Abstract
In association with NMDA receptors (NMDARs), neuronal α7 nicotinic ACh receptors (nAChRs) have been implicated in neuronal plasticity as well as neurodevelopmental, neurological, and psychiatric disorders. However, the role of presynaptic NMDARs and their interaction with α7 nAChRs in these physiological and pathophysiological events remains unknown. Here we report that axonal α7 nAChRs modulate presynaptic NMDAR expression and structural plasticity of glutamatergic presynaptic boutons during early synaptic development. Chronic inactivation of α7 nAChRs markedly increased cell surface NMDAR expression as well as the number and size of glutamatergic axonal varicosities in cortical cultures. These boutons contained presynaptic NMDARs and α7 nAChRs, and recordings from outside-out pulled patches of enlarged presynaptic boutons identified functional NMDAR-mediated currents. Multiphoton imaging of presynaptic NMDAR-mediated calcium transients demonstrated significantly larger responses in these enlarged boutons, suggesting enhanced presynaptic NMDAR function that could lead to increased glutamate release. Moreover, whole-cell patch clamp showed a significant increase in synaptic charge mediated by NMDAR miniature EPSCs but no alteration in the frequency of AMPAR miniature EPSCs, suggesting the selective enhancement of postsynaptically silent synapses upon inactivation of α7 nAChRs. Taken together, these findings indicate that axonal α7 nAChRs modulate presynaptic NMDAR expression and presynaptic and postsynaptic maturation of glutamatergic synapses, and implicate presynaptic α7 nAChR/NMDAR interactions in synaptic development and plasticity.
Keywords: silent synapse, synaptic development, synaptic plasticity, alpha bungarotoxin, cytisine
As the major excitatory neurotransmitter systems in the CNS, the nicotinic and glutamatergic systems have been implicated in a variety of neurological, neurodevelopmental, and psychiatric disorders as well as learning and memory (1, 2). Glutamate exerts its effects through a series of postsynaptic receptors named for their prototypic agonists. The most common ionotropic receptors are the NMDA and AMPA receptors. The importance of NMDA receptor (NMDAR)-mediated neural transmission is illustrated by its role in models of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD). One of the crucial biochemical mechanisms mediating these processes is trafficking of glutamate receptors to and from the synapse/cell surface. Once these receptors are placed into the membrane, they contribute to the generation of additional biochemical or structural events leading to more permanent alterations in synaptic strength.
Similarly, nicotinic ACh receptors (nAChRs) have crucial roles in a variety of CNS processes, including neuronal plasticity, nicotine addiction, Alzheimer's disease, Down syndrome, and schizophrenia (3–8). The α7 and α4β2 subtypes are the predominant nAChRs in the central nervous system. Interestingly, there are many interactions among both nAChR subtypes and glutamate receptors, particularly NMDARs, in physiological and pathological events. For example, nAChRs mediate neuroprotection against cell death induced by NMDAR stimulation (excitotoxicity) (9, 10). nAChR stimulation facilitates glutamatergic transmission at selected CNS synapses and enhances a synapse selective form of LTP in the amygdala (11). This facilitation is commonly mediated by presynaptic receptors and likely involves regulation of transmitter release, including the release of glutamate (12–14). nAChRs also regulate the downstream turnover of selected glutamate receptors such as the AMPA receptor GluR1 subunit (15).
Investigations of synaptic plasticity have concentrated on postsynaptic mechanisms, especially on postsynaptic NMDA and AMPA receptors. Presynaptic NMDARs have recently been implicated in cortical synaptic function and plasticity (16). They exist at higher levels early in development, and are involved in regulation of transmitter release and forms of LTD (16–20). However, the mechanisms controlling the expression of presynaptic NMDARs, how they affect synaptic development, and why they decrease with development are unknown. In the present study, we have identified a previously uncharacterized structural component of presynaptic plasticity reflecting interactions of axonal α7 nAChRs and presynaptic NMDARs in glutamatergic presynaptic bouton formation during early synaptic development.
Results
Chronic Inactivation of α7 nAChR Increases Surface NMDAR Expression and Numbers of Presynaptic Boutons Containing NMDARs in Cortical Cultures.
