Abstract
Whether grocery shopping or choosing words to express a thought, selecting between options can be challenging, especially for people with anxiety. We investigate the neural mechanisms supporting selection during language processing and its breakdown in anxiety. Our neural network simulations demonstrate a critical role for competitive, inhibitory dynamics supported by GABAergic interneurons. As predicted by our model, we find that anxiety (associated with reduced neural inhibition) impairs selection among options and associated prefrontal cortical activity, even in a simple, nonaffective verb-generation task, and the GABA agonist midazolam (which increases neural inhibition) improves selection, whereas retrieval from semantic memory is unaffected when selection demands are low. Neural inhibition is key to choosing our words.
Keywords: anxiety, choice, midazolam, ventrolateral prefrontal cortex, neural network model
People claim to love the freedom of unlimited choices, but in reality we are often stymied by too many options and disconcerted by not knowing what the outcomes of our choices will be. Selecting among multiple options is effortful and time consuming, whether choosing among fruit jams (1), retirement plans (2), or medical treatments (3). This problem is particularly pervasive during language production, when we must constantly choose words to express a thought (4–6). People with anxiety disorders find coping with too many options particularly difficult, and struggle with decision-making problems (7), indecisiveness (8), and intolerance of uncertainty (9). What mechanisms allow us to select among multiple options when speaking, and why does this process break down in people with anxiety? Current psychological theories of selection focus on the importance of cognitive control (5) and prefrontal cortical regions (4, 6, 10), but do not address questions at the level of specific neural mechanisms. We address these questions by implementing a unified, biologically plausible computational model of selection, and testing its predictions about both brain and behavior in humans making choices in a well-controlled language production task.
Our model demonstrates how competitive, inhibitory dynamics among neurons in prefrontal cortical networks (11) support selection between alternatives. Specifically, these competitive dynamics serve to sharpen cognitive representations by amplifying activity in the most active, task-relevant, representations (e.g., the most appropriate word to complete a sentence) and suppressing competing representations (e.g., for the many other word possibilities). A tenet of the model is that these critical dynamics occur via inhibitory, GABAergic interneurons (12–14). Here we test the predictions of the model regarding selection processes that are supported by the left ventrolateral prefrontal cortex. We do so for the following reasons: (i) this region has been implicated in selecting among competing alternatives during language processing (6, 15–18); (ii) this region shows altered activity in individuals who suffer from anxiety (particularly anxious apprehension, characterized by worry, and hereafter referred to as “anxiety” (19); and (iii) GABAergic function is reduced in individuals with anxiety (20–23). Hence, our model provides a unified framework that can link these formerly disconnected observations.
Specifically, our model demonstrates how reduced GABAergic function can lead to reduced competitive dynamics in prefrontal cortical networks, allowing nonwinning competitors (alternative responses that are not selected) to become more active and to compete over a longer period, which impairs selection. Conversely, increased GABAergic function leads to lower and briefer activation of these competitors and to improvements in selection. These basic mechanisms provide a unified framework for understanding how we make choices in language processing and how this process relates to worrying about the future, to a degree of precision that allows us to test (and confirm) predictions through neuropharmacological manipulation, links to psychopathology, and levels of brain activity. Whereas decision-making deficits in persons with anxiety have previously been shown in complex or affective tasks, our model predicts that selection deficits that lie at the core of these problems should be observed even in a simple language-production task, whereas other cognitive processes should remain intact.
We provide a previously undescribed direct test of this model, and confirm its predictions. We demonstrate that participants high in anxiety, which has been linked to reduced GABAergic function, have more difficulty selecting between competing word options and exhibit reduced VLPFC activation during such selection. In addition, we demonstrate that drugs that increase GABAergic function improve selection in a nonclinical population. Furthermore, such effects are specific, as the retrieval of words from semantic memory is unaffected by GABAergic function when selection demands are low, despite the importance of retrieval processes in language processing and the role of VLPFC in retrieval (4).
Results
We investigated selection and retrieval of words from semantic memory with a verb generation task, in which participants are presented with a noun and say the first associated verb that comes to mind (5, 6). This task allows selection and retrieval demands to be precisely quantified and independently manipulated (Fig. 1A and Methods). We present the basic behavioral effects of selection and retrieval demands and their simulation in a neural network model first, followed by predictions from the model and empirical tests of the effects of increasing and decreasing neural inhibition.
