Abstract
Bacteriophage φ29 DNA polymerase is a unique enzyme endowed with two distinctive properties, high processivity and faithful polymerization coupled to strand displacement, that have led to the development of protocols to achieve isothermal amplification of limiting amounts of both circular plasmids and genomic DNA. To enhance the amplification efficiency of φ29 DNA polymerase, we have constructed chimerical DNA polymerases by fusing DNA binding domains to the C terminus of the polymerase. The results show that the addition of Helix-hairpin-Helix [(HhH)2] domains increases DNA binding of the hybrid polymerases without hindering their replication rate. In addition, the chimerical DNA polymerases display an improved and faithful multiply primed DNA amplification proficiency on both circular plasmids and genomic DNA and are unique φ29 DNA polymerase variants with enhanced amplification performance. The reported chimerical DNA polymerases will contribute to make φ29 DNA polymerase-based amplification technologies one of the most powerful tools for genomics.
Keywords: DNA amplification, processivity, protein engineering, strand displacement, helix-hairpin-helix domain
A cornerstone of molecular biology, as genomic studies and phylogenetic and epidemiological analysis, is the amplification of limited amounts of DNA (1).
Presently, the polymerase chain reaction (PCR) (2) is still the most widely used methodology for DNA amplification. However, PCR depends on a previous knowledge of the sequence to be amplified, and it yields relatively short amplicons. Whole genome amplification technologies have been recently developed to generate large amounts of high quality DNA for genomic studies. Although several of the approaches are still based on PCR cycling, as primer extension preamplification (PEP) (3) or degenerated oligonucleotide primed PCR (DOP-PCR) (4, 5), isothermal multiple displacement amplification (MDA) by bacteriophage φ29 DNA polymerase has successfully gained ground in the DNA amplification area (6, 7). Due to the use of random hexamer primers, no previous sequence information is required, thus, any DNA is susceptible to be amplified; on the other hand, amplification performed by the φ29 DNA polymerase can generate large DNAs of hundreds of Kbs. In addition, multiply primed rolling circle amplification (RCA) has been shown to be the most robust technology to amplify circular templates of variable size required for genome sequencing (7), for the efficient amplification and detection of known and unknown circular viral genomes, for genotyping of single nucleotide polymorphisms (8), for whole genome analysis of noncultivable viruses (9), for detection and identification of circular plasmids in zoonotic pathogens (10), and very recently also for the description of new metagenomes (11). The combination of plasmid amplification and further direct sequencing represents a simple, fast, and low-cost method to get results comparable to those obtained with the use of other methods available (12). The success of MDA relies on the intrinsic and distinctive features of φ29 DNA polymerase: outstanding processivity and ability to couple polymerization to strand displacement (13).
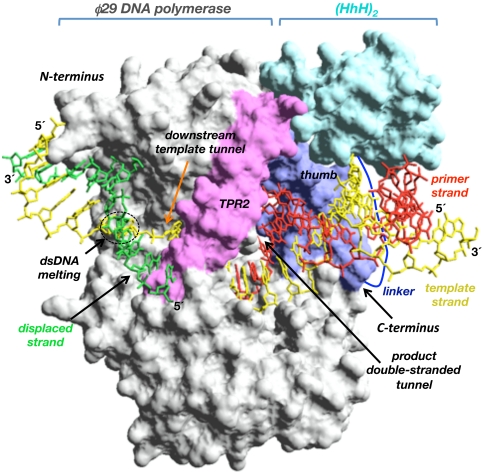
Crystallographic and biochemical studies gave insights into the molecular mechanism by which φ29 DNA polymerase is singular in coupling processive polymerization to strand displacement (14, 15). Thus, an amino acid insertion specifically conserved in the polymerization domain of the protein-primed subgroup of DNA polymerases, named TPR2 (16, 17), contributes to form a narrow tunnel around the template strand, forcing melting of the duplex ahead of the polymerase (strand displacement) to gain access to the polymerase active site (see Fig. 1) (15). Additionally, in the polymerization domain, the TPR2 insertion along with the palm and thumb subdomains form a closed doughnut-shaped structure that encircles the upstream duplex product, in contrast to the open polymerization domain of most DNA polymerases, endowing φ29 DNA polymerase with high processivity in a manner analogous to sliding-clamp factors (14, 15, 18).
Fig. 1.
Modeling of the chimerical DNA polymerase. The figure represents the structural model of a (HhH)2 domain (colored in cyan) joint through a linker peptide (in dark blue) to the C terminus of φ29 DNA polymerase (colored in gray). φ29 DNA polymerase TPR2 insertion and thumb subdomain are colored in pink and dark blue, respectively. The modeled primer, template, and displaced strands are colored in red, yellow, and green, respectively. The upstream tunnel that encircles the newly synthesized dsDNA, the downstream template tunnel, as well as the N and C termini of φ29 DNA polymerase are indicated. Crystallographic data are from Protein Data Bank ID codes 2PZX (φ29 DNA polymerase binary complex) (18) and 1BPX [(HhH)2 domain of DNA polymerase β (residues 91–148) (39)]. Figure was made by using the Swiss-PdbViewer software (www.expasy.org/spdbv).
