Abstract
Upon antigen (Ag) encounter, B cells require T-cell help to enter the germinal center (GC). They obtain this help by presenting Ag-derived peptides on MHC class II (MHCII) for recognition by the T-cell receptor (TCR) of CD4+ T cells. Peptides are loaded onto MHCII in endosomal compartments in a process catalyzed by the MHCII-like protein H2-M (HLA-DM in humans). This process is modulated by another MHCII-like protein, H2-O (HLA-DO in humans). H2-O is a biochemical inhibitor of peptide loading onto MHCII; however, on the cellular level, it has been shown to have varying effects on Ag presentation. Thus, the function of H2-O in the adaptive immune response remains unclear. Here, we examine the effect of H2-O expression on the ability of Ag-specific B cells to enter the GC. We show that when Ag specific WT and H2-O−/− B cells are placed in direct competition, H2-O−/− B cells preferentially populate the GC. This advantage is confined to Ag-specific B cells and is due to their superior ability to obtain Ag-specific T-cell help when T-cell help is limiting. Overall, our work shows that H2-O expression reduces the ability of B cells to gain T-cell help and participate in the GC reaction.
Keywords: antigen processing, antigen presentation, H2-M, follicular helper T cells, HLA-DO
Antigen (Ag) processing and presentation on MHCII is central to T cell-dependent Ab-mediated immunity. During an immune response, Ag-specific naïve B cells recognize and internalize Ag via the B-cell receptor (BCR). The Ag is subsequently transported to late endosomes and lysosomes, where it is degraded into peptides, some of which are loaded onto MHC class II (MHCII) (1). These MHCII-peptide complexes are then displayed on the cell surface for recognition by cognate CD4+ T cells. This recognition event allows B cells to obtain T-cell help from a rare population of T cells known as CD4+ follicular helper T cells (TFH). The interaction between the Ag-specific B cells and TFH cells is essential for B-cell entry into the germinal center (GC) (2).
Peptide loading of MHCII is catalyzed by the nonclassical MHCII-like protein H2-M (HLA-DM in humans) (3–5). H2-M, which localizes to endosomes (6), promotes the removal of class II-associated invariant chain peptides (CLIP) from the peptide-binding groove of MHCII. H2-M also stabilizes empty MHCII molecules and edits the final MHCII-bound peptide repertoire that is displayed at the cell surface to ensure that only long-lived MHCII-peptide complexes are presented (7). Another nonclassical MHCII-like protein, H2-O (HLA-DO in humans), also localizes to endosomes in B cells (8). H2-O forms a complex with H2-M and has been shown to inhibit or modulate the ability of H2-M to catalyze and edit MHCII peptide exchange (9–12). Surprisingly, H2-O–deficient mice exhibit only minor abnormalities in Ag presentation (11, 13).
Ag presentation is important for many aspects of immunity, including initiating the GC response. Admission of B cells into the GC is a competitive process that is dependent upon help from Ag-specific TFH cells. The effectiveness of T-cell help is directly dependent upon MHCII-mediated Ag presentation by individual B cells. B cells expressing BCRs with high avidity for Ag have been shown to be at an advantage, as higher densities of Ag-derived MHCII-peptide complexes will be displayed at the cell surface. It is possible that H2-O, which modulates MHCII peptide loading, might also influence this critical step in effector B-cell development.
To test the hypothesis that H2-O affects the ability of Ag-specific B cells to compete for T-cell help, we developed an adoptive transfer system in which WT and H2-O−/− Ag-specific B cells were placed in direct competition. Our results showed that H2-O−/− Ag-specific B cells preferentially populated the GC in comparison with WT Ag-specific B cells. This preference was dependent on immunization with cognate Ag and was eliminated when recipient mice were preimmunized to expand the endogenous population of TFH cells. Finally, the ability of H2-O−/− B cells to out-compete WT B cells for GC occupancy was reversed to favor WT B cells by cotransferring TCR Tg T cells specific for a peptide that is presented better in the presence of H2-O. Together, these results show that Ag-specific H2-O−/− B cells preferentially occupy the GC due to a greater ability to present Ag-derived peptides via MHCII and obtain T-cell help. Thus, H2-O-mediated modulation of B-cell Ag presentation regulates the ability of B cells to gain T-cell help and participate in the GC reaction.
Results
NP-Specific H2-O−/− B Cells Preferentially Populate the GC When Placed in Competition with WT B Cells.
Studies using MHCII-peptide–specific Abs, TCR Tg T cells, or T-cell hybridomas have shown that H2-O variably and unpredictably affects the presentation of individual peptides (9, 14, 15). Therefore, rather than further study T-cell responses to the presentation of individual peptides, we measured the ability of GC B cells to obtain T-cell help from the pool of endogenous polyclonal CD4+ T cells. This sensitive and physiological approach allows the detection of overall differences in the efficiency of presentation of a polyvalent Ag by WT versus H2-O−/− B cells.