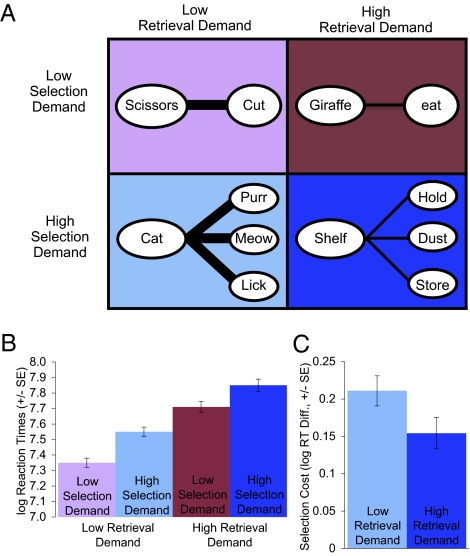
Fig. 1.
Design and basic behavioral findings for the verb generation task. (A) Selection demands (high vs. low competition) are crossed with retrieval demands (high vs. low association strength) (SI Methods 2.1). (B) Participants take longer to generate a response when retrieval demands are high and when selection demands are high. (C) Selection costs (RT difference between high and low selection demand conditions) are greater when retrieval demands are low than when they are high. All error bars are SEs.
For the basic behavioral data, verb generation data were analyzed with a 2 × 2 repeated-measures ANOVA. We replicated previous results showing participants are slowed by (i) greater selection demand, measured in terms of the competition between alternative responses (e.g., cat brings to mind many possible competing verbs such as purr, meow, and lick, and so has high selection demand, whereas scissors generally brings to mind the single verb cut); and (ii) greater retrieval demand, measured as the weakness of the association strength between the noun and the appropriate response (e.g., scissors is strongly associated with cut and so has low retrieval demand, whereas giraffe is only weakly associated with any verb). Specifically, reaction times were longer in the high selection demand [log reaction time (RT) mean (M) = 7.70, SE = 0.03; RT 2208 ms, SE = 68] than low selection demand conditions (log RT M = 7.53, SE = 0.03; RT 1,863 ms, SE = 57) [F(1,82) = 215.9, P < 0.001], and longer in the high retrieval demand (log RT M = 7.78, SE = 0.04; RT 2,392 ms, SE = 98) than low retrieval demand (log RT M = 7.45, SE = 0.03; RT 1,720 ms, SE = 52) conditions [F(1,82) = 387.9, P < 0.001] (Fig. 1B and Table S1). In addition, we found an interaction between selection and retrieval demands: selection costs were greater under low retrieval demands [log RT difference (diff.) M = 0.20, SE = 0.02; RT diff. 352 ms] than under high retrieval demands (log RT diff. M = 0.13, SE = 0.02; RT diff. 324 ms) [F(1,82) = 12.1, P = 0.001] (Fig. 1C).
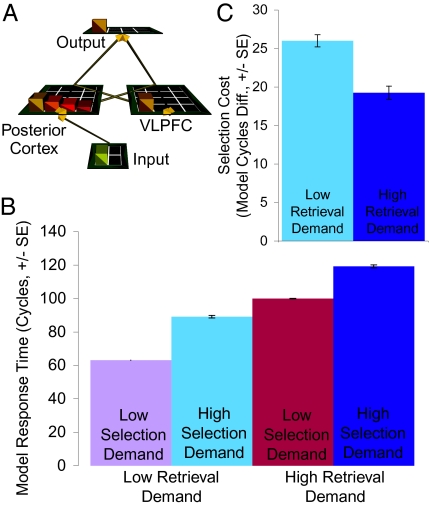
Our model simulates and provides a framework for understanding these findings. The model uses a powerful framework that simulates the electrophysiological properties of neurons and can use networks of such neurons to simulate human behavior, including language and cognitive control (SI Methods 2.2–2.3 and Tables S2 and S3 provides details of the modeling framework, architecture, and simulations). The model contains layers (simulated brain areas) that simulate the following: (i) presentation of noun stimuli, (ii) activation of associated verb responses in the posterior cortex, (iii) selection of responses in the ventrolateral prefrontal cortex (VLPFC), and (iv) output of a response (Fig. 2A). The strength of connections between nouns and associated verb responses and between alternative verb responses were set according to the known association strengths observed in humans (24); these connections support spreading activation between related semantic representations like that observed in posterior cortex. Simulated neurons in the posterior cortex layer then activate verb representations in the VLPFC layer, which implements competitive lateral inhibition, selecting one response for output.
Fig. 2.
Neural network model. (A) Network architecture. (B) Model simulates human performance, showing independent effects of selection demand (driven by competition between active representations) and retrieval demand (driven by synaptic weight strength). (C) Model simulates interaction between selection and retrieval (driven by benefit of spreading activation when retrieval demands are high). All error bars are SEs.