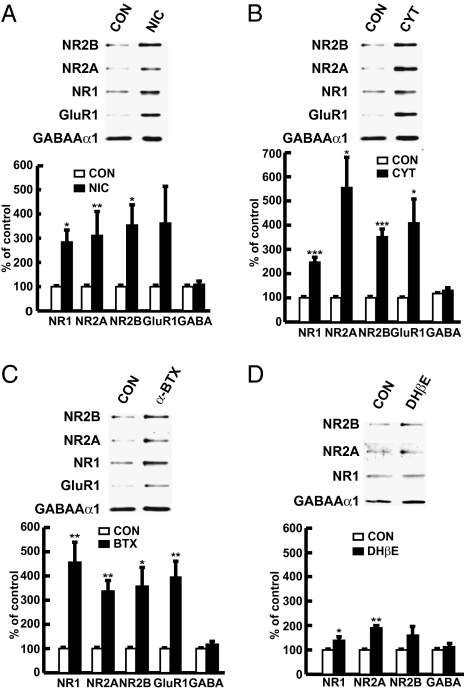
To explore the possible interactions of nAChRs and NMDARs, we examined the effects of nicotine on cell surface expression levels of NMDARs in cortical neurons. Nicotine markedly increased such levels (Fig. 1A). As nicotine is an agonist at both α4β2 and α7 nAChRs with a higher affinity for α4β2 receptors, we also investigated the subtype selective agents cytisine, α-bungarotoxin (α-BTX), and dihydro-β-erythrodine (DHβE). Cytisine is a full agonist at α7 and partial agonist at α4β2 nAChRs. α-BTX and DHβE are specific antagonists at α7 and α4β2 nAChRs, respectively. Cytisine and α-BTX treatment markedly increased surface levels of NMDAR subunits NR1, NR2A, NR2B, and the AMPAR GluR1 subunit in cortical cultures (Fig. 1 B and C), whereas DHβE had a smaller effect on surface levels of NMDARs (Fig. 1D). The surface level of the GABAAα1 subunit of GABA receptors was unchanged following exposure to any of these agents (Fig. 1 A–D).
Fig. 1.
Chronic inactivation of α7 nAChR, but not α4β2 nAChR, leads to marked increases in surface level of NR1, NR2, and GluR1 subunits in cortical neurons. Representative blots and quantification showing surface level of NR1, NR2A, NR2B, GluR1, and GABAAα1 in cortical cultures treated with nicotine (A), cytisine (B), α-BTX (C) or DHβE (D). Each experiment was repeated at least three times.
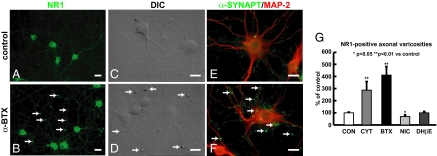
We then examined the distribution of glutamate receptors in cortical cultures. α-BTX or cytisine treatment markedly increased the number and size of NR1-positive axonal varicosities or boutons in treated cortical cultures (Fig. 2). Axonal varicosities or boutons are swellings along an axon and are frequently the sites that release transmitters and form synapses. We compared NR1-positive axonal varicosities (Fig. 2 A and B) and differential interference contrast (DIC) images of enlarged presynaptic boutons (Fig. 2 C and D) between control and α-BTX–treated cultures. The presynaptic terminal marker α-synaptophysin was also present in the enlarged boutons (Fig. 2 E and F). Although NR1-positive presynaptic boutons were increased and enlarged in α-BTX– or cytisine-treated cultures, no significant increases were observed in DHβE- or nicotine-treated cultures (Fig. 2G). The increases in surface NMDAR expression and in presynaptic boutons were blocked by the transcriptional inhibitor actinomycin D and the translational inhibitor cycloheximide (Fig. S1). These data suggest that α-BTX– or cytisine-induced increases in presynaptic boutons containing NMDARs largely reflect presynaptic increases in surface NMDAR expression, whereas nicotine-induced increases in surface NMDAR expression are not clearly associated with presynaptic terminals. NR2B and GluR1 colocalized with NR1-positive presynaptic boutons in treated cultures (Fig. S2), consistent with the α-BTX– or cytisine-induced increase in the surface levels of glutamate receptors largely reflecting enlarged axonal varicosities containing presynaptic glutamate receptors.
Fig. 2.
Chronic inactivation of α7 nAChR increases number and size of NR1-positive axonal varicosities in cortical cultures. Representative images showing increased and enlarged NR1-positive axonal varicosities (B), DIC images of enlarged boutons (D), and α-synaptophysin–positive presynaptic terminals (F) in α-BTX–treated cultures compared with those boutons in control cultures (A, C, and E). Quantification (G) showed that α-BTX, cytisine, but not DHβE or nicotine, increases the number of NR1-positive axonal varicosities in cortical cultures (n = 3–5). (Scale bars, 20 μm.)