The GC reaction is constrained by B-cell–extrinsic factors, including the size of the TFH population and Ag availability (16, 17). B cells bearing high-affinity BCR out-compete lower affinity clones for access to T cell-mediated survival signals (18). Because of the competitive nature of B-cell entry into the GC and the dependence on T-cell help for this process, GC entry provides a sensitive measure of the ability of WT and H2-O−/− B cells to obtain T-cell help when placed in direct competition.
The affinity of the BCR for Ag is a major contributing factor to the ability of B cells to enter and compete successfully in the GC (18). To eliminate differences in BCR affinity, H2-O−/− mice were crossed with B1-8hi (B1-8) mice, a 4-hydroxy-3-nitrophenyl acetyl (NP)-specific BCR heavy chain knock-in strain of mice (19). In these mice, when the B1-8 heavy chain pairs with a lambda light chain, ∼5–10% of the total splenic B cells express a BCR specific for NP.
Splenocytes containing a 1:1 mixture of NP-specific B cells from congenically marked B1-8 (CD45.1/2) and B1-8.H2-O−/− (CD45.1) mice were cotransferred into B6 (CD45.2) recipient mice (Fig. 1A). The following day, recipient mice were immunized with the T-dependent Ag NP-chicken gamma globulin (CGG). B-cell responses were monitored by flow-cytometric analysis of splenocytes on days 3, 7, 10, 14, and 21 to determine the fate of B1-8 and B1-8.H2-O−/− cells. To identify the transferred NP-specific B1-8 B cells, splenocytes were stained with fluorphore-labeled NP to distinguish the NP-binding B cells together with Abs to define B-cell subsets among the transferred cells (Fig. S1A). B1-8 B cells made up ∼0.5% of the total B cells in unimmunized recipients (Fig. S1B).
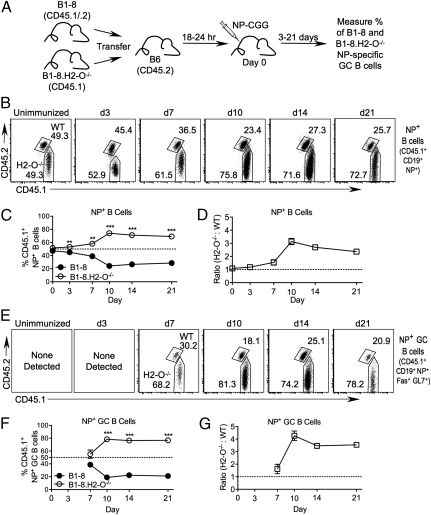
Fig. 1.
H2-O−/− cells preferentially populate the GC. (A) Schematic representation of experimental setup. Mice were transferred with a 1:1 mixture of congenically marked B-8 and B1-8.H2-O−/− splenocytes and immunized with NP-CGG. Analysis was performed on days 0–21. (B) Contribution of transferred B1-8 and B1-8.H2-O−/− cells to donor-derived NP+ B-cell population over time, gated as shown in Fig. S1A. Numbers indicate percentage of each gated population. (C) Quantification of plots shown in B for multiple mice and experiments. (D) Ratio of B1-8.H2-O−/− to B1-8 B cells, normalized to ratio present in transferred mixture for data shown in B. (E) Contribution of transferred B1-8 and B1-8.H2-O−/− cells to donor-derived NP+ GC B-cell populations; gated as shown in Fig. S1A. (F) Quantification of plots shown in E for multiple mice and experiments. (G) Ratio of B1-8.H2-O−/− to B1-8. GC B cells, normalized to ratio in transferred mixture for data shown in E. Statistical significance was determined by two-tailed paired t test between B1-8 and B1-8.H2-O−/−.
Analysis of recipient mice on the day of immunization (unimmunized), confirmed that a 1:1 mixture of B1-8 and B1-8.H2-O−/− B cells had been transferred (Fig. 1 B and C). At 3 d postimmunization, a significantly higher percentage of NP+ B1-8.H2-O−/− (53.1 ± 1.3%) than NP+ B1-8 (45.0 ± 1.6%) B cells were present in the recipient mice (Fig. 1 B and C). By day 7, the preferential accumulation of B1-8.H2-O−/− B cells increased, and NP+ B1-8.H2-O−/− B cells represented 58.2 ± 6.0% of the total NP-specific B1-8 B-cell pool. By day 10, NP+ B1-8.H2-O−/− B cells rose to 74.1 ± 5.1% and were maintained near this level on day 14 (71.16 ± 3.5%) and day 21 (69.2 ± 2.8%). Conversion of percentages to ratios of NP+ B1-8.H2-O−/− to B1-8 B cells (normalized to the input ratio to account for small differences in the transferred mixtures in individual experiments; Fig. 1D) showed that at 3 and 7 d posttransfer, B1-8.H2-O−/− B cells were found to be 1.2-fold and 1.5-fold more abundant, respectively, in the total B1-8 NP+ B-cell pool. This increased to 3.1-fold on day 10 and then decreased to 2.7-fold and 2.4-fold on days 14 and 21, respectively. These data show that after immunization, NP+ B1-8.H2-O−/− B cells preferentially accumulate relative to NP+ B1-8 B cells.