These structural data also unraveled the specific and delicate spatial arrangement of the different polymerization subdomains and the 3′–5′ exonuclease domain of φ29 DNA polymerase, allowing the efficient coupling of processive DNA polymerization to strand displacement, neither impairing the polymerization catalytic rate nor the proofreading of the insertion errors.
Due to their structural complexity and the multiple interactions with the substrates, acquisition of new properties by DNA polymerases is likely to require large structural modifications, which is difficult to tackle with point mutations. In this work we show that fusion of specific DNA binding domains to the C terminus of φ29 DNA polymerase provides the enzyme with a better DNA binding that leads to an improvement in its amplification efficiency.
Results and Discussion
Design of Chimerical φ29 DNA Polymerases.
The most promising strategies to confer new properties to a DNA polymerase are domain swapping and domain tagging (19). In this sense, efforts have been focused to increase the low processivity of the thermostable enzymes used in PCR, as Taq and Pfu DNA polymerases. Thus, Motz et al. (20) fused the PCNA binding domain of Archaeoglobus fulgidus DNA polymerase B to the C terminus of Taq DNA polymerase. The hybrid polymerase was stimulated in the presence of PCNA, although it was less active than the original enzyme in PCR. Davidson et al. (21) inserted the thioredoxin-binding domain of T3 DNA polymerase at an analogous position of Taq DNA polymerase. Despite the fact that addition of the processivity factor thioredoxin increased the processivity of the hybrid DNA polymerase from 80 nt to 300 nt, it was very inefficient to carry out PCR of fragments larger than 5 kb. An alternative and encouraging approach to improve PCR performance consists in the fusion of DNA binding proteins to the amplification polymerase. Thus, Wang et al. (22) were successful in conferring a higher processivity to Taq (from 22 nt to 104 nt) and Pfu (from 6 nt to 55 nt) DNA polymerases by linking the polymerase domain to the dsDNA binding protein of Sulfolobus solfataricus. Additionally, it has been reported that the fusion of (HhH)2 domains of Methanopyrus kandleri Topoisomerase V to the C or N terminus of Taq and Pfu DNA polymerases produced hybrid enzymes that retained the intrinsic low processivity at high levels of salt and other inhibitors of DNA synthesis (19, 23).
Because isothermal MDA using φ29 DNA polymerase is the most promising alternative to PCR, a major goal is the construction of φ29 DNA polymerase variants with improved amplification efficiency. φ29 DNA polymerase is provided with a nearly unlimited processivity (13). Thus, our efforts have been focused to get new φ29-based DNA polymerases with an enhanced DNA binding to increase the DNA amplification performance exhibited by this enzyme. In this sense, M. kandleri Topo V (HhH)2 domain H (residues 696–751) or H and I (residues 696–802) (24–27) have been fused to the C terminus of φ29 DNA polymerase because it lies just at the exit of the upstream dsDNA product (see Fig. 1) (14, 18). Fusion of a (HhH)2 domain at the N terminus of φ29 DNA polymerase could hinder its intrinsic strand displacement capacity, because biochemical and structural data demonstrated that unwinding of parental DNA takes place close to it (Fig. 1) (14, 15). Either one or two (HhH)2 domains were fused to the polymerase through the flexible linker Gly-Thr-Gly-Ser-Gly-Ala (28) to preserve the structural folding of the enzyme and the DNA binding domains, rendering the chimerical polymerases φ29-H and φ29-HI (H and I stand for Topo V domains H and I, respectively; see Materials and Methods). A modeling of a (HhH)2 domain linked to the C-terminus of φ29 DNA polymerase is shown in Fig. 1.
Chimerical DNA Polymerases Show a Higher DNA Binding.
To analyze whether fusion of the (HhH)2 domains to φ29 DNA polymerase enhances its DNA binding, DNA gel retardation assays were performed, using as substrate a labeled hybrid 15-mer/21-mer DNA molecule (see Materials and Methods).
As shown in Fig. 2, φ29 DNA polymerase produces a single retardation band corresponding to a stable enzyme–DNA complex competent for polymerization (29) whose intensity depends on the concentration of enzyme added. Chimeras φ29-H and φ29-HI showed an improved DNA binding, requiring about twofold less enzyme concentration than the wild-type polymerase to give a similar shifted band. Thus, addition of (HhH)2 domains H and HI from Topo V to the C-terminal end of the φ29 DNA polymerase endows the enzyme with an improved DNA binding.
Fig. 2.
Chimerical DNA polymerases have an improved DNA binding. The 5′-labeled hybrid molecule 15mer/21mer (dsDNA) was incubated with φ29 DNA polymerase or with the indicated chimerical DNA polymerase, under the conditions described in Materials and Methods. After gel electrophoresis, the mobility of free dsDNA and the polymerase-DNA complex was detected by autoradiography. The experiment shown is representative of several experiments.
Rolling Circle Replication (RCR) by Chimerical DNA Polymerases.
As mentioned above, the use of φ29 DNA polymerase to perform successful DNA amplification relies on its faculty to couple processive polymerization to strand displacement by virtue of a simultaneous binding and translocation of the primer, template, and displaced strands through different regions of the polymerization domain (see Fig. 1). Thus, any alteration in this fine-tuned binding equilibrium could hinder the replication rate. To ascertain whether the improvement in DNA binding displayed by the chimerical polymerases affected the distinctive hallmarks of φ29 DNA polymerase, RCR assays were carried out using as substrate singly primed circular ssDNA of bacteriophage M13 (see Materials and Methods). In this assay the first replication round does not require any unwinding activity. However, to proceed through the next replication rounds the polymerase will have to deploy its active strand displacement (rolling circle type) (see Materials and Methods).
