of information via its widespread projections to other brain regions, including the hippocampus and neocortical regions (5, 6). In support of the view that glucocorticoid effects on working memory and memory consolidation are intrinsically linked because of a necessary interaction between the mPFC and BLA, we previously found that neurotoxic lesions of the BLA also blocked working memory impairment induced by a glucocorticoid administered either systemically or directly into the mPFC (3).
In summary, acute stress and glucocorticoids do not affect all memory functions in the same manner. Stress-level glucocorticoids typically enhance memory consolidation, yet impair working memory. The present study revealed that glucocorticoid administration into the mPFC impairs working memory and enhances memory consolidation via a common activation of the noradrenergic signaling pathway, thus strongly suggesting that glucocorticoid effects on these two memory functions are intrinsically related. These findings may also have important consequences for the development of effective strategies for treating mPFC cognitive dysfunction. Several lines of investigation proposed the use of GR antagonists, β-adrenoceptor antagonists, or PKA inhibitors as an avenue for the clinical treatment of mPFC-dependent cognitive deficits during chronic stress conditions, aging, or pathophysiology (45–47). However, because the present findings indicate that the different pharmacological treatments that blocked glucocorticoid effects on working memory also abolished the enhancement of memory consolidation, such drugs targeting preservation of working memory may inherently have undesirable effects on the consolidation of memory of emotionally significant experiences.
Materials and Methods
Subjects.
Male Sprague-Dawley rats (270–320 g) from Charles River Breeding Laboratories were housed individually in a temperature-controlled (22 °C) vivarium room (0700–1900 hours lights on). Rats trained on the working memory task were fed a diet of rat chow (15–17 g/rat per day) immediately after behavioral testing. Training and testing were performed during the light phase of the cycle between 1000 and 1500 hours. All experimental procedures were in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine and the University of Groningen.
Surgery.
Animals were adapted to the vivarium for 1 wk before surgery. They were anesthetized with sodium pentobarbital (50 mg/kg of body weight, i.p.), given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration, and were subsequently injected with 3 mL of saline to facilitate clearance of these drugs and prevent dehydration. The skull was positioned in a stereotaxic frame (Kopf Instruments), and bilateral stainless-steel guide cannulae (23 gauge) were implanted with the cannula tips 1.5 mm above the prelimbic region of the mPFC [11 mm; anteroposterior, +3.7 mm from Bregma; mediolateral, ±0.7 mm from midline; dorsoventral, −2.4 mm from skull surface; ref. 48]. For unilateral cannula placement, a cannula was placed 1.5 mm above the left mPFC. The cannulae were fixed to the skull with two anchoring screws and dental cement. Stylets (11-mm-long 00-insect dissection pins) inserted into each cannula to maintain patency were removed only for drug infusions.
Delayed Alternation.
Rats were initially habituated to the T-maze (dimensions, 100 × 90 cm) for 5 d until they were readily eating chocolate chips placed in food wells at the end of each arm. The rats were then trained on a delayed alternation task as previously described (3). Rats were placed in the start box of the maze, the gate was opened, and the animal was allowed to run to a choice point in the maze. On the first trial, food was placed in both arms and the rat was rewarded for entering either arm. Then the rat was placed back in the start box for the intertrial delay. On the subsequent trials, the rat was only rewarded for entering the arm not chosen on the previous trial. Between each trial, the choice point was wiped clean with 10% alcohol to remove olfactory cues. Each day consisted of 11 trials, although the first trial was not included in the analyses. Accuracy of response and response time were scored, with response time being the time from when the start gate was opened to when the rat made its choice and reached one food well. Rats were tested once daily, five times per week. The intertrial delay was initially set at “0” s (approx. 2–3 s) and increased in 5-s increments as needed to stabilize performance at ≈80% accuracy. Training took place until a rat scored between 70% and 90% correct for 3 consecutive days. Once a stable performance was achieved, drug treatment was initiated. Each rat received vehicle and the two doses of RU 28362 or cort:BSA, randomized across rats. Different cohorts of rats were used for each of the different antagonist treatment conditions. There was a minimum of 1 wk between drug treatments. The average intertrial delay was 14.7 ± 0.7 s for the first drug treatment (after ≈3–4 wk of training) and was increased over the study to an average of 19.2 ± 0.6 s (P < 0.0001; paired t test). Control groups of rats were trained with the intertrial delay always set at “0” s.
Inhibitory Avoidance.