To determine whether the preferential accumulation of NP+ B1-8.H2-O−/− B cells was due to a higher percentage of H2-O−/− B cells populating GCs, we analyzed the relative contribution of the two populations to GCs. On day 7 postimmunization, at the beginning of the detectable GC response (Fig. S1C), there was a slight but reproducible accumulation of the transferred B1-8.H2-O−/− GC B cells (CD45.1+CD19+Fas+GL7+) (55.2 ± 14.1%) compared with B1-8 GC B cells (38.8 ± 9.4%) (Fig. 1 E and F). At the peak of the GC response (days 10 and 14) the selective accumulation of B1-8.H2-O−/− B cells was further enhanced. B1-8.H2-O−/− B cells represented 78.4 ± 5.4% and 76.3 ± 4.4% of the GC on days 10 and 14 post immunization, respectively. By day 21, when the GC response waned, B1-8.H2-O−/− B cells still constituted the majority of GC B cells (76.8 ± 1.3%). Conversion to ratios of B1-8.H2-O−/− to B1-8 B cells showed that 7 d after immunization there were 1.6-fold more B1-8.H2-O−/− than B1-8 B cells among the NP-specific GC B cell pool (Fig. 1G). By day 10, the difference increased to 4.3-fold and was maintained at 3.5-fold on days 14 and 21 (Fig. 1 E, F, and G). These data support that upon engagement with specific Ag, H2-O−/− B cells have an advantage in entering GCs. However, once the B cells have entered, H2-O deficiency no longer imparts a further advantage.
Importantly, B1-8.H2-O−/− B cells contributed normally to the GC reaction in a noncompetitive transfer system in which B1-8.H2-O−/− or B1-8 B cells were transferred into separate recipients (Fig. S2). Analysis of mice 10 d post immunization showed that there was no significant difference in the mean percentage of total GC B cells in mice that had received WT or H2-O−/− B1-8 cells (Fig. S2 A and B). Furthermore, the vast majority of GC B cells in the recipient mice were derived from the transferred B1-8 B cells regardless of H2-O expression (Fig. S2 A and C). Thus, in the absence of competition, similar to WT B cells, B1-8.H2-O−/− B cells are constricted by the limited availability of Ag and T-cell help for access to GCs.
Preferential Expansion of H2-O−/− B Cells Is Specific to Ag-Experienced B Cells.
H2-O modulates H2-M function thereby altering the final set of peptides presented in the context of MHCII (15). Thus, the advantage that B1-8.H2-O−/− B cells have in populating the GC is likely due to differences in Ag presentation. If this is correct, then among the pool of transferred nonspecific B cells (i.e., B cells that did not carry the B1-8 BCR; NP−), the NP− B1-8.H2-O−/− and B1-8 B cells should be present at similar ratios. Indeed, NP− non-GC B cells derived from NP− B1-8.H2-O−/− and B1-8 donor splenocytes were present at similar percentages and at a near 1:1 ratio throughout the 21 d of analysis (Fig. 2 A, B, and D). In addition, CD4+ T cells derived the individual donor splenocyte populations were present at similar percentages and at a ratio of ∼1:1 (Fig. 2 A, C, and D). Thus, the selective advantage of B1-8.H2-O−/− B cells was largely confined to Ag-experienced B cells, and in particular to NP+B1-8.H2-O−/− B cells that participated in the GC reaction.
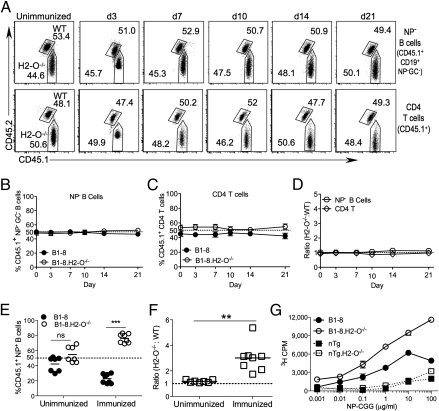
Fig. 2.