Like people, the model takes longer to respond when retrieval or selection demands are high (SI Methods 2.3, SI Results 3.1, Table S3, Fig. 2B, and Figs. S1 and S2). The effects of retrieval demand are a direct consequence of the strength of the synaptic weights between a stimulus and its response representation in the posterior cortex layer (25); weaker weights cause a slower buildup of activation, requiring more time to reach the threshold for generating a response. Selection demand increases when multiple alternative responses become simultaneously active and competition must be resolved to select a single response. In the model, this resolution is accomplished through strong lateral inhibition in the VLPFC layer, simulating the effects of GABAergic interneurons.
In addition, the model replicates the interaction between selection and retrieval demands, and provides insight into why such an interaction occurs (Fig. 2C). When responses are easily retrieved, activating multiple responses serves only to generate competition, imposing a large selection cost. However, when it is difficult to retrieve any response, activating multiple responses aids retrieval, as spreading activation between these weakly associated alternatives (e.g., between hold and store when generating a response for shelf) boosts their activation levels. Thus, when retrieval demands are high, selection costs are partially offset by the advantage multiple responses confer on retrieval (SI Results 3.1 and Fig. S2).
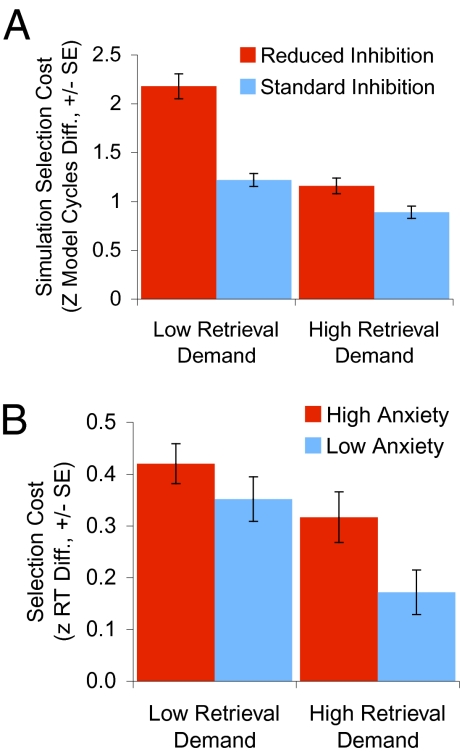
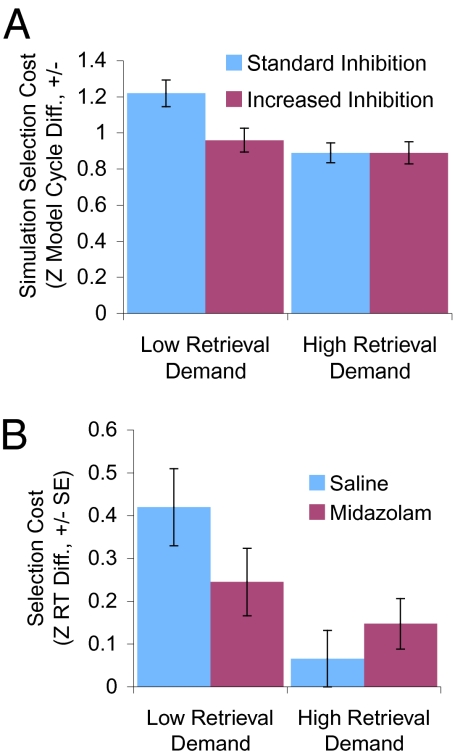
Manipulations of competitive inhibition in the VLPFC layer of the model enabled us to generate novel predictions regarding the effects of reduced GABAergic function associated with anxiety, and increased GABAergic function under GABA agonists, which we then tested empirically (SI Methods 2.4–2.6 and SI Results 3.2–3.4). These simulations showed that competitive inhibition is critical for selection between competing alternatives, and that the effect of competitive inhibition on selection is modulated by retrieval demands. Decreasing competitive inhibition (as in anxiety) impairs selection (Fig. 3A), whereas increasing competitive inhibition (as under GABA agonists) improves selection (Fig. 4A). These effects of competitive inhibition on selection are more robust when retrieval demands are low. When retrieval demands are high, increased neural inhibition increases competitive dynamics that support selection, but also reduces spreading activation that aids retrieval, leading to weaker effects (Figs. 3A and 4A). In contrast, changes in competitive inhibition do not affect retrieval when selection demands are low (i.e., there is one associated response and thus no spreading activation). Thus, the simulations predict that reduced neural inhibition associated with anxiety will impair selection and associated VLPFC activity (26, 27) (SI Discussion 1.2), whereas increased neural inhibition under the GABA agonist midazolam will improve selection. These effects may be more apparent when retrieval demands are low. In addition, retrieval should not be affected by changes in neural inhibition when selection demands are low. These predictions were supported in three empirical investigations.