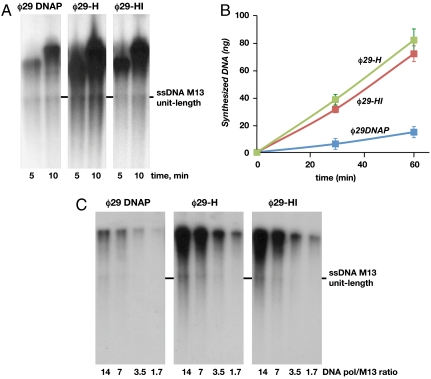
As it is shown in Fig. 3A, the length of the products synthesized by the three DNA polymerases was comparable. The fact that at the shortest reaction time products migrate at the resolving part of the gel indicates that the replication rate displayed by the chimeras was similar to that of φ29 DNA polymerase. This result indicates that, on the one hand, the (HhH)2 domains interfere with neither the proper placement of the primer/template junction at the polymerization active site nor the strand displacement in chimerical DNA polymerases and, on the other hand, that the enhancement in DNA binding described above does not imply a tighter DNA polymerase/DNA interaction that could halt translocation of the enzyme along the DNA. Thus, considering the almost identical length of the products obtained, the increase in the total amount of DNA synthesized by chimeras φ29-H and φ29-HI with respect to φ29 DNA polymerase (five- and fourfold, respectively) could be explained by a meliorated usage of the primer/template junctions, in agreement with their improved DNA binding.
Fig. 3.
Chimerical DNA polymerases show an enhanced RCR efficiency. (A) Strand displacement-coupled M13 DNA replication by φ29 DNA polymerase and chimerical DNA polymerases. Replication of 250 ng of singly primed M13 DNA was carried out as described in Materials and Methods using 60 nM of φ29 wild-type or chimerical DNA polymerases. The position of unit-length M13 DNA is shown at the right. (B) Multiply primed RCR of M13 DNA by φ29 DNA polymerase and chimerical DNA polymerases. The assay was performed as described in Materials and Methods, in the presence of 250 ng of multiply primed M13 DNA as input and 60 nM of either φ29 DNA polymerase or the indicated chimerical DNA polymerase. Data are represented as Mean ± SD. (C) Processive synthesis by φ29 DNA polymerase and chimerical DNA polymerases. The assay was performed as described in the text in the presence of 250 ng of singly primed M13 DNA and decreasing concentrations of the indicated DNA polymerase. After incubation at 30 °C for 20 min, samples were processed as described in A.
The current φ29 DNA polymerase-based amplification protocols make use of random primers as short as hexamers. Thus, to ascertain whether the presence of (HhH)2 domains confers a benefit on chimerical DNA polymerases also under these conditions, RCR assays were performed using as primers multiple hexamers (see Materials and Methods). As shown in Fig. 3B, chimerical DNA polymerases φ29-H and φ29-HI carried out RCR more efficiently than φ29 DNA polymerase, increasing the amount of DNA synthesized five- and sixfold, respectively.
Besides its intrinsic strand displacement capacity, the other specific feature that differentiates φ29 DNA polymerase is its processivity (13). To evaluate DNA polymerase processivity, the extension of each primer to one round of synthesis has to be assured so that the number of nucleotides added to each primer is equivalent to the processivity of the polymerase (30). For extremely processive DNA polymerases as φ29 DNA polymerase, this condition can be achieved by analyzing the distribution of products in a singly primed RCR assay under a range of ratios of DNA polymerase to substrate, a commonly used methodology (13, 30). A distributive (nonprocessive) pattern of elongation should give rise to a decrease in the DNA size concomitant with the polymerase dilution, because at low DNA polymerase concentrations the chance of dissociated DNA polymerase molecules to reassociate to an elongated primer is prevented. As shown in Fig. 3C, the reduction in the DNA polymerase/DNA molar ratio diminishes the total amount of DNA synthesized by φ29 DNA polymerase and by the two chimeras without encompassing shortening of the products, indicating that the same enzyme that starts replication proceeds with elongation of the growing chain without dissociation, i.e., processively.
Therefore, the fusion of the (HhH)2 domains to the C-terminal end of φ29 DNA polymerase significantly improved its polymerization potential.
Multiply Primed RCA of Plasmid DNA by Chimerical DNA Polymerases.
