Abstract
The Notch signaling pathway plays an important role in cellular proliferation, differentiation, and apoptosis. Unregulated activation of Notch signaling can result in excessive cellular proliferation and cancer. Human T-cell leukemia virus type 1 (HTLV-I) is the etiological agent of adult T-cell leukemia (ATL). The disease has a dismal prognosis and is invariably fatal. In this study, we report a high frequency of constitutively activated Notch in ATL patients. We found activating mutations in Notch in more than 30% of ATL patients. These activating mutations are phenotypically different from those previously reported in T-ALL leukemias and may represent polymorphisms for activated Notch in human cancers. Compared with the exclusive activating frameshift mutations in the proline, glutamic acid, serine, and threonine (PEST) domain in T-ALLs, those in ATLs have, in addition, single-substitution mutations in this domain leading to reduced CDC4/Fbw7-mediated degradation and stabilization of the intracellular cleaved form of Notch1 (ICN1). Finally, we demonstrated that inhibition of Notch signaling by γ-secretase inhibitors reduced tumor cell proliferation and tumor formation in ATL-engrafted mice. These data suggest that activated Notch may be important to ATL pathogenesis and reveal Notch1 as a target for therapeutic intervention in ATL patients.
Notch signaling plays an important role in cellular differentiation, proliferation, and apoptotic events (1, 2). Notch proteins are transmembrane receptors noncovalently joined through a structural motif, the heterodimerization (HD) domain. Notch signaling, initiated by receptor–ligand interactions, requires subsequent proteolytic cleavage of the receptor, resulting in the intracellular cleaved form of Notch1 (hereafter referred to as “ICN1”), which translocates to the nucleus and up-regulates the transcription of Notch-regulated genes (3–5). In normal physiological conditions, activation of Notch is transient, because ICN1 interacts with the hCDC4/FBW7 ubiquitin ligase and is degraded rapidly through the proteasome (6, 7). Activated Notch signaling contributes to ~50% of human T-cell acute lymphoblastic leukemia (T-ALL) cases through gain-of-function mutations in the Notch1 gene (8). Notch1-activating mutations identified to date cluster at the HD and the proline, glutamic acid, serine, and threonine (PEST) domains, leading to ligand-independent cleavage and activation of Notch1 and increased stability of ICN1, respectively (8). Human T-cell leukemia virus type 1 (HTLV-I) is a human oncoretrovirus associated with the development of an aggressive and fatal lymphoproliferative disease (9, 10). Transformation of human T cells by HTLV-I coincides with the constitutive activation of the JAK/STAT pathway (11). The potential role of Notch in the pathogenesis of adult T-cell leukemia (ATL) has not been investigated previously. In this study, we report that ATL frequently harbors the constitutively activated Notch signaling pathway. The rate of activating mutation found in Notch in ATL was similar to that previously reported for T-ALL. However, mutations were never found in the HD domain or exon 28, and ICN1 was produced through normal processing of the NOTCH receptor by the γ-secretase complex. Surprisingly, 70% of mutations found in the PEST domain did not produce early termination but still significantly reduced CDC4-mediated ubiquitination, degradation, and turnover of ICN1. Constitutive activation of Notch signaling was biologically relevant to ATL, and its inhibition reduced proliferation and survival of ATL cells in vitro and significantly reduced tumors in engrafted mice.
Results
Constitutive Activation of Notch in HTLV-1–Associated ATL.