As α7 nicotinic receptors desensitize almost fully on chronic exposure to agonists such as cytisine, this, coupled with similar effects of α-BTX, suggests that the increase in NMDAR levels results from α7 inactivation (either by desensitization or blockade). The time course of events was consistent with inactivation of α7 being the primary site action of cytisine, as brief (3 min) application of cytisine slightly decreased NMDAR levels, whereas more prolonged application (3–24 h) increased levels (Fig. S3A). Coapplication of either α-BTX or DHβE had no effect on cytisine-induced changes in NMDARs (Fig. S3 B and C), suggesting that cytisine does not act as an agonist at either subtype in this paradigm, but instead may act through desensitization. The concentration–response curve of cytisine on surface NMDAR expression demonstrated that the EC50 of cytisine is ∼200 μM (Fig. S4), consistent with the dose–response curve of cytisine-evoked currents through α7 nAChR in cultured neurons (21), further suggesting that cytisine-induced increases in surface NMDAR expression and presynaptic bouton number result from desensitization of α7 nAChR. Taken together, our findings suggest that chronic inactivation of α7 nAChR increases presynaptic NMDAR expression and presynaptic boutons in cortical cultures.
Increased and Enlarged NR1-Positive Axonal Varicosities Are Glutamatergic Presynaptic Boutons.
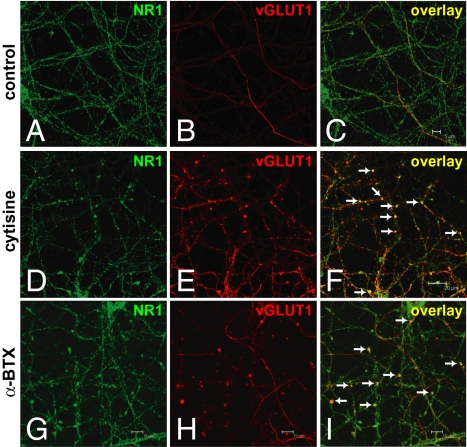
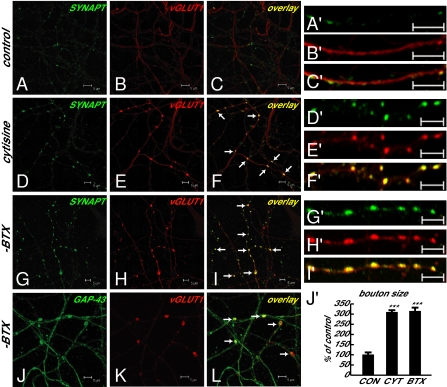
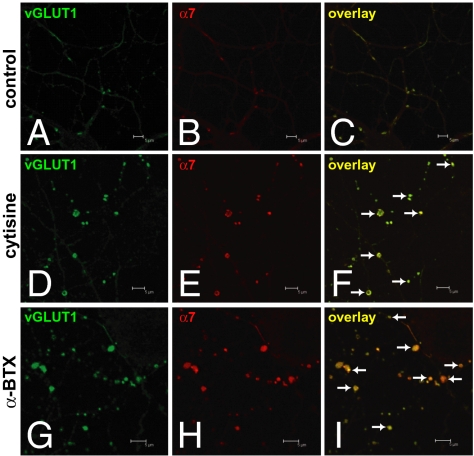
Previous work demonstrated an analogous pattern in developing cerebellar cultures in which NMDAR activation increases the size of GABAergic presynaptic boutons through presynaptic NMDARs (18). This suggests that a similar control mechanism might exist in cortical neurons. To assess the neurotransmitter identity in NR1-positive presynaptic boutons, we examined the immunoreactivities of GAD-65 (a GABAergic neuronal marker), the vesicular glutamate transporter 1 (vGLUT1, a glutamatergic presynaptic terminal marker), and α-synaptophysin (a presynaptic terminal marker) in cortical cultures. The increased and enlarged NR1-positive presynaptic boutons were vGLUT1 positive (Fig. 3) but not GAD-65 positive. These vGLUT1-positive presynaptic boutons also contained the presynaptic terminal marker α-synaptophysin and were noted to be largely en passant boutons (discussed later here) along axonal shafts (Fig. 4). Furthermore, quantitative measurement of size of boutons containing both α-synaptophysin and vGLUT1 confirmed the enlargement of glutamatergic boutons in cytisine- or α-BTX–treated cultures compared with those in control cultures (Fig. 4J′). α7 nAChRs also localized to α-synaptophysin– and vGLUT1-positive glutamatergic presynaptic boutons (Fig. 5). The findings provide structural evidence linking α7 nAChRs and presynaptic NMDARs in the growth and formation of glutamatergic boutons.