Fig. 3.
Effects of reduced neural inhibition (A) Model predictions: Reduced competitive neural inhibition in the VLPFC layer, simulating increasing anxiety, impairs selection (i.e., increases selection cost) under high and low retrieval demands, and suggests that effects of anxiety on selection may be most robust under low retrieval demands. (B) Empirical results. Higher anxiety participants show impaired selection under high and low retrieval demands (model fit is further discussed in Results). All error bars are SEs.
Fig. 4.
Effect of increased neural inhibition. (A) Model predictions. Increased neural inhibition (simulating GABA agonist drugs) improves selection (i.e., reduces selection cost) only when retrieval demands are low. (B) Empirical results. Midazolam improves selection only when retrieval demands are low. RTs are z transformed to remove baseline differences between conditions. All error bars are SEs.
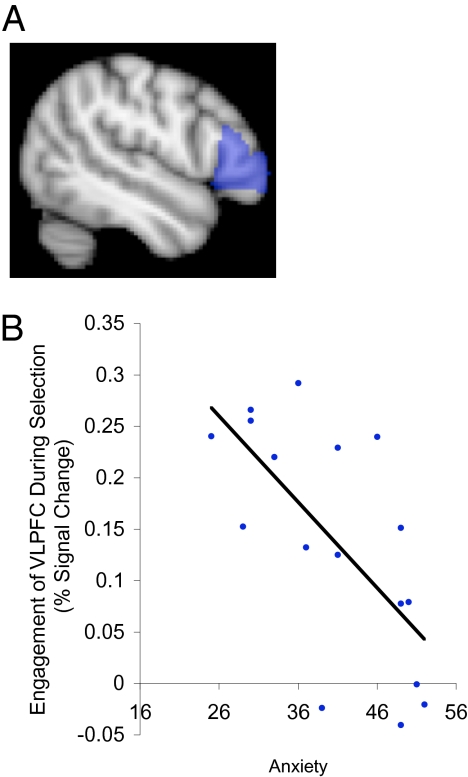
The effects of anxiety on selection were investigated in separate behavioral and functional MRI (fMRI) studies in nonclinical populations that varied in levels of anxious apprehension, which should in turn influence the level of GABAergic activity. Anxious apprehension was assessed by standard questionnaires in which individuals rated how well statements such as “many situations make me worry” applied to them (Methods). Behavioral data were analyzed with a 2 × 2 × 2 mixed factorial ANOVA (Table S4). As predicted, participants higher in anxiety had larger selection costs (z-transformed RT diff. M = 0.37, SE = 0.03; RT diff. 421 ms) than lower-anxiety participants (z-transformed RT diff. M = 0.26, SE = 0.03; RT diff. 269 ms) [F(1,57) = 6.32 P = 0.015] (Fig. 3B, Fig. S3, and Table S5), but the retrieval costs were equivalent across the groups [F(1,57) = 0.72, P = 0.4]. Also as predicted, left VLPFC activity correlated with anxiety during selection when retrieval demands were low (r = −0.663, P = 0.004, n = 17) (Fig. 5 and Fig. S4), but not during retrieval when selection demand was low (r = −0.181, P = 0.5, n = 16, Fisher's z = −1.60, P = 0.05, one-tailed). (The brain activity thus confirms the model's prediction of larger effect of anxiety on selection when retrieval demands are low, whereas the behavioral data show similar effects of anxiety on selection under high and low retrieval demands, perhaps reflecting a lower sensitivity of RT measures or the role of other brain mechanisms or compensatory strategies.) These findings suggest that higher anxiety individuals lack sufficient competitive dynamics in VLPFC for efficiently selecting between competing options (SI Discussion 1.2).
Fig. 5.
Engagement of left VLPFC during selection, as a function of anxiety. (A) Anatomically defined region of interest (mid and anterior left VLPFC, shown in blue) was chosen based on prior work establishing its role in selection. Engagement of this region during selection was computed for each participant as the difference in fMRI activation between the high and low selection demand conditions. (B) Higher anxiety participants showed reduced engagement of left VLPFC during selection under low retrieval demands, which may reflect reduced activity of GABAergic interneurons (SI Discussion).