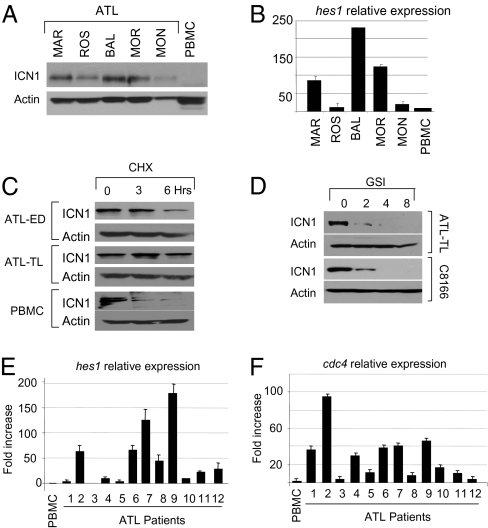
The fact that Notch signaling is essential in T-ALL prompted us to investigate activation of Notch signaling in HTLV-I ATL. Increased expression of ICN1 was found in uncultured, freshly isolated samples from ATL patients when compared with normal peripheral blood mononuclear cells (PBMC) (Fig. 1A). ICN1 was transcriptionally active in ATL, because its expression levels correlated reasonably with that of hes1, a downstream transcriptional target of Notch (Fig. 1B). Consistent with these data, we found that the half-life of ICN1 was significantly increased, from 1.5 h in normal PBMCs to more than 3 h in several ATL-derived HTLV-I–transformed cell lines (Fig. 1C). Treatment of HTLV-I–transformed T cells (C8166) with an inhibitor of the γ-secretase complex (GSI) (12) resulted in decreased expression of ICN1, indicating that in HTLV-I–infected cells ICN1 is produced mainly from normal receptor cleavage (Fig. 1D). Similar results were obtained with additional HTLV-I–infected cells, suggesting that, in contrast to T-ALL, aberrant Notch receptor processing through mutation of the HD domain is unlikely to contribute to increased expression of ICN1 in HTLV-I–infected cells. We next measured the levels of Hes1 and hCDC4, an E3 ubiquitin ligase involved in degradation of ICN1 (13, 14). Up-regulated expression of Hes1 was found in 7 of 12 ATL samples (Fig. 1E), indicating constitutive activation of Notch signaling in a majority of ATL patients. Stabilization of ICN1, as shown by an increased half-life (Fig. 1C), was not caused by a lack of hCDC4 expression (Fig. 1F). Together, these data demonstrate constitutive activation of the Notch1 signaling pathway in almost half of ATL patients. This observation is similar to the numbers previously reported in T-ALL (8) and suggests an important role for Notch signaling in ATL.
Fig. 1.
Constitutive activation of Notch in HTLV-1–infected cells. (A) Western blot analysis for expression of ICN1 in freshly isolated, uncultured samples from patients diagnosed with acute ATL. (B) Corresponding ATL patients were analyzed for hes 1 gene expression by real-time RT-PCR. (C) Half-life of ICN1 was measured by Western blot in ATL-derived cells and PBMC after cycloheximide treatment (100 μg/mL for 0, 3, and 6 h). Actin is shown as loading control. (D) Intracellular Notch is processed by receptor cleavage. Decreased ICN1 in ATL-TL–derived cells and HTLV-I–transformed C8166 cells after treatment with 1 μM GSI (Calbiochem) for 0, 2, 4, and 8 d. (E and F) Relative expression of HES1 and CDC4 mRNA, determined by real-time quantitative RT-PCR analysis, in samples from patients with acute ATL.
Mutations in the PEST Domain of ICN1 Lead to its Stabilization.
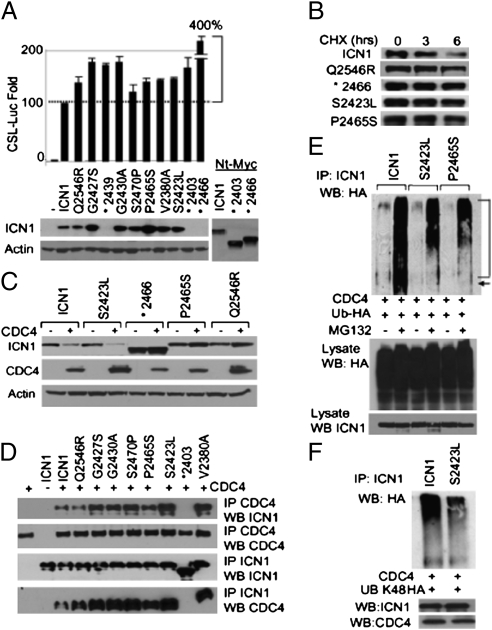
Activating mutations of Notch generally are clustered within the HD domain (responsible for linking the extracellular ligand binding and transmembrane domains) and the PEST domain (involved in regulating degradation and receptor turnover). Recently, additional mutations and microdeletions also have been reported in exon 28. Mutations in the HD domain generally are single-bp mutations which result in weakened association or complete dissociation of the receptor subunits and thus lead to ligand-independent activation (15). We investigated the presence of mutations in Notch in 32 ATL samples (Table 1). In contrast to previous observations made in T-ALL (8), mutations were never found in the HD domain of ATL patients. This finding is consistent with the observation that, in HTLV-I–infected cells, ICN1 appears to be produced through the processing of the Notch receptor by the γ-secretase complex (Fig. 1D). Instead, we found mutations in the PEST domain in 10 of 32 ATL patients. Previous studies have reported that functional mutations in the PEST domain of ICN1 correspond to an early termination codon, thereby preventing proteasome degradation and reducing turnover of ICN1 (8). In contrast, only 30% of the PEST mutations from our ATL samples (*2439, *2403, and *2466) corresponded to a premature termination codon, and 70% were missense amino acid substitutions (Table 1). We did not find alterations in exon 28 in any ATL patients. We next investigated the transcriptional activity of ATL ICN1 mutants by transient transfection of U2OS cells with ICN or ICN mutants along with a CBF-1, Su(H), LAG-1 (CSL)-Luciferase reporter construct (16). Results showed that the transcriptional activity of ICN1 mutants found in ATL was increased by 50% or more over that of wild-type ICN1 (Fig. 2A). Of note, Western blot analysis suggested that some ICN1 point mutants also were expressed at higher levels than in wild-type ICN1 (Fig. 2A). To extend these findings, we investigated the half-life of several ICN1 mutants. U2OS cells were transfected with Myc-tagged wild-type ICN1 or ICN1-mutant expression vectors, and after 36 h cells were treated with cycloheximide to prevent de novo protein synthesis. Western blot analyses confirmed reduced turnover of most ICN1 mutants, even though these mutations were not termination codons (Fig. 2B).