NP− B cells and CD4+ T cells maintain a 1:1 ratio. (A) Contribution of transferred B1-8 and B1-8.H2-O−/− cells to donor-derived NP− non-GC B-cell and CD4+ T-cell populations over time. Donor-derived cells were gated as shown in Fig. S1A. Numbers indicate percentage of each gated population. (B and C) Quantification of data shown in A for multiple mice. (D) Ratio of H2-O−/− to WT cells for populations shown in A, normalized to ratio present in transferred mixture. (E) B1-8 and B1-8.H2-O−/− B cells (1:1 mix) were transferred into recipient mice and either immunized with NP-CGG or not immunnized. Ten days later, the percentage of WT and H2-O−/− B1-8 cells were measured as shown in Fig. S2B and quantified for multiple mice. (F) Ratio of H2-O−/− to WT cells normalized to ratio of original mixture for data shown in E. (G) Purified and irradiated B cells from B1-8, B1-8.H2-O−/−, and nontransgenic (nTg) littermate mice were pulsed for 30 min on ice with increasing concentrations of NP-CGG, washed extensively, and cocultured for 72 h with NP-CGG–specific CD4 T cells. T cell proliferation was determined by [3H]thymidine incorporation during the last 18 h of culture. Data are representative of four independent experiments. Statistical significance determined by two-tailed paired t test for E and a two-tailed unpaired t test for F.
We also confirmed that immunization was required for the preferential expansion of the B1-8.H2-O−/− B cells. B1-8 and B1-8.H2-O−/− B-cell (1:1) recipients were either immunized or left unimmunized. As expected, at 10 d postimmunization, unimmunized recipients lacked a substantial NP+ GC B-cell population (Fig. S3A). Among the total NP+ B cells in immunized recipients, as before, B1-8.H2-O−/− B cells constituted a greater percentage and a higher ratio (3-fold more) of the transferred NP+ B cells than B1-8 B cells (Fig. 2 E and F and Fig. S3B). In contrast, in unimmunized recipients, B1-8 B cells and B1-8.H2-O−/− cells were present at similar percentages and ratios among the transferred NP-specific B cells (Fig. 2 E and F and Fig. S3B). These data support that B-cell interactions with Ag are necessary for NP+ H2-O−/− B cells to expand relative to WT NP+ B cells. Furthermore, these data confirm that a homeostatic expansion of the B1-8.H2-O−/− B cells does not account for the preferential skewing of H2-O deficient B cells.
Finally, we directly measured the ability of B1-8.H2-O−/− and B1-8 B cells to process and present peptides derived from NP-CGG. Purified B1-8.H2-O−/− and B1-8 B cells were pulsed with various concentrations of NP-CGG for 30 min on ice, washed, and cocultured with purified polyclonal CD4+ T cells that were generated by injecting mice 7–10 d prior with NP-CGG in alum. The B1-8.H2-O−/− B cells stimulated NP-CGG-specific T cells ∼2-fold better than B1-8 B cells (Fig. 2G). Low levels of presentation by nontransgenic littermate control B cells demonstrate that Ag presentation was mediated by the B1-8 BCR. Collectively, our data support that the preferential expansion of B1-8.H2-O−/− B cells is likely due to enhanced MHCII presentation of CGG peptides in the absence of H2-O.
Providing Extra T-Cell Help Allows WT and H2-O−/− B Cells to Populate GC More Equally.
B-cell entry into the GC is normally limited by the availability of TFH (17). Thus, if the preferential accumulation of B1-8.H2-O−/− B cells in GCs is due to their superior ability to obtain T-cell help, then eliminating the competition for T-cell help should allow WT and H2-O−/− B cells to compete more effectively. Previous studies have shown that preimmunization of mice to expand the endogenous TFH population before the introduction of Ag-specific B cells resulted in the activation of more Ag-specific B cells (20). Therefore, expansion of the polyclonal TFH population by preimmunization should eliminate B1-8 B-cell competition for T-cell help and entry into GCs.
B6 mice were preimmunized with NP-CGG in alum (Fig. 3A). One week later, B1-8 and B1-8.H2-O−/− splenocytes (1:1) were transferred into the preimmunized mice or as a control, into mice that had not been preimmunized (“naïve”). The following day, recipient mice were immunized with NP-CGG, and 10 d later, the GC composition and the size of the TFH cell pool were measured.
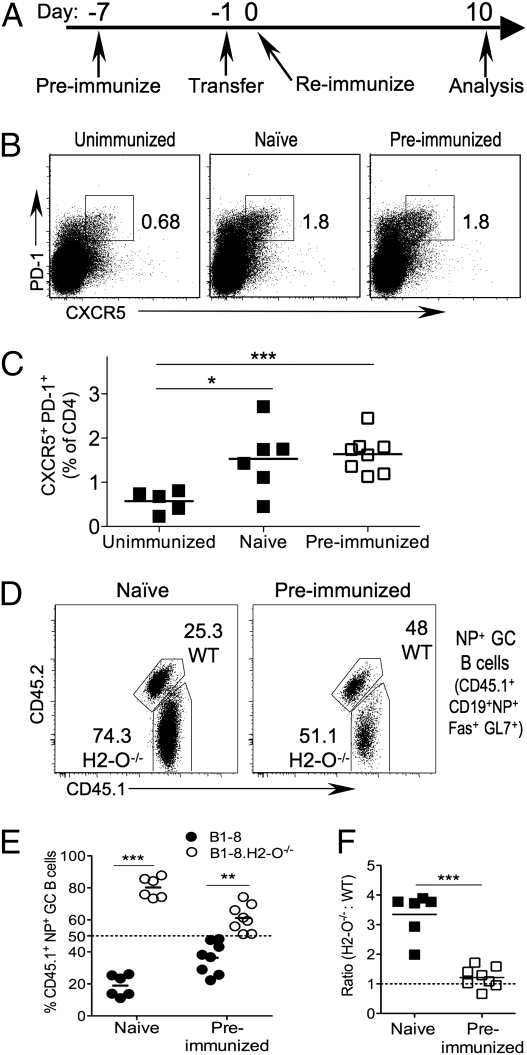
Fig. 3.