We tested the predicted effects of increased neural inhibition in a double-blind, placebo-controlled study in which participants completed the verb generation task after injection of the GABA agonist midazolam as compared with a saline control in two counterbalanced sessions (Methods, SI Methods 2.6, and SI Discussion 1.3). Data were analyzed with a 2 × 2 × 2 repeated-measures ANOVA (Table S6). There was an interaction between drug condition, selection demand, and retrieval demand [F(1,19) = 5.67, P = 0.028]. As predicted, when retrieval demands were low, midazolam improved selection (with selection costs lower under midazolam, z-transformed RT diff. M = 0.15, SE = 0.06; RT diff. 267 ms, than under saline, z-transformed RT diff. M = 0.37, SE = 0.07; RT diff. 355 ms) [t(19) = −2.95, P = 0.008], whereas midazolam did not improve selection when retrieval demands were high [t(19) = 1.05, P = 0.3] (Fig. 4B, Fig. S5, and Table S7). Also as predicted, there was no effect of midazolam on retrieval when selection demands were low [t(19) = −0.53, P = 0.6].
Discussion
In daily life, we often face a tyranny of choice (28), and this problem is ubiquitous when we choose words to express a thought. The current studies demonstrate that competitive neural inhibition, via GABAergic interneurons in prefrontal circuits, likely plays an important role in selecting among alternatives during language processing. As predicted by neural network simulations, selection and associated prefrontal activity in a verb-generation task are impaired by anxiety (associated with reduced GABAergic function), whereas selection is improved under the drug midazolam (which increases GABAergic function). Of note, retrieval is unaffected by GABAergic function; instead, other mechanisms [e.g., sustained neuronal activation, enabled by recurrent connections in PFC networks (SI Discussion 1.1)] may support retrieval of weakly active representations. These findings shed light on why choosing among many options can be difficult for anyone, and why it can be paralyzing for people with anxiety.
In complex decision-making tasks, choice-overload is believed to increase when there are many options (1–3), the options are similar (29), or there is conflict between equally good options (30). Similarly, in language production, the difficulty of selecting among words has been described as a function of the number of alternatives (31), or similarity of activation levels across alternatives (5, 32). Each of these factors can be seen as increasing the amount of time necessary to resolve competition among options through neural inhibition, as each increases the degree to which multiple options are represented with equal strength.
Although we are normally able to use cognitive control to overcome these selection difficulties, this process becomes more difficult for persons with anxiety. Our modeling and empirical work suggest that the reduced GABAergic function associated with anxiety leads to impaired competitive neural inhibition and contributes to difficulty in selection. Although GABA agonists are widely used to treat the affective symptoms of anxiety disorders (33), we demonstrate that midazolam improves the cognitive process of selection in a nonclinical population, suggesting that GABA agonists may also be effective in treating the cognitive control and decision-making deficits in anxiety disorders.
Even for individuals without anxiety disorders, the difficulty of selecting between options has important real-world consequences in domains beyond language production. When people are faced with too many options, they may use suboptimal heuristics to reduce the number of alternatives (34), make a decision they regret (1, 28), or delay making a decision altogether (2, 29), often with negative consequences. For example, the more retirement plans that employees must choose among, the less likely they are to join any plan at all (2). Likewise, when physicians are asked to choose between two similar pain medications, they are less likely to prescribe either (3). In these complex domains, and in language production as well, selection is likely to depend on many processes in addition to neural inhibition, such as assigning values to different options (35), which are supported by additional brain areas. A complete model of selection will thus need to incorporate additional processes (e.g., generating potential response options and dynamically increasing control when there is response competition) supported by a larger network of brain areas [e.g., anterior cingulate cortex, presupplementary motor area (36, 37)], which may also be affected by anxiety. Therefore, an important goal for future research will be to investigate how these processes interact with competitive inhibition processes in ventrolateral prefrontal cortex to support selection in language processing, and whether these processes generalize across other prefrontal regions to support decision making in other domains (38, 39). Future work should also include imaging methods (e.g., SPECT) and modeling frameworks [e.g., detailed neurophysiological models (40)] that provide more direct measures and simulations of GABAergic activity. Whether in the grocery store or speaking a sentence, there is no escaping the necessity of selecting among competing alternatives. In language processing, and perhaps beyond, competitive neural inhibition is critical in helping us to cope with this tyranny of choice.
Methods
All participants were from the University of Colorado and wider Boulder and Denver communities, spoke English as a first language, and did not report any reading disorder. All participants gave informed consent and were treated in accordance with procedures approved by the University of Colorado institutional review board.