Table 1.
Samples from 32 ATL patients were analyzed for mutations in the HD domain, the PEST domain, and exon 28 of Notch
| Patient | ICN1 PEST domain | ICN1 HD domain | ICNI exon 28 |
| ATL 1 | No mutation | No mutation | No mutation |
| ATL 2 | No mutation | No mutation | No mutation |
| ATL 3 | No mutation | No mutation | No mutation |
| ATL 4 | No mutation | No mutation | No mutation |
| ATL 5 | Point mutation Q2546R | No mutation | No mutation |
| ATL 6 | No mutation | No mutation | No mutation |
| ATL 7 | Point mutation G2427S | No mutation | No mutation |
| ATL 8 | STOP after amino acid 2440 - TAG | No mutation | No mutation |
| ATL 9 | No mutation | No mutation | No mutation |
| ATL 10 | Point mutation G2430A | No mutation | No mutation |
| ATL 11 | Point mutation S2470P | No mutation | No mutation |
| ATL 12 | No mutation | No mutation | No mutation |
| ATL 13 | No mutation | No mutation | No mutation |
| ATL 14 | No mutation | No mutation | Not detected |
| ATL 15 | No mutation | No mutation | No mutation |
| ATL 16 | No mutation | No mutation | No mutation |
| ATL 17 | Point mutation P2465S | No mutation | No mutation |
| ATL 18 | No mutation | No mutation | No mutation |
| ATL 19 | No mutation | No mutation | No mutation |
| ATL 20 | No mutation | No mutation | No mutation |
| ATL 21 | STOP after amino acid 2403 - TAG | No mutation | No mutation |
| ATL 22 | No mutation | No mutation | No mutation |
| ATL 23 | STOP after a amino acid 2466 - TAG | No mutation | No mutation |
| ATL 24 | No mutation | No mutation | No mutation |
| ATL 25 | No mutation | No mutation | No mutation |
| ATL 26 | Point mutation V2380A | No mutation | No mutation |
| ATL 27 | Point mutation S2423L | No mutation | No mutation |
| ATL 28 | No mutation | No mutation | No mutation |
| ATL 29 | No mutation | No mutation | No mutation |
| ATL 30 | No mutation | No mutation | No mutation |
| ATL 31 | No mutation | No mutation | No mutation |
| ATL 32 | No mutation | No mutation | No mutation |
Fig. 2.
Mutations in the PEST domain of ICN1 led to its stabilization and reduced degradation by CDC4/FBW7. (A) Transcriptional activity of ICN1 and ATL ICN1 mutants using the 12XCSL luciferase reporter construct. Luciferase values were normalized and expressed compared with wild-type ICN1 set at 100%. All experiments were performed in duplicate and were repeated independently at least twice; representative data are shown as the average with SDs. Western blot showing expression of ICN1 mutants with a C-terminal Myc tag. Asterisk indicates the presence of an early termination codon at that amino acid position. *2439, *2403, and *2466 were detected with using a N-terminal Myc tag. (B) Increased half-life of several ICN1 mutants shown by Western blot analyses after cycloheximide treatment (100 μg/mL for 0, 3, and 6 h). (C) The same ATL ICN1 mutants were analyzed for CDC4-mediated degradation and compared with wild-type ICN1 after transient expression in U2OS cells in the presence of CDC4-Flag and treatment with cycloheximide (100 μg/mL for 5 h). Expression of CDC4 using Flag antibody and actin-loading controls is shown. (D). Interactions between ATL ICN1 mutants and wild-type FLAG-tagged CDC4. 293T cells were transfected with ICN1 or ICN1 mutants in the presence or absence of CDC4. IP Flag (CDC4) and WB Myc (ICN1) show interactions between ICN1, ICN1 mutants, and CDC4. ICN1 PEST domain early stop *2403 was used as negative control. WB Flag revealed equivalent amounts of CDC4 IP. Reverse IP Myc (ICN1) and WB Flag (CDC4) is shown underneath IP Myc; WB Myc is shown for control. (E) CDC4-mediated ubiquitination assay for ATL ICN1 mutants G2427S and S2423L in U2OS cells cotransfected with HA-Ub vector. Experiments were performed in the presence and absence of MG132 (10 μM). Controls for ICN1 and HA expression from lysates are shown. (F) ICN1 S2423L and wild-type ICN1 ubiquitination assay in U2OS cells transfected with HA-Ub (K48R). IP, immunoprecipitation; WB, Western blot.