The rats were trained in an inhibitory avoidance apparatus consisting of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top, and 6.4 cm wide at the bottom) divided into two compartments, separated by a sliding door that opened by retracting into the floor (18). The starting compartment (31 cm) was made of opaque white plastic and well lit; the shock compartment (60 cm) was made of dark, electrifiable metal plates and was not illuminated. Training and testing were conducted in a sound- and light-attenuated room. For training, the rats were placed in the starting compartment, facing away from the door, and were allowed to enter the shock compartment. The sliding door was closed and a single inescapable footshock (0.35 mA) was delivered for 1 s. The rats were removed from the shock compartment and, after drug treatment, returned to their home cages. On the 48-h retention test, the latency to reenter the shock compartment with all four paws (maximum latency of 600 s) was recorded. Each rat received only a single drug infusion and was never trained twice on the inhibitory avoidance task.
Drug Treatment.
The specific GR agonist RU 28362 (11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20yn-3-one, 3 or 10 ng in 0.5 μL; a gift from Aventis) was infused into the mPFC either alone or together with the specific β1-adrenoceptor antagonist atenolol (1.25 μg; Sigma-Aldrich), the specific α1-adrenoceptor antagonist urapidil (0.3 μg; Sigma-Aldrich), the cAMP inhibitor Rp-cAMPS (10 μg; Sigma-Aldrich), or the cell-permeable PKA inhibitor PKI 14-22 amide (0.3 μg; Calbiochem). Receptor binding studies have shown that RU 28362 has selective and high affinity for GRs (32). Drugs were first dissolved in 100% ethanol and subsequently diluted in 0.9% saline to reach a final ethanol concentration of 0.5%. The selective PKA activator Sp-cAMPS (5 μg; Sigma-Aldrich) was dissolved in saline. Drug infusions or an equivalent volume of vehicle into the mPFC were given by using 30-gauge injection needles connected to 10-μL Hamilton microsyringes with polyethylene (PE-20) tubing. The injection needles protruded 1.5 mm beyond the tip of the cannula, and a 0.5-μL injection volume per hemisphere was infused over a period of 35 s by an automated syringe pump (Sage Instruments). The injection needles were retained within the cannulae for 20 s after drug infusion.
Cort:BSA (3 or 10 ng in 0.5 μL; Sigma-Aldrich) was infused into the mPFC either alone or together with the specific GR antagonist RU 38486 (5 ng; Roussel-Uclaf) or the mineralocorticoid receptor antagonist RU 28318 (5 ng; Roussel-Uclaf). The use of BSA-conjugated steroid hormones is a standard approach in steroid biology to demonstrate specific membrane effects (49). The vehicle solution contained 0.5% ethanol and 0.1% BSA (Sigma-Aldrich) in saline.
Corticosterone (1 mg/kg; Sigma-Aldrich) or vehicle (5% ethanol in saline) was injected s.c. in a volume of 2 mL/kg. This dose of corticosterone is known to induce plasma corticosterone levels that resemble moderate stress (50).
Histology.
The rats were deeply anesthetized with an overdose of sodium pentobarbital and perfused transcardially with 0.9% saline followed by 4% formaldehyde solution. After decapitation, the brains were removed and immersed in fresh 4% formaldehyde. At least 24 h before sectioning, the brains were transferred to a 20% sucrose solution for cryoprotection. Coronal sections of 50 μm were cut on a freezing microtome, mounted on gelatin-coated slides, and stained with cresyl violet. For all experiments, only rats with needle tips within the boundaries of the mPFC were included in the data analysis.
Statistics.
Delayed alternation data were analyzed with one- or two-way ANOVAs with doses of RU 28362, cort:BSA, or Sp-cAMPS as repeated measure. Paired and unpaired t tests determined the source of the detected significance. Inhibitory avoidance training and retention test latencies were analyzed with one- or two-way ANOVAs with different doses of RU 28362, cort:BSA, or Sp-cAMPS and the different antagonists both as between-subject variables. Further analysis used Fisher’s post hoc tests. A probability level of <0.05 was accepted as statistical significance. Data are presented as mean ± SEM.
Immunohistochemistry.