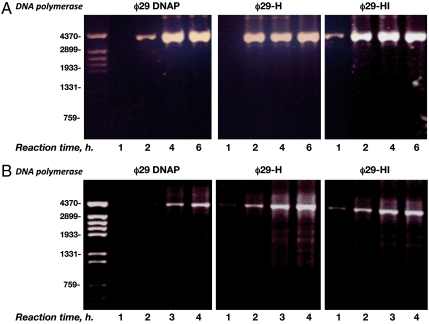
The use of φ29 DNA polymerase in combination with 3′-protected random hexamers that allow synthesis of both strands has been the basis for the development of one of the most efficient procedures for isothermal dsDNA amplification of circular plasmids, named RCA (6, 7). In this methodology, the number of input DNA molecules used as substrate is reduced 105-fold with respect to the RCR assays. Due to this significant difference between both protocols, an increase in the efficiency of chimerical DNA polymerases to perform RCA could not be anticipated. Therefore, RCA assays were set up in the presence of 1 ng (Fig. 4A) and 1 pg (Fig. 4B) of a dsDNA circular plasmid (see also Materials and Methods) and 3′-protected random hexamers. As shown in Fig. 4A, φ29 DNA polymerase gave rise to detectable amplification products using EtBrd staining at 2 h, and amplification continued for two more hours before reaching a plateau. Interestingly, such an amplification plateau was attained by both chimerical DNA polymerases in only 2 h. At this reaction time the amount of amplified DNA by the chimeras was fourfold higher than that rendered by φ29 DNA polymerase. Furthermore, chimera φ29-HI produced detectable amplification products at 1 h. In all cases the final amount of amplified material after 4 h was similar, most likely due to the exhaustion of the dNTPs provided. In the presence of 103-fold lower input DNA (1 pg; Fig. 4B) φ29 DNA polymerase required 3 h to give an amplification product. Chimerical DNA polymerases rendered a perceptible amplification product from 1 h, reaching the amplification plateau at 3 h, the total amplification output at this reaction time being three- to fourfold higher than that obtained with φ29 DNA polymerase. With the three DNA polymerases, more than 80% of the amplified DNA was linearized after digestion with the endonuclease EcoRI rendering a 4.2 kbp dsDNA fragment, thus, most of the amplification products were tandem repeats of the original plasmid.
Fig. 4.
Multiply primed RCA of plasmidic DNA by φ29 DNA polymerase and chimerical DNA polymerases. The assay was performed as described in Materials and Methods, in the presence of 1 ng (A) or 1 pg (B) of plasmidic DNA as input and 50 nM of φ29 DNA polymerase or the indicated chimerical DNA polymerase. At the left, linear DNA fragments obtained after digesting φ29 DNA with HindIII, used as DNA length markers.
Additionally, 4,918 nonoverlapping nucleotides from the amplification reactions were sequenced as described (31) (see also Materials and Methods). φ29 DNA polymerase and the chimeras performed faithful DNA amplification because no polymerization errors were detected.
The results described above indicate that the addition of (HhH)2 domains to φ29 DNA polymerase improves the amplification performance in chimerical DNA polymerases, at least under the current RCA conditions. Because chimeras exhibit a similar polymerization rate as φ29 DNA polymerase (see above), the faster amplification burst could be mainly explained by their enhanced interaction with DNA, most likely affecting recognition/stabilization of the primer/template junction.
Multiply Primed DNA Amplification of Genomic DNA by φ29 Wild-Type and Chimerical DNA Polymerases.
Besides the development of RCA technology, a simple and reliable whole genome amplification method named multiple displacement amplification (MDA) has been also developed (6). To investigate whether fusion of (HhH)2 domains boosts the intrinsic efficiency of φ29 DNA polymerase to amplify genomic DNA, MDA assays were set up, using Bacillus subtilis genomic DNA as substrate (see Materials and Methods).
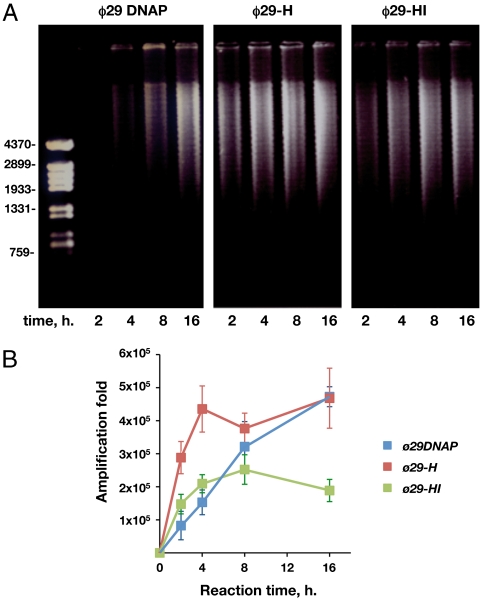
As shown in Fig. 5A, in the presence of 1 ng of genomic DNA as input, φ29 DNA polymerase yielded amplification products after 4 h of incubation, whereas chimeras φ29-H and φ29-HI rendered higher amounts of amplification products from the shortest time assayed (2 h). At this reaction time chimera φ29-H produced an amount of amplified product comparable to that observed after 8 h with φ29 DNA polymerase, whereas the DNA synthesized by chimera φ29-HI was comparable to that rendered by the wild-type enzyme after 4 h of reaction. Although both chimeras reached an amplification plateau after 4 h, the total amount of DNA synthesized by chimera φ29-HI was smaller than that produced by chimera φ29-H and by the φ29 DNA polymerase at the longest reaction time.
Fig. 5.
Multiply primed whole genome amplification of genomic DNA with φ29 DNA polymerase and chimerical DNA polymerases. The assay was performed as described in Materials and Methods, in the presence 1 ng of genomic DNA from B. subtilis and 50 nM of each polymerase. After the indicated reaction times, 1 μl of each sample was loaded in a Etd bromide-containing 0.7% agarose gel (A). At the left, linear DNA fragments obtained after digesting φ29 DNA with HindIII, used as DNA length markers. 1 μl of a 250-fold dilution of each sample was subjected to qPCR as described in Materials and Methods. The specific amplification factors are represented as Mean ± SD in B.