Experiment 1: Basic Behavioral Effects and Effects of Anxiety.
Participants
Participants were 85 young adults (52 female and 33 male); a subset (n = 60) also completed anxiety and depression measures. An additional eight participants were excluded for not following task directions (n = 4), self-reported reading disorders (n = 2), and equipment failure (n = 2). In addition, two outliers with negative selection and/or retrieval effects were excluded from analysis, because the basic effects of the task manipulations are very robust, occurring for the vast majority of subjects, making it difficult to interpret individual differences in cases in which there is a clear manipulation failure. With the inclusion of these subjects, all significant effects remain significant.
Design, procedure, and analysis.
Verb generation stimuli were 100 nouns in a 2 × 2 design: high and low retrieval demand (association strength between nouns and possible verb responses) crossed with high and low selection demand (degree of competition among alternative responses). Association strength and competition were calculated as in previous work (5), using latent semantic analysis (24). High and low association strength conditions were matched on competition, whereas high and low competition conditions were matched on association strength. The full stimulus set is available upon request.
Participants were instructed to say the first verb that came to mind when presented with a noun (e.g., “meow” or “feed” for “cat”), and were given an example and eight practice trials before completing the task. A fixation-cross appeared for 500 ms, followed by a noun. Participants responded by speaking into a microphone that recorded voice-triggered reaction times (RTs), and advanced the computer to the next trial. Trial order was randomized for each participant. When the microphone was accidentally triggered (e.g., by a cough) or an error made (a nonverb), the trial was eliminated from analysis. The data were trimmed to remove trials with RTs less than 200 ms or greater than three SDs above each participant's mean. For within-subject analyses, RTs were log transformed to normalize the data. For individual differences analyses, RTs were further z transformed to remove baseline differences.
Participants completed four standardized questionnaires to assess anxious apprehension: (i) NEO Five Factor Inventory (NEO-FFI) neuroticism subscale (41), (ii) Lehrer Woolfolk Anxiety Symptom Questionnaire cognitive factor (LWASQ) (42), (iii) Penn State Worry Questionnaire (PSWQ) (43), and (iv) Behavioral Inhibition Scale/Behavioral Activation Scale (BIS/BAS) (44) Behavioral Inhibition subscale. These questionnaires were combined into a summary score (SI Methods). Participants were classified as characterized by high or low anxious apprehension using a median split (SI Results describes converging results from a continuous analysis). In addition, participants completed the Mood and Anxiety Symptom Questionnaire (MASQ) (45) to control for depression and anxious arousal symptoms (SI Discussion and SI Results).
Experiment 2: Effects of Anxiety on VLPFC Activity during Selection.
Experiment 2 was part of a larger fMRI study focused on prefrontal organization. Here we focus on the critical test of our theory, the effect of anxiety on VLPFC activity.
Participants.
Participants were 18 right-handed young adults (9 female and 9 male). Three additional participants were excluded because of excessive (>25%) error rates.
Design, procedure, and analysis.
The verb generation task was identical to experiment 1, except that, to adapt the procedure for fMRI scanning, trail length was fixed to 4,000 ms rather than being self-paced. Verbal responses were recorded with a fiberoptic microphone (Optoacoustics Ltd.). A rapid event-related paradigm and standard blood oxygen level-dependent (BOLD) imaging techniques were used to collect functional data in one run lasting ∼9 min (SI Methods). Participants completed the PSWQ and MASQ outside of the scanner (refer to experiment 1).
Nonverb errors were removed from analysis, and instead included as a nuisance covariate in the GLMs. (RTs are not available because the scanner environment was too noisy to accurately determine voice-onset RTs.) First, GLMs were conducted estimating signal change for contrasts of high vs. low selection demand and high vs. low retrieval demand conditions (SI Methods). Next, an anatomical region of interest (ROI) consisting of left inferior frontal gyrus pars triangularis and pars orbitalis (mid and anterior VLPFC) was defined using the Harvard–Oxford Cortical Structures (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html) and Duvernoy atlases (46), and signal change in each contrast within this ROI was extracted for each participant and correlated with anxiety and depression scores. Outlier analyses were conducted (Cook's D > 3 SD above mean), resulting in the exclusion of no more than two participants from each correlation analysis.
Experiment 3: Effects of Increased Neural Inhibition under Midazolam.
Participants.