Fig. 3.
Increased and enlarged NR1-positive axonal varicosities are glutamatergic presynaptic boutons. (A–C) NR1 and vGLUT1 immunoreactivities in control cultures. (D–I) Colocalization of glutamatergic presynaptic terminal marker, vesicular glutamate transporter 1 (vGLUT1), with NR1-positive axonal varicosities in cytisine- or α-BTX–treated cultures. (Scale bars as indicated.)
Fig. 4.
Glutamatergic presynaptic boutons containing α-synaptophysin are significantly enlarged and are largely en passant boutons. (A–C and A′–C′) α-Synaptophysin and vGLUT1 immunoreactivities in control cultures. (D–I and D′–I′) Colocalization of α-synaptophysin in increased and enlarged vGLUT1-positive glutamatergic presynaptic boutons in cytisine- or α-BTX–treated cultures. (J–L) Glutamatergic presynaptic boutons (vGLUT1 as glutamatergic presynaptic terminal marker) along axons (GAP-43 as axonal marker) in α-BTX–treated cultures. (Scale bars as indicated.) (J′) Quantitative measurement of bouton size, showing significant enlargement of glutamatergic boutons containing both α-synaptophysin and vGLUT1 in cytisine-treated (1.21 ± 0.05 μm2, n = 61, P < 0.001 vs. control) or α-BTX–treated (1.23 ± 0.06 μm2, n = 60, P < 0.001 vs. control) cultures compared with those in control cultures (0.39 ± 0.04 μm2, n = 31). n, Number of boutons in three to four fields from different cultures in each group. (Scale bars, 5 μm.)
Fig. 5.
α7 nAChRs are present in enlarged glutamatergic presynaptic boutons. (A–C) vGLUT1 immunoreactivity and α7 nAChR labeled by tetramethylrhodamine α-BTX in control axons. (D–I) Colocalization of α7 nAChR in enlarged vGLUT1-positive glutamatergic presynaptic boutons in cytisine- or α-BTX–treated cultures. (Scale bars as indicated.)
Presynaptic NMDAR Function Is Enhanced in Enlarged Glutamatergic Boutons and Likely Associated with Selective Enhancement of Postsynaptically Silent Synapses.
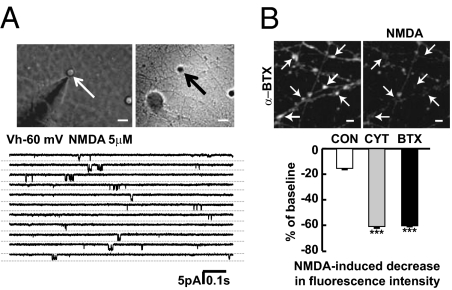
We then assessed whether presynaptic NMDARs in the enlarged boutons are functional. Direct voltage clamp recordings from outside-out pulled membrane patches of enlarged boutons revealed single channel currents in response to NMDA (5 μM) application at a holding potential of −60 mV, demonstrating the presence of functional NMDARs in the enlarged boutons (Fig. 6A). In addition, calcium imaging using Fura-2 as the calcium indicator was conducted using two-photon microscopy and 780-nm excitation; at this wavelength, fluorescence emission decreases upon calcium entry. In the presence of tetrodotoxin and voltage-dependent calcium channel blockers, bath application of NMDA produced a greater decrease in fluorescence intensity in boutons of α-BTX– or cytisine-treated cultures than those in control cultures (Fig. 6B). These results indicate that presynaptic NMDAR-mediated calcium entry is increased in boutons of treated cultures, suggesting that presynaptic NMDAR function is enhanced in the enlarged glutamatergic boutons.
Fig. 6.
Presynaptic NMDAR function is enhanced in enlarged presynaptic boutons. (A) Examples of NMDA-activated channel currents recorded in outside-out membrane patches excised from enlarged presynaptic boutons in treated cultures. Similar channel currents were recorded upon application of 5 μM NMDA from five distinct terminals, and displayed a chord conductance of 58 ± 4 pS. (B) Fura-2 calcium imaging using two-photon microscopy at 780-nm excitation wavelength showed that NMDA stimulation led to calcium entry as indicated by decreased fluorescence intensity in presynaptic boutons of α-BTX–treated cultures. Quantification showed that NMDA stimulation produced a greater decrease in fluorescence intensity in the presence of tetrodotoxin and calcium channel blockers in boutons of α-BTX– (n = 48, P < 0.001 vs. control) or cytisine-treated (n = 44, P < 0.001 vs. control) cultures compared with those of control cultures (n = 12). (Scale bars, 5 μm.)