To analyze the specificity of the amplified DNA, real-time quantitative PCR (qPCR) of each sample was carried out, using primers that amplify a 700 bp region of the B. subtilis gene yshC (see Materials and Methods). After 2 h, the number of copies of gene yshC rendered by chimeras φ29-H and φ29-HI was four- and twofold higher, respectively, than those obtained with the wild-type enzyme (Fig. 5B). After 4 h, an amplification plateau of 4 × 105- and 2 × 105-fold was reached by φ29-H and φ29-HI, respectively, yielding 2.7- and 1.5-fold more copies than φ29 DNA polymerase. As observed in Fig. 5B, achievement of a specific amplification level similar to that obtained by chimera φ29-H at 4 h took φ29 DNA polymerase a longer time (8–16 h). These results indicate that the presence of the (HhH)2 domain is also beneficial to amplify genomic DNA by the chimerical DNA polymerases. Although chimera φ29-HI also reached the amplification plateau after 4 h, the specific amplification (2 × 105) was twofold lower than that reached by φ29 DNA polymerase and chimera φ29-H. Thus, despite the high capacity exhibited by chimera φ29-HI in amplifying specifically circular plasmidic DNA, the presence of two (HhH)2 domains could be hampering amplification once a certain amount of linear genomic DNA has been produced, maybe trapping the hybrid enzyme at unspecific dsDNA regions.
Notwithstanding its advantages to amplify DNA, MDA is hampered by nonspecific synthesis due either to the presence of contaminant DNA that competes with the input template or to the formation of DNA molecules via direct elongation of the hexamers as well as by some amplification bias. To overcome these drawbacks, it has been shown that diminishing of the reaction volume to the nanoliter scale largely improves specificity as well as provides an even representation of all the sequences (32, 33). Recently, new primers that suppress the template-independent amplification have been reported and, in combination with an increase in the reaction temperature, also reduce the amplification bias (34). To date, MDA improvements have been achieved by exploring different technical modifications of the current protocols. Here, we report previously undescribed enhancement of DNA synthesis by modifying the isothermal amplification enzyme instead of the reaction conditions. The combination of the reported chimerical DNA polymerases with those modified protocols will contribute to make φ29 DNA polymerase-based amplification technologies one of the most powerful tools for genomics.
Materials and Methods
Construction of Chimerical DNA Polymerases.
To make chimera φ29-HI, a DNA fragment containing the residues that code for the (HhH)2 domains H (56 amino acids) and I (51 amino acids) of the Topoisomerase V from M. kandleri (GenBank code AF311944 and ref. 23), was synthesized by the GenScript Corporation and cloned between the EcoRV sites of commercial vector pUC57. The resulting plasmid pUC57-(HhH)2 was used as template to amplify by PCR a DNA fragment that will code for domains H and I. Thus, antisense primer 2 (5′-GGCGGGATCCTTAATGATGATGATGATGATGGCC) together with the sense primer 1 (5′GGCACCGGCTCTGGCGCCTGGAAAGAATGGCTGGAACG) rendered the 369 bp DNA fragment I. Primer 1, in addition to a KasI site, also introduced the sequence coding for the linker GTGSGA (a derivative of the GTGSGT linker previously described) (28). Antisense primer 2 contained the sequence that will code for a stop codon and a BamHI site (see in Fig. 6 a simplified scheme of the construction of the chimerical DNA polymerases). In parallel, a derivative of the pJLw2 plasmid (35), which contains the gene that codes for φ29 DNA polymerase (573 amino acids), was used as template for a PCR reaction performed with sense primer 3 (5′-CCGTCTCCGGGAGCTGCATGTG) that includes a 5′ HindIII site, and antisense primer 4 (5′- GGCGCCAGAGCCGGTGCCTTTGATTGTGAATGTGTCATCAACC), to obtain the 1757 bp fragment II that will contain the DNA coding for the φ29 DNA polymerase followed by the sequence GTGSGA that also includes a KasI site. Fragments I and II were purified from 0.7% agarose gels and further digested with KasI. The digested DNA fragments were ligated with T4 DNA ligase to obtain a 2108 bp linear DNA coding for chimera φ29-HI. The ligated products were purified from 0.7% agarose gels and further digested with BamHI and HindIII endonucleases. The digested product was purified from agarose gel and finally cloned into the pT7-4 vector (36). The gene coding for chimera φ29-HI (φ29 DNA polymerase + linker GTGSGA + (HhH)2 domains H and I from topoV) was used as template to construct chimera φ29-H by inserting a stop codon after the TopoV fragment H by the QuikChange® site-directed mutagenesis kit (Stratagene). Confirmation of the DNA sequence and absence of additional mutations was carried out by sequencing the entire gene. Chimerical DNA polymerases were expressed in Escherichia coli BL21(DE3) cells harboring the chimerical gene cloned into the plasmid pT7-4, and further purified essentially as described (35). The chimerical DNA polymerases obtained were φ29-H [φ29 DNA polymerase-GTGSGA- (HhH)2 H (635 aa; ∼73 kDa)] and φ29-HI (φ29 DNA polymerase-GTGSGA- (HhH)2 H-I (692 aa; ∼80 kDa).