B1-8 and B1-8.H2-O−/− B cells populate the GC equally in the presence of abundant T-cell help. (A) Schematic representation of experiment setup. Marked B1-8 and B1-8.H2-O−/− cells were mixed at a 1:1 ratio and transferred into either naïve hosts or hosts that had been immunized 7 d prior with NP-CGG. The next day, mice were immunized with NP-CGG in alum for the first time (naïve) or second time (preimmunized), or were left unimmunized. (B) Size of TFH population was examined on day 10 postimmunization. Plots shown are gated on CD4+ T cells. Numbers indicate size of gated population as percentage of CD4+ cells. (C) Quantification of data shown in B for multiple mice. (D) Contribution of WT and H2-O−/− B1-8 cells to NP+ GC B-cell pool upon preimmunization. (E and F) Quantification of population defined in D as percentage of donor-derived NP+ GC B cells (E), and as a ratio of H2-O−/− to WT populations, normalized to the ratio in the transferred mixture (F). Statistical significance was calculated using two-tailed paired t test in E and a two-tailed unpaired t test in C and F.
The frequency of the TFH population (CD4+ CXCR5+ PD-1+) was similar in recipients that had been immunized once (naïve) or twice (preimmunized), but significantly larger than in unimmunized mice (Fig. 3 B and C). Thus, the expanded TFH population was present in preimmunized recipients when B1-8 cells were transferred, whereas in naïve mice TFH cells developed during the GC response of the transferred B1-8 B cells.
In mice that were immunized only after B1-8 transfer (naïve), 80.2 ± 6.4% of the transferred NP+ GC B cells were derived from B1-8.H2-O−/− B cells. In preimmunized mice, the percentage NP+ B1-8.H2-O−/− GC B cells decreased to 61.2 ± 8.5% (Fig. 3 D and E). Although there was a significantly higher percentage of NP+B1-8.H2-O−/− GC B cells than B1-8 B cells in preimmunized recipients, the difference was reduced compared with that in recipients that had not been preimmunized to generate more TFH cells (Fig. 3 D and E). Conversion of the percentages to ratios showed that there were 3.3-fold more B1-8.H2-O−/− B cells in the mice immunized once, whereas the ratio for preimmunized mice decreased to 1.2 (Fig. 3F). Once again, this change in relative abundance was confined to NP+ B cells (Fig. S4). Similar results were obtained when mice were preimmunized with azobenzene arosonate (ABA)-CGG so that no prior NP-specific B-cell response was in progress at the time of B1-8–cell transfer (Fig. S5). These data support that H2-O−/− B cells have an advantage at entering GCs due to a superior ability to obtain T-cell help in a competitive environment, because in the presence of an expanded CGG-specific TFH cell population, the advantage is lost.
B-Cell Population That Preferentially Populates the GC Is Determined by B-Cell Ag Presentation.
The experiments presented above collectively support that T-cell help mediates the selective advantage that H2-O−/− B cells have in populating the GC. We reasoned, therefore, that this advantage could be altered to favor WT B cells if an excess of TFH cells specific for an Ag that is presented better by WT B1-8 B cells were present. The ovalbumin (OVA) peptide recognized by CD4+ OT-II TCR Tg T cells is presented better by Ag-specific WT B cells than by H2-O−/− B cells (14) (Fig. 4A). TFH cells develop from T cells with high affinity for Ag only upon Ag encounter (16). Therefore, OT-II T cells cotransferred with WT B1-8 and B1-8.H2-O−/− B cells into recipient mice should join the TFH cell pool and preferentially provide T-cell help to WT B1-8 B cells after immunization with NP-OVA. To test this idea, B1-8 and B1-8.H2-O−/− B cells were cotransferred into B6 recipients together with OT-II T cells or, as a nonspecific T-cell control, Eα-specific TEa TCR Tg T cells (21). As before, recipient mice were immunized the next day, but this time with NP-OVA.