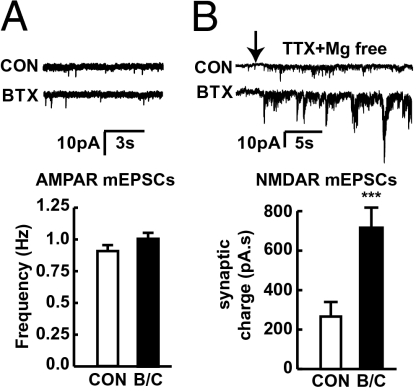
Furthermore, we assessed whether the enhanced presynaptic NMDAR function alters transmission. Surprisingly, whole-cell patch clamp of cortical neurons in α-BTX– or cytisine-treated cultures showed no alteration in the frequency of AMPAR mEPSCs (Fig. 7A), In contrast, we found a significant increase in the synaptic charge mediated by NMDAR mEPSCs (P < 0.001) (Fig. 7B). NMDA-mEPSCs were assessed as synaptic charge as their long duration and degree of overlap prevented individual detection. Combined, this lack of change in AMPAR and enhanced NMDAR mEPSCs suggests that the enlarged boutons are contained within postsynaptically silent synapses containing NMDARs but lacking AMPARs.
Fig. 7.
Selective enhancement of postsynaptically silent synapses upon inactivation of α7 nAChR. (A) Whole-cell voltage-clamp recordings illustrating examples of AMPAR mEPSCs in cortical cultures. Frequency of AMPAR mEPSCs was not altered in α-BTX– and cytisine-treated cortical neurons (n = 12) compared with control (n = 14). (B) Examples of robust increase in synaptic activity upon removal of Mg2+ due to overlapping NMDAR-mediated mEPSCs. Synaptic charge mediated by NMDAR mEPSCs was significantly increased in α-BTX– or cytisine-treated cortical neurons (n = 16, P < 0.001 vs. control) compared with control neurons (n = 13).
We also measured the total pool of NMDARs in cortical neurons by examining the whole-cell currents evoked by exogenous application of distinct concentrations (5 and 200 μM) of NMDA in the presence of tetrodotoxin and in the absence of Mg2+ in control and treated cultures. Whole-cell NMDA current density exhibited no significant increase between control and treated cultures (Fig. S5). Because we measured the total pool of postsynaptically localized NMDARs including extrasynaptic and synaptic receptors, we observed only a slight increase. This discrepancy may reflect the abundant extrasynaptic NMDARs during early synaptic development (22) and the possibility that bath application of exogenous NMDA may preferentially activate extrasynaptic receptors but have limited access to synaptic NMDARs. Taken together, these results suggest the selective enhancement of postsynaptically silent synapses upon inactivation of α7 nAChR.
Discussion
The present studies show that, in cortical neuronal cultures, chronic inactivation of α7 nAChRs increases the number and size of glutamatergic presynaptic boutons containing presynaptic NMDARs. Presynaptic NMDAR function is enhanced in these boutons. Although a variety of nicotinic agents altered NMDAR levels, the effects on presynaptic NMDARs and boutons were most prominent with exposure to compounds that block or desensitize α7 nicotinic receptors. The biochemical effects appear to reflect mainly structural changes in the presynaptic membrane, with appearance of enlarged axonal boutons containing large numbers of NMDARs. These presynaptic NMDARs can alter glutamate release and are potentially involved in altering postsynaptic neurotransmission and development, thus demonstrating a pharmacological, structural, and physiological interaction among presynaptic α7 nAChR and NMDAR during early synaptic development. A similar regulatory mechanism for analogous events has been observed in cultured cerebellar neurons. Presynaptic NMDARs influence development of GABAergic presynaptic boutons in developing cerebellar neurons (18), whereas, as we describe here, axonal α7 nAChRs alter development of glutamatergic presynaptic boutons in developing cortical neurons. Such parallel results suggest that presynaptic events control development of axonal bouton size and function in multiple neurotransmitter systems. These findings implicate presynaptic α7 nAChR/NMDAR interactions in synaptic development and plasticity.