Fig. 6.
Scheme of the different steps followed to construct chimeras φ29-H and φ29-HI. Details are given in the Materials and Methods.
DNA Binding Assay.
Oligonucleotides 15-mer (5′-GATCACAGTGAGTAC) and 21-mer (5′-TCTATTGTACTCACTGTGATC), which has a 5′-extension of 6 nucleotides in addition to the sequence complementary to the 15-mer, were supplied by Isogen. Oligonucleotide 15-mer was 5′-labeled with [γ-32P]ATP and T4 polynucleotide kinase. 5′-labeled 15-mer was hybridized to 21-mer in the presence of 0.2 M NaCl and 60 mM Tris-HCl, pH 7.5. The resulting 5′-labeled 15-mer/21-mer hybrid molecule was used as DNA primer/template to analyze the interaction with either φ29 DNA polymerase or chimerical DNA polymerases. The incubation mixture contained, in a final volume of 20 μl, 12 mM Tris-HCl, pH 7.5, 1 mM EDTA, 20 mM ammonium sulphate, 0.1 mg/ml BSA, 1 nM of the 15-mer/21-mer molecule and the indicated concentrations of the corresponding enzyme. After incubation for 5 min at 30 °C, the samples were subjected to electrophoresis in 4% (w/v) polyacrylamide gels (80∶1, monomer∶bis), containing 12 mM Tris-acetate, pH 7.5, and 1 mM EDTA, and run at room temperature in the same buffer at 8 V/cm, essentially as described in ref. 37. After autoradiography, the polymerase complexed with dsDNA was detected as a mobility shift (retardation) in the migrating position of the labeled DNA.
Rolling Circle Replication Assay.
M13mp18 ssDNA was hybridized to a specific 17mer (singly primed) or to primer HNCCTC (where H is not G) that hybridizes in 61 sites (multiply primed) in the presence of 0.2 M NaCl and 60 mM Tris-HCl, pH 7.5, and the resulting molecule was used as a primer/template to analyze processive DNA polymerization coupled to strand displacement by chimerical DNA polymerases. The incubation mixture contained, in 25 μl, 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT, 4% (v/v) glycerol, 0.1 mg/ml BSA, 40 μM each dCTP, dGTP, dTTP, and [α-32P]dATP (1 μCi), 4.3 nM of primed M13mp18 ssDNA, and 30 nM of φ29 DNA polymerase or chimerical DNA polymerases. After incubation for the indicated times at 30 °C, the reactions were stopped by adding 10 mM EDTA-0.1% SDS, and the samples were filtered through Sephadex G-50 spin columns. The synthesized DNA was calculated from the Cerenkov radiation corresponding to the excluded volume. For size analysis, the labeled DNA was denatured by treatment with 0.7 M NaOH and subjected to electrophoresis in alkaline 0.7% agarose gels, as described (38). After electrophoresis, unit-length M13mp8 ssDNA was detected by Etd bromide staining and gels were dried and autoradiographed.
Processivity Assay.
The assay was performed using as substrate 4.3 nM singly primed M13mp18 ssDNA as described above, incubating the reactions 20 min at 30°. Polymerization processivity was assessed by analysis of the length of the replication products under the decreasing DNA polymerase/DNA ratios indicated.
Multiply Primed RCA.
The incubation mixture contained, in 12.5 μl, 37 mM Tris-HCl, pH 7.5, 0.025% Tween-20, 50 mM KCl, 5 mM (NH4)2SO4, 10 mM MgCl2, 50 μM of 3′-protected random hexamers, 500 μM dNTPs and the indicated amount of plasmid DNA (4.2 kbp) as input. To denature DNA, samples were incubated for 3 min at 95 °C and afterward chilled on ice for 5 min. Reactions were started by adding 50 nM of φ29 DNA polymerase or the corresponding chimerical DNA polymerase. After incubation at 30 °C for the indicated times, reactions were stopped by incubating the samples for 10 min at 65 °C. One μl of each reaction was digested with EcoRI and further analyzed by electrophoresis in 0.7% agarose gels. After electrophoresis, the amplified DNA was detected by Etd bromide staining.
Multiply Primed DNA Amplification of Genomic DNA.
The incubation mixture contained, in 12.5 μl, 37 mM Tris-HCl, pH 7.5, 0.025% Tween-20, 50 mM KCl, 5 mM (NH4)2SO4, 10 mM MgCl2, 50 μM of 3′-protected random hexamers, 500 μM dNTPs and 1 ng of B. subtilis genomic DNA. To denature DNA, the samples were incubated for 3 min at 95 °C and afterward chilled on ice for 5 min. Reactions were started by adding 50 nM of φ29 DNA polymerase or the corresponding chimerical DNA polymerase. After incubation at 30 °C for the indicated times, reactions were stopped by incubating samples for 10 min at 65 °C. One μl of each reaction was analyzed by electrophoresis in 0.7% agarose gels. After electrophoresis, the amplified DNA was detected by Etd bromide staining. In parallel, qPCR was carried out with a Roche Applied Science LightCycler following the manufacturer standard protocols, analyzing 1 μl of a 250-fold dilution of each sample. The pair of specific primers amplifies a 700 bp region of the B. subtilis gene yshC. The data obtained for the samples were interpolated to a standard curve obtained with known amounts of B. subtilis genome in the same experiment. The results are represented as the amplification fold versus reaction time.