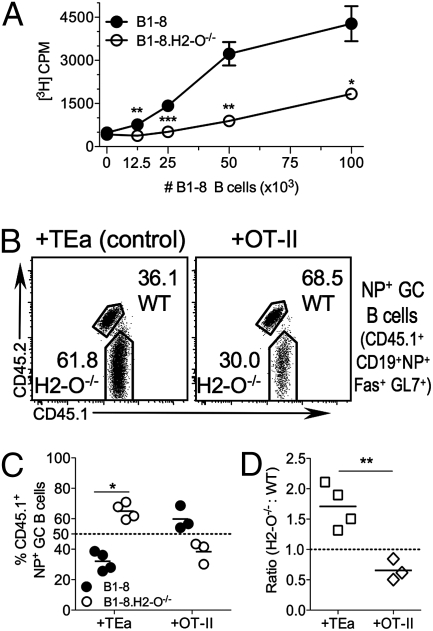
Fig. 4.
WT B cells preferentially populate the GC when T cells specific for a peptide presented better by WT cells are available to provide help. (A) B1-8 B cells present OVA peptide better than B1-8.H2-O−/− B cells to OT-II T cells. B1-8 and B1-8.H2-O−/− mice were primed in vivo by immunization i.p. with NP-OVA. Forty-eight hours later, level of OVA presentation was measured by incubating titrated numbers of purified and irradiated NP+B1-8.H2-O−/− and WT B cells with purified OT-II T cells. T cell proliferation was determined by [3H]thymidine incorporation during last 18 h of culture. Data are representative of four independent experiments. (B–D) Mixture of splenocytes containing equal numbers of NP+ B cells from B1-8 and B1-8.H2-O−/− mice were transferred into B6 hosts, along with either Eα-specific TEa (control) or OVA-specific OT-II T cells, and mice were immunized with NP-OVA. Ten days later, GC composition was examined. (B) Contribution of each donor to donor-derived Ag-specific GC population. (C) Quantification of data shown in B for multiple mice. (D) Ratio of H2-O−/− to WT B cells, normalized to ratio in transferred mixture, for the populations shown in B. Statistical significances were calculated using two-tailed paired t test for C and a two-tailed unpaired t test for A and D.
After immunization with NP-OVA, control mice cotransferred with TEa T cells, had greater percentages of NP+ B1-8.H2-O−/− GC B cells (65.0 ± 5.3%) than B1-8 GC B cells (32.1 ± 6.3%) (Fig. 4 B and C). After normalization to the input population, there were 1.7-fold more H2-O−/− than WT B1-8 B cells in the NP+ GC population (Fig. 4D). When OT-II T cells were cotransferred with the WT and H2-O−/− B1-8 B cells, a larger percentage of B1-8 (59.8 ± 7.7%) than B1-8.H2-O−/− B cells (38.4 ± 7.2%) were present in the NP+ GC population (Fig. 4 B and C). In the presence of OT-II T cells, the NP+ B1-8.H2-O−/− B cells were exceeded by WT B1-8 B cells in the GC at a ratio of 0.66:1 (H2-O−/−:WT) (Fig. 4D). This result was confined to NP+ GC B cells, and there was no change in the percentages or ratios of the transferred NP− GC− B cells or CD4 T cells (Fig. S6).
These results support that the mechanism that promotes H2-O−/− B-cell entry into the GC is directly related to the ability of the B-cell to present the specific peptides that result in TFH cell help. When T cells specific for a peptide that is presented better in the presence of H2-O than in its absence are overrepresented, the advantage that H2-O−/− B cells previously had in entering the GC is lost. Instead, H2-O–expressing B cells gain an advantage in entering the GC, because they more efficiently present the peptide recognized by the predominant population of TFH cells.
Discussion
We found that placing Ag-specific WT and H2-O−/− B cells in direct competition in an environment where Ag presentation on MHCII plays an important role revealed an important functional difference in H2-O−/− cells. H2-O−/− B cells had a distinct T cell-dependent advantage over WT cells in populating the GC after immunization. The advantage was evident as early as 7 d postimmunization but remained constant from day 10 on (Fig. 1). These data show that, upon Ag encounter, H2-O−/− B cells have an advantage in entering the GC; however, once they have entered, H2-O−/− and WT B cells have a similar ability to contribute to the expanding GC B-cell pool. This is consistent with data showing that H2-O is down-regulated in WT GC B cells, making them functionally more similar to H2-O−/− B cells (22–24).
Importantly, the relative expansion of cells from B1-8.H2-O−/− donors was confined to the pool of NP+ B1-8 GC B cells. NP+ B cells in B1-8 mice constitute only 5–10% of the total B cells (18, 19). Therefore, large numbers of donor-derived nonspecific B cells were present in recipient mice. The nonspecific B cells provided an excellent point of comparison because they were largely not exposed to their cognate Ags and thus did not participate in the GC reaction. Further supporting the importance of GC entry, the very small pool of B1-8–derived NP− B cells that did take on a GC phenotype (most likely CGG- or OVA-specific cells) displayed a trend similar to that of the NP+ B1-8 cells in that more H2-O−/− cells than WT cells were present in the GC population. In contrast, the ratio of nonspecific, non-GC B cells from B1-8 WT and H2-O−/− mice remained similar to the input ratio throughout the course of the immune response (Fig. 2). These data highlight the importance of Ag encounter in the process that allows the specific expansion of the H2-O−/− B-cell population. This was confirmed when unimmunized recipient mice were examined (Fig. 2 and Fig. S3). In the absence of immunization, Ag-specific B cells did not encounter their cognate Ag and the H2-O−/− B cells did not selectively expand; rather, the H2-O−/− B-cell population remained similar in size to the WT B-cell population. In addition, upon immunization, the ratio of donor-derived CD4 T cells, which do not express H2-O, remained constant throughout the course of the experiment.