As the present data identify changes in both presynaptic NMDAR levels and function induced by α7 blockade, altered presynaptic function may be a direct consequence of α7 nAChR/glutamate receptor interactions. Because our studies also reveal enhancement in presynaptic NMDAR-mediated glutamate release in these enlarged boutons, the potential consequences of α7 blockade may include a variety of downstream events, such as alteration of postsynaptic transmission and synaptic strength (16). Immunocytochemical studies revealed the presence of NR1, NR2 and GluR1 subunits in the enlarged glutamatergic presynaptic boutons. Considerable anatomical evidence supports the presence of NR1, NR2, and GluR1 in the presynaptic terminals or boutons in different brain regions, especially during early development (16, 23). NR2B is preferentially accumulated in axonal growth cones and varicosities in immature neurons (24), suggesting a role of presynaptic NMDAR in development. Physiologically, presynaptic NMDARs enhance the probability of spontaneous and evoked neurotransmitter release at cortical, hippocampal, and cerebellar synapses (16, 18–20). Although we identified enhanced presynaptic NMDAR levels and physiological responses, our findings further suggest the selective association of chronic α7 nAChR blockade and presynaptic NMDAR function with increases in postsynaptically silent synapses, as AMPAR-mediated synaptic currents were not altered, whereas those mediated by NMDARs were increased in treated cultures. As α7 nAChRs are preferentially located at presynaptic terminals incorporated in postsynaptically silent synapses (14, 25), our data suggest that presynaptic α7 nAChR/NMDAR interactions may be involved in establishing the conversion of silent to fully functional synapses and play crucial roles in presynaptic and postsynaptic development.
The present data also demonstrate specific structural and functional changes that could influence synaptic maturation. Cortical cultures develop in a manner that models synaptic maturation in vivo. The time course of synapse formation was delayed due to lack of glial cells and low density of cortical neurons in our cortical cultures, and thereby enables us to examine the events occurring in bouton formation during early synaptic development. Regulation of α7 nAChR could provide a physiological mechanism that mediates the development or plasticity of glutamatergic presynaptic boutons. Structural plasticity of presynaptic boutons has crucial roles in developmental circuit assembly processes and neural circuit remodeling in adult cortex (26–29). Axonal varicosities or boutons are a series of swellings along the axons, and are typically the sites that release transmitters and form synapses. They are usually divided into two subtypes: en passant and terminaux. En passant varicosities are swellings in the axonal shaft, whereas terminaux varicosities are swellings at the terminals that connect to the axonal shaft by a short neck. Here, chronic inactivation of axonal α7 nAChRs increased the number and size of glutamatergic presynaptic boutons, particularly those with the appearance of en passant boutons along glutamatergic axons. Studies in Caenorhabditis elegans and in rodent cortical cultures suggest that initial formation of presynaptic terminals can occur preferentially at predefined sites within axons in the absence of postsynaptic targets such as neuronal or glial contacts, suggesting that many en passant synapses form specifically and autonomously at predefined sites in developing axons (30–32). As α7 nAChRs are present within these enlarged boutons, and as en passant boutons are increased and enlarged upon inactivation of α7 nAChR, axonal α7 nAChRs may be an intrinsic factor within glutamatergic axons that modulates the formation of presynaptic boutons. Considerable anatomical and functional evidence supports the presence of α7 nAChRs in glutamatergic axonal terminals in different cortical regions (14, 25, 33–36). Thus, α7 may be a structural determinant for presynaptic development in glutamatergic synapses throughout the brain.
Although our data focus on modifications of the presynaptic membrane, there may be postsynaptic interactions of NMDAR and nicotinic receptors as well. In our studies, nicotine itself increased NMDAR levels but did not produce notable presynaptic changes, suggesting that nicotine exposure may increase postsynaptic NMDAR levels. Pharmacologically, this could reflect the high affinity of nicotine as a α4β2 agonist, and is in accordance with previous findings in which the effects of nicotine on NMDAR expression reflect the alterations in postsynaptic NMDARs (37, 38). Still, it is possible that nicotine could create presynaptic abnormalities as well with longer exposure or exposure at higher concentrations.
As the present data implicate presynaptic α7 nAChR/NMDAR interactions in synaptic development and plasticity during early development, abnormal presynaptic interactions due to genetic variations in α7 nAChR may thus produce permanent changes that could contribute to cognition deficits in schizophrenia and Alzheimer's disease (AD). A convergence of recent genetic evidence identifies α7 nAChR as a potential modifier gene for schizophrenia and AD patients (39–41). Similarly, deleting the α7 nAChR gene leads to impairment of working/episodic-like memory (42) and alters the synaptic development and cognition in AD transgenic mice (43, 44). α7 nAChRs thus are therapeutic targets for cognitive deficits in schizophrenia and AD (45–48). Moreover, both α7 nAChR agonists and antagonists have similar effects on enhancing LTP and cognition in animals (49, 50). Desensitization of α7 nAChRs may play an important role in both normal information processing and in various disease states and thus be used as a strategy for drug development (51, 52). Our findings implicate desensitization of α7 nAChRs in synaptic development and plasticity and thereby provide important insights into development of therapeutic drugs for treatment of cognitive deficits in schizophrenia and AD.