Measurement of the Fidelity of the Chimerical Polymerases.
The protocol used is essentially as described in ref. 31. Thus, samples from the experiment corresponding to multiply primed RCA of plasmidic DNA by φ29 DNA polymerase and chimerical DNA polymerases, were taken (3 μl) and mixed with 17 μl of restriction mix (2 μl New England Biolabs (NEB) 10X EcoRI Buffer, 0.5 μl NEB EcoRI enzyme [10 units] and 14.5 μl H2O) to get linear monomers of amplified plasmid. After incubation for 1 h at 37 °C, DNA was purified through Qiagen Gel-Extraction Kit Columns and eluted in 30 μl of buffer 50 mM Tris-HCl pH 7.5, 1 mM EDTA. 10 μl of each of the eluted samples were religated by mixing with 2 μl NBE 10X Ligase Buffer, 8 μl H2O, and 0.5 μl NEB Ligase (200 units). After overnight incubation at 16 °C, 2 μl of each of the ligation reactions were transformed onto chemically competent E. coli cells XL-1 Blue. About 1,000 transformants were obtained using each of the amplified samples, whereas no transformant was obtained from control samples that contained the initial amount of plasmid pT7-4 and underwent the same procedure as the amplified samples. Two clones from each transformation were selected and the plasmid was prepared and sequenced according to standard procedures. In total, 4,918 nonoverlapping nucleotides were sequenced for each polymerase.
Acknowledgments.
This work was supported by Spanish Ministry of Science and Innovation grants BFU 2008-00215, Consolider-Ingenio CSD2007-00015 (M.S.), and PET2007-0160 (to M.V.); by the Autonomous Community of Madrid grant P-MAT-0283-0505 (M.S.); and by an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa.
Footnotes
Conflict of interest statement: A patent application related to this work has been filed for which the authors are inventors.
References
- 1.Demidov VV, Broude NE. In: DNA Amplification. Current Technologies and Applications. Demidov VV, Broude NE, editors. Norfolk, United Kingdom: Horizon Bioscience; 2004. pp. ix–x. [Google Scholar]
- 2.Mullis KB, Faloona FA. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, et al. Whole genome amplification from a single cell: Implications for genetic analysis. Proc Natl Acad Sci USA. 1992;89:5847–5851. doi: 10.1073/pnas.89.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins TL, Detter JC, Richardson PM. Whole genome amplification—Applications and advances. Curr Opin Biotechnol. 2002;13:65–67. doi: 10.1016/s0958-1669(02)00286-0. [DOI] [PubMed] [Google Scholar]
- 5.Telenius H, et al. Degenerate oligonucleotide-primed PCR: General amplification of target DNA by a single degenerate primer. Genomics. 1992;13:718–725. doi: 10.1016/0888-7543(92)90147-k. [DOI] [PubMed] [Google Scholar]
- 6.Dean FB, et al. Comprehensive human genome amplification using multiple displacement amplification. Proc Natl Acad Sci USA. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean FB, Nelson JR, Giesler TL, Lasken RS. Rapid amplification of plasmid and phage DNA using Phi 29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res. 2001;11:1095–1099. doi: 10.1101/gr.180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Bakht S, Devos KM, Gale MD, Osbourn A. L-RCA (ligation-rolling circle amplification): A general method for genotyping of single nucleotide polymorphisms (SNPs) Nucleic Acids Res. 2001;29:e116. doi: 10.1093/nar/29.22.e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johne R, Müller H, Rector A, van Ranst M, Stevens H. Rolling-circle amplification of viral DNA genomes using phi29 polymerase. Trends Microbiol. 2009;17:205–211. doi: 10.1016/j.tim.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Gao S, Bruno JF, Luft BJ, Dunn JJ. Rapid detection and identification of a pathogen’s DNA using Phi29 DNA polymerase. Biochem Biophys Res Commun. 2008;375:522–525. doi: 10.1016/j.bbrc.2008.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Bueno A, et al. High diversity of the viral community from an Antarctic lake. Science. 2009;326:858–861. doi: 10.1126/science.1179287. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama F, Nozawa T, Aikawa C, Sakurai A, Nakagawa I. Cost effective DNA sequencing and template preparation from bacterial colonies and plasmids. J Biosci Bioeng. 2009;107:471–473. doi: 10.1016/j.jbiosc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Blanco L, et al. Highly efficient DNA synthesis by the phage φ29 DNA polymerase. Symmetrical mode of DNA replication. J Biol Chem. 1989;264:8935–8940. [PubMed] [Google Scholar]
- 14.Kamtekar S, et al. Insights into strand displacement and processivity from the crystal structure of the protein-primed DNA polymerase of bacteriophage φ29. Mol Cell. 2004;16:609–618. doi: 10.1016/j.molcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez I, et al. A specific subdomain in φ29 DNA polymerase confers both processivity and strand-displacement capacity. Proc Natl Acad Sci USA. 2005;102:6407–6412. doi: 10.1073/pnas.0500597102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasco MA, Blanco L, Parés E, Salas M, Bernad A. Structural and functional analysis of temperature-sensitive mutants of the phage φ29 DNA polymerase. Nucleic Acids Res. 1990;18:4763–4770. [PMC free article] [PubMed] [Google Scholar]