To determine the mechanism that favored entry of H2-O−/− cells into the GC, we manipulated the pool of TFH cells. Introducing B1-8.H2-O−/− and B1-8 B cells into an environment containing an expanded population of polyclonal CGG specific TFH cells equalized the WT and H2-O−/− B-cell content of the GC (Fig. 3 E and F). This shows that H2-O−/− cells have an advantage only when TFH cell help is limiting, and thus a competitive environment was necessary to uncover the phenotype. Furthermore, inducing GC formation in the presence of a clonal T-cell population that recognized a peptide that is presented better by WT B cells allowed WT cells to populate the GC more efficiently than H2-O−/− cells (Fig. 4). Collectively, these data support that the mechanism determining which B-cell population enters and proliferates in GCs is the T-cell–B-cell interaction mediated by MHCII-peptide complexes. Direct measurement of Ag presentation of CGG derived peptides by B1-8 and B1-8.H2-O−/− B cells showed that B1-8.H2-O−/− B cells were ∼2-fold better at presentation than WT B1-8 B cells (Fig. 2G). Thus, together our data show that H2-O modulates the ability of B cells to obtain T-cell help, and that the mechanism of the modulation is dependent on Ag presentation on MHCII.
These experiments have furthered our understanding of the physiological function of H2-O. During the course of an immune response, Ag presentation takes place in the context of many peptides derived from many Ags; thus, an experimental system that allows us to probe overall levels of Ag presentation, rather than presentation of single peptides, clarifies the cellular and immunological function of H2-O in a way that measuring presentation of single peptides has not. H2-O−/− B cells out-competed WT B cells by ∼2- to 5-fold, but did not completely take over the GC niche. This suggests that H2-O–dependent modulation of Ag presentation may be subtle, which is supported by previous studies that examined presentation on the cellular level and found varying effects of H2-O depending on the specific epitope examined (9, 11–15, 25). Nevertheless, small differences in Ag presentation, especially when considering a complex Ag mix containing many individual epitopes, could have a considerable impact on the immune response. Recent studies from our lab have shown that modulation of Ag presentation by DO expression in NOD DCs results in the prevention of the autoimmune disease, type 1 diabetes (26). The mechanism of protection was likely due to an alteration of the MHCII presented on the surface of DCs in these mice. These findings further support that subtle alterations in MHCII peptide presentation can have a profound impact on the immune response and, in this case, an autoimmune response.
Our experiments also show that H2-O inhibits Ag presentation primarily in naïve B cells, limiting their ability to receive T-cell help and enter the GC. This suggests an important immunological function for H2-O in which H2-O establishes a higher threshold of Ag presentation for cells to commence the GC reaction. Down-regulation of H2-O upon GC entry allows Ag presentation to proceed more efficiently after the entry checkpoint has been passed (22–24). The GC reaction, albeit a vital part of the immune response, is also inherently dangerous because it combines the active mutagenic mechanisms of somatic hypermutation with rapid cellular proliferation (27). If cells were to enter the GC unnecessarily, without sufficient stimulus and immunological need, the result could be increased risk of B-cell lymphomas and leukemias or autoimmunity. H2-O may mitigate this risk by dampening Ag presentation in naïve B cells.
Methods
Mice.
H2-O−/− mice (11) were provided by Lars Karlsson (Johnson and Johnson Pharmaceutical Research and Development, San Diego, CA). B1-8 mice (19) were shared by Michel Nussenzweig (Rockefeller University, New York, NY). B1-8 mice were crossed to the B6.SJL and H2-O−/−.SJL strains. TEa mice (21) were provided Jonathan Bromberg (Mount Sinai Medical Center, New York, NY). C57BL6/J (B6) and B6.SJL mice were obtained from the Jackson Laboratory, and OT-II.2a/Rag1 mice were obtained from Taconic Farms through the National Institute of Allergy and Infectious Diseases Exchange Program, National Institutes of Health (28). Mice were used at 6–16 wk of age. Experiments were performed in accordance with protocols approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
Immunizations.
Immunizations were performed 1 d after cell transfer, except where noted. Recipient mice were immunized i.p. with 50 μg NP-CGG, ABA-CGG, or NP-OVA (Biosearch Technologies) in 200 μL as an alum precipitate.