Materials and Methods
Neuronal Cultures.
Primary cortical cultures from E17–E19 rats were prepared, maintained, and treated as described in detail in SI Text. Neurobasal medium for cultures contains choline chloride, a selective agonist at α7 nAChR. In addition, cholinergic neurons are present in cortical cultures as identified by choline acetyltransferase (Abcam) immunostaining as described in the manufacturer's instructions.
Two-Photon Calcium Imaging.
Cortical neurons were loaded with the calcium indicator dye 5 μM fura-2-acetoxymethyl ester and subjected to two-photon imaging as described in detail in SI Text. NMDAR-mediated calcium images were captured and analyzed off-line using ImageJ software (Fiji, an Open Source image processing package based on ImageJ), and data were presented as relative changes in fluorescence with respect to background fluorescence (Δf/f).
Electrophysiology.
Whole-cell patch-clamp and outside-out membrane patch recordings were performed as described in detail in SI Text. The frequency of AMPAR-mediated mEPSCs was measured, and NMDAR-mediated mEPSCs were assessed as synaptic charge from total current integrated during a 1-min time interval, as their long duration and degree of overlap prevented individual detection. Outside-out membrane patches were excised from visually identified enlarged boutons.
Statistical Analysis.
Data are shown as mean ± SEM. Experiments were analyzed using Student's t test to compare two conditions or by ANOVA followed by planned comparisons of multiple conditions. Significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Margaret Maronski (University of Pennsylvania) for cortical neuronal cultures, Dr. Robert Kalb (The Children's Hospital of Philadelphia) for confocal microscopy, Dr. Guoxiang Xiong (The Children's Hospital of Philadelphia) for technical suggestions on immunocytochemistry, and Dr. Jon Lindstrom (University of Pennsylvania) for α7 cell lines. The studies were supported by National Institutes of Health Grant NS45986 (to D.R.L) and the CHOP Trisomy 21 Program (to D.R.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007397107/-/DCSupplemental.
References
- 1.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: Multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 2.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 3.Placzek AN, Zhang TA, Dani JA. Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol. 2009;78:686–692. doi: 10.1016/j.bcp.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nashmi R, Lester HA. CNS localization of neuronal nicotinic receptors. J Mol Neurosci. 2006;30:181–184. doi: 10.1385/JMN:30:1:181. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Warren RA. Muscarinic and nicotinic presynaptic modulation of EPSCs in the nucleus accumbens during postnatal development. J Neurophysiol. 2002;88:3315–3330. doi: 10.1152/jn.01025.2001. [DOI] [PubMed] [Google Scholar]
- 6.Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence: Implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary KT, Leslie FM. Developmental regulation of nicotinic acetylcholine receptor-mediated [3H]norepinephrine release from rat cerebellum. J Neurochem. 2003;84:952–959. doi: 10.1046/j.1471-4159.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 9.Dajas-Bailador FA, Lima PA, Wonnacott S. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39:2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 10.Jonnala RR, Buccafusco JJ. Relationship between the increased cell surface alpha7 nicotinic receptor expression and neuroprotection induced by several nicotinic receptor agonists. J Neurosci Res. 2001;66:565–572. doi: 10.1002/jnr.10022. [DOI] [PubMed] [Google Scholar]
- 11.Huang YY, Kandel ER, Levine A. Chronic nicotine exposure induces a long-lasting and pathway-specific facilitation of LTP in the amygdala. Learn Mem. 2008;15:603–610. doi: 10.1101/lm.975308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Filippi G, Baldwinson T, Sher E. Nicotinic receptor modulation of neurotransmitter release in the cerebellum. Prog Brain Res. 2005;148:307–320. doi: 10.1016/S0079-6123(04)48024-8. [DOI] [PubMed] [Google Scholar]
- 13.Girod R, Barazangi N, McGehee D, Role LW. Facilitation of glutamatergic neurotransmission by presynaptic nicotinic acetylcholine receptors. Neuropharmacology. 2000;39:2715–2725. doi: 10.1016/s0028-3908(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 14.Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezvani K, Teng Y, Shim D, De Biasi M. Nicotine regulates multiple synaptic proteins by inhibiting proteasomal activity. J Neurosci. 2007;27:10508–10519. doi: 10.1523/JNEUROSCI.3353-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: Newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14:609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiszman ML, et al. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grilli M, et al. Exposure to an enriched environment selectively increases the functional response of the pre-synaptic NMDA receptors which modulate noradrenaline release in mouse hippocampus. J Neurochem. 2009;110:1598–1606. doi: 10.1111/j.1471-4159.2009.06265.x. [DOI] [PubMed] [Google Scholar]