- 17.Dufour E, et al. An aspartic acid residue in TPR-1, a specific region of protein-priming DNA polymerases, is required for the functional interaction with primer terminal protein. J Mol Biol. 2000;304:289–300. doi: 10.1006/jmbi.2000.4216. [DOI] [PubMed] [Google Scholar]
- 18.Berman AJ, et al. Structures of phi29 DNA polymerase complexed with substrate: the mechanism of translocation in B-family polymerases. EMBO J. 2007;26:3494–3505. doi: 10.1038/sj.emboj.7601780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI. Recent developments in the optimization of thermostable DNA polymerases for efficient applications. Trends Biotechnol. 2004;22:253–260. doi: 10.1016/j.tibtech.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Motz M, et al. Elucidation of an archaeal replication protein network to generate enhanced PCR enzymes. J Biol Chem. 2002;277:16179–16188. doi: 10.1074/jbc.M107793200. [DOI] [PubMed] [Google Scholar]
- 21.Davidson JF, Fox R, Harris DD, Lyons-Abbott S, Loeb LA. Insertion of the T3 DNA polymerase thioredoxin binding domain enhances the processivity and fidelityof Taq DNA polymerase. Nucleic Acids Res. 2003;31:4702–4709. doi: 10.1093/nar/gkg667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, et al. A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro. Nucleic Acids Res. 2004;32:1197–1207. doi: 10.1093/nar/gkh271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlov AR, Belova GI, Kozyavkin SA, Slesarev AI. Helix-hairpin-helix motifs confer salt resistance and processivity on chimeric DNA polymerases. Proc Natl Acad Sci USA. 2002;99:13510–13515. doi: 10.1073/pnas.202127199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao X, Grishin NV. Common fold in helix-hairpin-helix proteins. Nucleic Acids Res. 2000;28:2643–2650. doi: 10.1093/nar/28.14.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slesarev AI, Belova GI, Lake JA, Kozyavkin SA. Topoisomerase V from. Methanopyrus kandleri. Methods Enzymol. 2001;334:179–192. doi: 10.1016/s0076-6879(01)34467-1. [DOI] [PubMed] [Google Scholar]
- 26.Slesarev AI, Lake JA, Stetter KO, Gellert M, Kozyavkin SA. Purification and characterization of DNA topoisomerase V. An enzyme from the hyperthermophilic prokaryote Methanopyrus kandleri that resembles eukaryotic topoisomerase I. J Biol Chem. 1994;269:3295–3303. [PubMed] [Google Scholar]
- 27.Slesarev AI, et al. DNA topoisomerase V is a relative of eukaryotic topoisomerase I from a hyperthermophilic prokaryote. Nature. 1993;364:735–737. doi: 10.1038/364735a0. [DOI] [PubMed] [Google Scholar]
- 28.Sun S, Geng L, Shamoo Y. Structure and enzymatic properties of a chimeric bacteriophage RB69 DNA polymerase and single-stranded DNA binding protein with increased processivity. Proteins. 2006;65:231–238. doi: 10.1002/prot.21088. [DOI] [PubMed] [Google Scholar]
- 29.Méndez J, Blanco L, Lázaro JM, Salas M. Primer-terminus stabilization at the φ29 DNA polymerase active site. Mutational analysis of conserved motif TX2GR. J Biol Chem. 1994;269:30030–30038. [PubMed] [Google Scholar]
- 30.Bambara RA, Fay PJ, Mallaber LM. Methods of analyzing processivity. Methods Enzymol. 1995;262:270–280. doi: 10.1016/0076-6879(95)62023-0. [DOI] [PubMed] [Google Scholar]
- 31.Fujii R, Kitaoka M, Hayashi K. One-step random mutagenesis by error-prone rolling circle amplification. Nucleic Acids Res. 2004;32:e145. doi: 10.1093/nar/gnh147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchison CA, 3rd, Smith HO, Pfannkoch C, Venter JC. Cell-free cloning using phi29 DNA polymerase. Proc Natl Acad Sci USA. 2005;102:17332–17336. doi: 10.1073/pnas.0508809102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcy Y, et al. Nanoliter reactors improve multiple displacement amplification of genomes from single cells. PLoS Genet. 2007;3:1702–1708. doi: 10.1371/journal.pgen.0030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsmadi O, Alkayal F, Monies D, Meyer BF. Specific and complete human genome amplification with improved yield achieved by phi29 DNA polymerase and a novel primer at elevated temperature. BMC Res Notes. 2009;2:48. doi: 10.1186/1756-0500-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lázaro JM, Blanco L, Salas M. Purification of bacteriophage φ29 DNA polymerase. Methods Enzymol. 1995;262:42–49. doi: 10.1016/0076-6879(95)62007-9. [DOI] [PubMed] [Google Scholar]
- 36.Tabor S, Richardson CC. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carthew RW, Chodosh LA, Sharp PA. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985;43:439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- 38.McDonell MW, Simon MN, Studier FW. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977;110:119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]