Participants were 24 young adults (10 female and 14 male). One additional participant was excluded for not completing the second session. In addition, four outliers with negative selection and/or retrieval effects under saline were excluded from analysis, as in experiment 1, because it is difficult to interpret drug effects in cases in which there is a clear manipulation failure. Including these subjects does not change the overall pattern of results; the significant three-way interaction becomes marginally significant (P = 0.06), and all other significant effects remain significant.
Design, procedure, and analysis.
A double-blind, placebo-controlled design was used. Participants completed counterbalanced sessions, 1 wk apart, under midazolam (0.03 mg/kg body weight diluted to a total volume of 10 mL) and placebo (10 mL saline solution) (SI Methods). The verb generation task was identical to that in experiment 1, except that the stimulus set was divided at random into two lists (with an additional 50 filler items), counterbalanced across sessions and drug conditions. RT data were trimmed and transformed as in experiment 1.
Neural Network Model.
We used a biologically plausible neural network modeling framework, Leabra (47, 48) (SI Methods), implemented in Emergent (http://grey.colorado.edu/emergent/). On each trial, one noun input unit was turned on, activating the relevant verb response units in the posterior cortex layer, which then became active in the VLPFC layer, which implemented strong k-winners-take-all (kWTA) lateral inhibition to select one verb and biased the posterior cortex layer representations toward this response. Multiple simulations were run with differing levels of kWTA lateral inhibition in the VLPFC layer. The level of inhibition was reduced to test the effects of decreased neural inhibition (that is likely associated with anxious apprehension) and increased to test the effect of increased neural inhibition (under midazolam) (SI Methods and SI Results). Each trial ended when the network settled, producing a response in the output layer. Thus, the VLPFC layer of this model implements mechanisms similar to the leaky, competing accumulator model of perceptual choice (49, 50), in which lateral inhibition between units allows one response to emerge as the winner, whereas decay of unit activations is counteracted by recurrent excitatory connections.
Supplementary Material
Acknowledgments
We thank members of the P50 center on Executive Function and Dysfunction including Wendy Heller, whose ideas have influenced the present study. In addition we thank Michael Frank, Peter Todd, Susan Jung-Grant, Rosi Kaiser, Ranjani Prabhakaran, and Mark Whisman for discussions, Paula Villar, Kirsten Orcutt, and Luka Ruzic for assistance with the fMRI study, and the staff of the University of Colorado Boulder Clinical and Translational Research Center for medical assistance with the midazolam study. This research was supported by grants from the National Institutes of Health: The midazolam study was supported by RO1-MH64812 and by Clinical Translational Research Center Grants (M01-RR0051 and UL1-RR025780), and all other research by P50-MH079485 and 1F31-MH087073-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002291107/-/DCSupplemental.
References
- 1.Iyengar SS, Lepper MR. When choice is demotivating: Can one desire too much of a good thing? J Pers Soc Psychol. 2000;79:995–1006. doi: 10.1037//0022-3514.79.6.995. [DOI] [PubMed] [Google Scholar]
- 2.Sethi-Iyengar S, Huberman G, Jiang W. In: Pension design and structure: New lessons from behavioral finance. Mitchell OS, Utkus S, editors. Oxford: Oxford University Press; 2004. pp. 83–95. [Google Scholar]
- 3.Redelmeier DA, Shafir E. Medical decision making in situations that offer multiple alternatives. JAMA. 1995;273:302–305. doi: 10.1001/jama.1995.03520280048038. [DOI] [PubMed] [Google Scholar]
- 4.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Snyder HR, Munakata Y. So many options, so little time: The roles of association and competition in underdetermined responding. Psychon Bull Rev. 2008;15:1083–1088. doi: 10.3758/PBR.15.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachdev PS, Malhi GS. Obsessive-compulsive behaviour: A disorder of decision-making. Aust N Z J Psychiatry. 2005;39:757–763. doi: 10.1080/j.1440-1614.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- 8.Abramowitz JS, Foa EB. Worries and obsessions in individuals with obsessive-compulsive disorder with and without comorbid generalized anxiety disorder. Behav Res Ther. 1998;36:695–700. doi: 10.1016/s0005-7967(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 9.Starcevic V, Berle D. Cognitive specificity of anxiety disorders: A review of selected key constructs. Depress Anxiety. 2006;23:51–61. doi: 10.1002/da.20145. [DOI] [PubMed] [Google Scholar]
- 10.Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoriing of response conflict: Evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- 11.Herd SA, Banich MT, O'Reilly RC. Neural mechanisms of cognitive control: An integrative model of stroop task performance and FMRI data. J Cogn Neurosci. 2006;18:22–32. doi: 10.1162/089892906775250012. [DOI] [PubMed] [Google Scholar]