- 21.Varas R, et al. Electrophysiological characterization of nicotinic acetylcholine receptors in cat petrosal ganglion neurons in culture: Effects of cytisine and its bromo derivatives. Brain Res. 2006;1072:72–78. doi: 10.1016/j.brainres.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Petralia RS, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenk U, Matteoli M. Presynaptic AMPA receptors: More than just ion channels? Biol Cell. 2004;96:257–260. doi: 10.1016/j.biolcel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Herkert M, Röttger S, Becker CM. The NMDA receptor subunit NR2B of neonatal rat brain: Complex formation and enrichment in axonal growth cones. Eur J Neurosci. 1998;10:1553–1562. doi: 10.1046/j.1460-9568.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 25.Metherate R, Hsieh CY. Regulation of glutamate synapses by nicotinic acetylcholine receptors in auditory cortex. Neurobiol Learn Mem. 2003;80:285–290. doi: 10.1016/s1074-7427(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 26.Majewska AK, Sur M. Plasticity and specificity of cortical processing networks. Trends Neurosci. 2006;29:323–329. doi: 10.1016/j.tins.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–524. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martín-Peña A, et al. Age-independent synaptogenesis by phosphoinositide 3 kinase. J Neurosci. 2006;26:10199–10208. doi: 10.1523/JNEUROSCI.1223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 33.Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones IW, Wonnacott S. Precise localization of α7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong C, et al. Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J Neurosci. 2008;28:9111–9116. doi: 10.1523/JNEUROSCI.0381-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hancock ML, Canetta SE, Role LW, Talmage DA. Presynaptic type III neuregulin1-ErbB signaling targets alpha7 nicotinic acetylcholine receptors to axons. J Cell Biol. 2008;181:511–521. doi: 10.1083/jcb.200710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki Y, Jia Y, Niu R, Sumikawa K. Nicotine exposure in vivo induces long-lasting enhancement of NMDA receptor-mediated currents in the hippocampus. Eur J Neurosci. 2006;23:1819–1828. doi: 10.1111/j.1460-9568.2006.04714.x. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki Y, Jia Y, Wong JK, Sumikawa K. Chronic nicotine-induced switch in Src-family kinase signaling for long-term potentiation induction in hippocampal CA1 pyramidal cells. Eur J Neurosci. 2006;24:3271–3284. doi: 10.1111/j.1460-9568.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 39.Heinzen EL, et al. Genome-wide scan of copy number variation in late-onset Alzheimer's disease. J Alzheimers Dis. 2010;19:69–77. doi: 10.3233/JAD-2010-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinkus ML, et al. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carson R, et al. Genetic variation in the alpha 7 nicotinic acetylcholine receptor is associated with delusional symptoms in Alzheimer's disease. Neuromolecular Med. 2008;10:377–384. doi: 10.1007/s12017-008-8048-8. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain Behav. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 43.Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the α7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci. 2009;29:8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Yamada K, Nabeshima T, Sokabe M. alpha7 Nicotinic acetylcholine receptor as a target to rescue deficit in hippocampal LTP induction in beta-amyloid infused rats. Neuropharmacology. 2006;50:254–268. doi: 10.1016/j.neuropharm.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen MS, Christensen DZ, Hansen HH, Redrobe JP, Mikkelsen JD. alpha(7) Nicotinic acetylcholine receptor activation prevents behavioral and molecular changes induced by repeated phencyclidine treatment. Neuropharmacology. 2009;56:1001–1009. doi: 10.1016/j.neuropharm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: How the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302–311. doi: 10.1016/j.pharmthera.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Hajós M, Rogers BN. Targeting alpha7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr Pharm Des. 2010;16:538–554. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- 49.Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- 50.Fujii S, Ji Z, Sumikawa K. Inactivation of alpha7 ACh receptors and activation of non-alpha7 ACh receptors both contribute to long term potentiation induction in the hippocampal CA1 region. Neurosci Lett. 2000;286:134–138. doi: 10.1016/s0304-3940(00)01076-4. [DOI] [PubMed] [Google Scholar]
- 51.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 52.Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.