Cell Transfers.
Single-cell suspensions were made from spleens of B1-8 mice or pooled lymph nodes and spleens of OT-II or TEa TCR Tg mice. Red blood cells were lysed from splenocytes. Cells were stained to determine the percentage of NP+ B cells or T cells, mixed and resuspended in PBS for injection. For competitive transfers, splenocytes containing 106 NP+ B1-8 and 106 NP+ B1-8.H2-O−/− were transfered per recipient. For noncompetitive transfers, 106 NP+ B1-8 or 106 NP+ B1-8.H2-O−/− were transferred per recipient. For T-cell cotransfer, 5 × 105 OT-II or TEa T cells per recipient were added to the B1-8 splenocyte mixture. Mixtures were stained and examined by flow cytometry to confirm the cellular ratios. Cells were transferred into recipients by i.v. injection (200 μL).
Flow Cytometry and T-Cell Assays.
Flow cytometry and T-cell assays were performed using standard approaches and as detailed in SI Methods.
Supplementary Material
Acknowledgments
We thank Michel Nussenzweig (Rockefeller University, New York, NY), Jonathan Bromberg (Mount Sinai Medical Center, New York, NY), Lars Karlsson (Johnson and Johnson Pharmaceutical Research and Development, San Diego, CA), and Eric Meffre (Yale University School of Medicine, New Haven, CT) for providing mice; Sergio Quezada, Emily Corse, Eric Alonzo, Tom Martillotti, and Rachel Gottschalk for experimental support and helpful advice; and Derek Sant'Angelo, Gavin Porter, Armine Matevossian, and Woelsung Yi for providing advice, experimental support and critical reading of the manuscript. This project was funded by National Institutes of Health Grant R01-AI061484.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004664107/-/DCSupplemental.
References
- 1.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 2.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 3.Sloan VS, et al. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 4.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 5.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 6.Lindstedt R, Liljedahl M, Péléraux A, Peterson PA, Karlsson L. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity. 1995;3:561–572. doi: 10.1016/1074-7613(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 7.Jensen PE, Weber DA, Thayer WP, Chen X, Dao CT. HLA-DM and the MHC class II antigen presentation pathway. Immunol Res. 1999;20:195–205. doi: 10.1007/BF02790403. [DOI] [PubMed] [Google Scholar]
- 8.Liljedahl M, et al. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J. 1996;15:4817–4824. [PMC free article] [PubMed] [Google Scholar]
- 9.Alfonso C, Karlsson L. Nonclassical MHC class II molecules. Annu Rev Immunol. 2000;18:113–142. doi: 10.1146/annurev.immunol.18.1.113. [DOI] [PubMed] [Google Scholar]
- 10.Denzin LK, Sant'Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 11.Liljedahl M, et al. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 12.van Ham SM, et al. HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol. 1997;7:950–957. doi: 10.1016/s0960-9822(06)00414-3. [DOI] [PubMed] [Google Scholar]
- 13.Perraudeau M, et al. Altered major histocompatibility complex class II peptide loading in H2-O-deficient mice. Eur J Immunol. 2000;30:2871–2880. doi: 10.1002/1521-4141(200010)30:10<2871::AID-IMMU2871>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso C, et al. Analysis of H2-O influence on antigen presentation by B cells. J Immunol. 2003;171:2331–2337. doi: 10.4049/jimmunol.171.5.2331. [DOI] [PubMed] [Google Scholar]
- 15.Denzin LK, Fallas JL, Prendes M, Yi W. Right place, right time, right peptide: DO keeps DM focused. Immunol Rev. 2005;207:279–292. doi: 10.1111/j.0105-2896.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 16.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHeyzer-Williams LJ, Pelletier N, Mark L, Fazilleau N, McHeyzer-Williams MG. Follicular helper T cells as cognate regulators of B cell immunity. Curr Opin Immunol. 2009;21:266–273. doi: 10.1016/j.coi.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nat Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 19.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 20.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, et al. Regulated expression of human histocompatibility leukocyte antigen (HLA)-DO during antigen-dependent and antigen-independent phases of B cell development. J Exp Med. 2002;195:1053–1062. doi: 10.1084/jem.20012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glazier KS, et al. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J Exp Med. 2002;195:1063–1069. doi: 10.1084/jem.20012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallas JL, Yi W, Draghi NA, O'Rourke HM, Denzin LK. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J Immunol. 2007;178:1488–1497. doi: 10.4049/jimmunol.178.3.1488. [DOI] [PubMed] [Google Scholar]
- 25.Fallas JL, et al. Ectopic expression of HLA-DO in mouse dendritic cells diminishes MHC class II antigen presentation. J Immunol. 2004;173:1549–1560. doi: 10.4049/jimmunol.173.3.1549. [DOI] [PubMed] [Google Scholar]
- 26.Yi W, et al. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120:1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 28.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.