Brains were quickly removed and flash frozen in 2-methylbutane at −30 °C for 2 min. Frozen coronal sections (20 μm) from animals that had received vehicle or corticosterone were mounted together. Sections were fixed in 2% buffered paraformaldehyde (pH 7.0–8.0) for 5 min and then permeabilized with a 1:1 mixture of ice-cold acetone–methanol for 5 min. Endogenous peroxidase activity was blocked with a 2% hydrogen peroxide solution (pH 7.4) for 15 min. Background staining was blocked in tyramide signal amplification blocking buffer for 30 min. Sections were incubated overnight with the primary antibody pPKA-s rabbit monoclonal (1:200; 100G7E; Cell Signaling). The slides were rinsed in Trizma-buffered saline (TBS; pH 7.4; Sigma-Aldrich) between each step. Sections were incubated with AffiniPure goat anti-rabbit IgG conjugated with HRP (1:1,000; Jackson ImmunoResearch) for 2 h, and the conjugate was detected by incubating sections in TSA-cyanine3-tyramide (CY3) (PerkinElmer) for 30 min. Sections were counterstained with DAPI for 20 min.
Data Analysis.
Slides were scanned with a Typhoon Variable Mode Imager (Amersham) at a 10-μm resolution to detect CY3 fluorescence. Photomultiplier tube settings were varied from 470 to 490 V to achieve a broad distribution of fluorescent intensity. Images were analyzed using ImageQuant TL v2005 (Amersham). The prelimbic region of the mPFC was roughly outlined in both hemispheres, and its mean fluorescent intensity was normalized to the ipsilateral olfactory bulb. Normalized values were then averaged across hemispheres. Measurements from animals treated with corticosterone were normalized again to the corresponding vehicle control on each slide. Data were analyzed using paired t tests.
Acknowledgments
We thank Gabriel Hui for preparation of the figures and Teiko Miyashita for assistance with the immunofluorescence study. Research was supported by National Science Foundation Grant IOB-0618211 (to B.R.) and National Institute of Mental Health Grant MH12526 (to J.L.M.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 7.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 8.Fuster JM. The prefrontal cortex and its relation to behavior. Prog Brain Res. 1991;87:201–211. doi: 10.1016/s0079-6123(08)63053-8. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res. 1991;564:97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- 11.Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 12.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amat J, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 14.Roozendaal B, et al. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor JR, Birnbaum S, Ubriani R, Arnsten AFT. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapur S, et al. Functional role of the prefrontal cortex in retrieval of memories: A PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 20.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- 21.Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor-cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 22.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Stegeren AH, et al. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Bernabeu R, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sindreu CB, Scheiner ZS, Storm DR. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oitzl MS, Reichardt HM, Joëls M, de Kloet ER. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc Natl Acad Sci USA. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol. 2008;29:273–291. doi: 10.1016/j.yfrne.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 30.Karst H, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill MN, McEwen BS. Endocannabinoids: The silent partner of glucocorticoids in the synapse. Proc Natl Acad Sci USA. 2009;106:4579–4580. doi: 10.1073/pnas.0901519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teutsch G, et al. 17 alpha-alkynyl-11 beta, 17-dihydroxyandrostane derivatives: A new class of potent glucocorticoids. Steroids. 1981;38:651–665. doi: 10.1016/0039-128x(81)90084-2. [DOI] [PubMed] [Google Scholar]
- 33.Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 34.Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 35.Yuen EY, et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Micheau J, Destrade C, Soumireu-Mourat B. Time-dependent effects of posttraining intrahippocampal injections of corticosterone on retention of appetitive learning tasks in mice. Eur J Pharmacol. 1984;106:39–46. doi: 10.1016/0014-2999(84)90675-7. [DOI] [PubMed] [Google Scholar]
- 39.Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem. 2002;78:458–464. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]
- 40.Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 41.van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93:56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo LA, et al. A link between role of two prefrontal areas in immediate memory and in long-term memory consolidation. Neurobiol Learn Mem. 2007;88:160–166. doi: 10.1016/j.nlm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Belanoff JK, Jurik J, Schatzberg LD, DeBattista C, Schatzberg AF. Slowing the progression of cognitive decline in Alzheimer’s disease using mifepristone. J Mol Neurosci. 2002;19:201–206. doi: 10.1007/s12031-002-0033-3. [DOI] [PubMed] [Google Scholar]
- 46.Ramos BP, et al. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 47.Ramos BP, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. San Diego: Academic; 2005. [DOI] [PubMed] [Google Scholar]
- 49.Zheng J, Ali A, Ramirez VD. Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. J Psychiatry Neurosci. 1996;21:187–197. [PMC free article] [PubMed] [Google Scholar]
- 50.de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]