- 12.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- 13.Bagary M, et al. Is benzodiazepine-induced amnesia due to deactivation of the left prefrontal cortex? Psychopharmacology (Berl) 2000;150:292–299. doi: 10.1007/s002130000419. [DOI] [PubMed] [Google Scholar]
- 14.Jansen JF, et al. Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav. 2006;9:181–185. doi: 10.1016/j.yebeh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Kan IP, Kable JW, Van Scoyoc A, Chatterjee A, Thompson-Schill SL. Fractionating the left frontal response to tools: Dissociable effects of motor experience and lexical competition. J Cogn Neurosci. 2006;18:267–277. doi: 10.1162/089892906775783723. [DOI] [PubMed] [Google Scholar]
- 16.Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn Affect Behav Neurosci. 2004;4:43–57. doi: 10.3758/cabn.4.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Persson J, et al. Selection requirements during verb generation: Differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Thompson-Schill SL, et al. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engels AS, et al. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 20.Sen S, et al. Serotonin transporter and GABAA alpha 6 receptor variants are associated with neuroticism. Biol Psychiatry. 2004;55:244–249. doi: 10.1016/j.biopsych.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Smoller JW, et al. Genetic association analysis of behavioral inhibition using candidate loci from mouse models. Am J Med Genet. 2001;105:226–235. doi: 10.1002/ajmg.1328. [DOI] [PubMed] [Google Scholar]
- 22.Zai G, et al. Evidence for the gamma-amino-butyric acid type B receptor 1 (GABBR1) gene as a susceptibility factor in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:25–29. doi: 10.1002/ajmg.b.30152. [DOI] [PubMed] [Google Scholar]
- 23.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 24.Landauer TK, Dumais ST. A solution to Plato's problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychol Rev. 1997;104:211–240. [Google Scholar]
- 25.Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 26.Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz B. The tyranny of choice. Sci Am. 2004;290:70–75. doi: 10.1038/scientificamerican0404-70. [DOI] [PubMed] [Google Scholar]
- 29.Fasolo B, Hertwig R, Huber M, Ludwig M. Size, entropy, and density: What is the difference that makes the difference between small and large real-world assortments? Psychol Mark. 2009;26:254–279. [Google Scholar]
- 30.Tversky A, Shafir E. Choice under conflict: The dynamics of deferred decision. Psychol Sci. 1992;3:358–361. [Google Scholar]
- 31.Desmond JE, Gabrieli JD, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- 32.Thompson-Schill SL, Botvinick MM. Resolving conflict: A response to Martin and Cheng (2006) Psychon Bull Rev. 2006;13:402–408. doi: 10.3758/bf03193860. discussion 409–411. [DOI] [PubMed] [Google Scholar]
- 33.Mula M, Pini S, Cassano GB. The role of anticonvulsant drugs in anxiety disorders: A critical review of the evidence. J Clin Psychopharmacol. 2007;27:263–272. doi: 10.1097/jcp.0b013e318059361a. [DOI] [PubMed] [Google Scholar]
- 34.Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 35.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 36.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 37.Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- 38.O'Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: A neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 40.Deco G, Rolls ET, Horwitz B. Integrating fMRI and single-cell data of visual working memory. Neurocomputing. 2004;58:729–737. doi: 10.1162/089892904323057380. [DOI] [PubMed] [Google Scholar]
- 41.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 42.Lehrer PM, Woolfolk RL. Self-report assessment of anxiety: Semantic, cognitive and behavioral modalities. Behav Assess. 1982;4:167–177. [Google Scholar]
- 43.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 44.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 45.Watson D, et al. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 46.Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. New York: Springer-Verlag Wien; 1999. [Google Scholar]
- 47.O'Reilly RC. Six principles for biologically based computational models of cortical cognition. Trends Cogn Sci. 1998;2:455–462. doi: 10.1016/s1364-6613(98)01241-8. [DOI] [PubMed] [Google Scholar]
- 48.O'Reilly RC, Munakata Y. Computational explorations in cognitive neuroscience: Understanding the mind by simulating the brain. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 49.Bogacz R, Usher M, Zhang J, McClelland JL. Extending a biologically inspired model of choice: Multi-alternatives, nonlinearity and value-based multidimensional choice. Philos Trans R Soc Lond B Biol Sci. 2007;362:1655–1670. doi: 10.1098/rstb.2007.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Usher M, McClelland JL. The time course of perceptual choice: The leaky, competing accumulator model. Psychol Rev. 2001;108:550–592. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.