PEST-Substitution Mutants Have Reduced CDC4/FBW7-Mediated Degradation.
This intriguing observation prompted us to investigate whether ATL ICN1 PEST mutants were more resistant than wild-type ICN1 to CDC4/FBW7-induced ubiquitination and proteasome degradation. Coexpression of wild-type ICN1 and CDC4 in U2OS cells resulted in reduced expression of ICN1, whereas ICN1 mutants generally were more resistant to CDC4-mediated degradation (Fig. 2C). Although the half-life of ICN1 S2423L is longer than that of the wild type (Fig. 2B), the results shown in Fig. 2C suggested that this mutant still is targeted for degradation by CDC4. However, in that experiment, CDC4 was expressed at much higher levels in ICN1 mutant S2423L than in the wild type and presumably accounts for the reduced S2423L ICN1 levels. We then investigated whether the lack of degradation was caused by reduced or lack of interaction between ICN1 and CDC4. Coexpression of wild-type ICN1 and CDC4 in U2OS cells and coimmunoprecipitation assays showed that all ICN1 mutants retained binding to CDC4 similar to that of the wild-type ICN1 (Fig. 2D). For these assays, the PEST domain early termination codon (*2403) was used as the negative control because it is known not to bind CDC4. To gain further insight into why these ICN1 mutants are resistant to CDC4-mediated degradation, we analyzed the extent of CDC4-mediated ubiquitination of ICN1 and several ICN1 mutants. Our results demonstrate that, despite efficient binding to CDC4, the ICN1 mutants P2465S and S2423L were still ubiquitinated, albeit at much lower efficiency (Fig. 2E). These data are consistent with those reported in Fig. 2C. Ubiquitination regulates a wide range of cellular processes, from transcriptional activity to degradation. Ubiquitination on Lysine 48, usually considered a mark for proteasomal degradation (17), also was less abundant on ICN1 mutant S2423L than on wild type ICN1 (Fig. 2F). The possibility that this mutation favored CDC4-K63 ubiquitination, and therefore reduced degradation, warrants further investigation. Together, our results describe a high prevalence of ICN1-activating mutations in ATL.
Inhibition of Notch Signaling Reduces Proliferation and Survival of ATL Cells in Vitro.
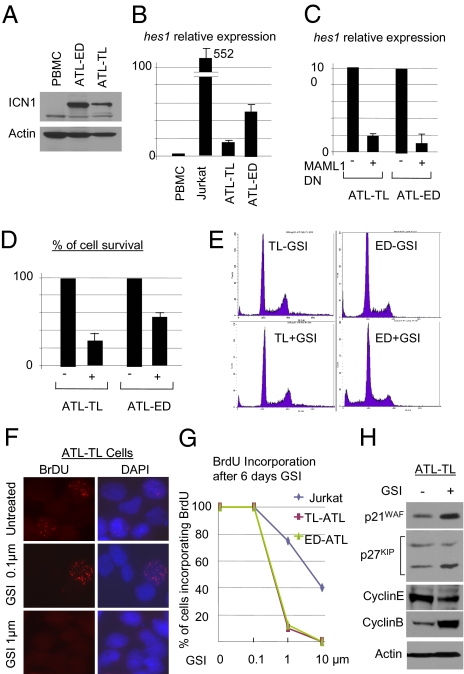
To determine the physiological relevance of our studies, we next investigated whether constitutive activation of Notch1 plays a role in ATL tumor cell growth or survival. ATL-derived HTLV-I–transformed cell lines readily expressed ICN1, which correlated with hes1 expression (Fig. 3 A and B). Interestingly, treatment of T-ALL–transformed cell lines with GSI leads to G0/G1 arrest, demonstrating that the cells are dependent on Notch signaling for their growth. Additional studies have demonstrated that Notch signaling also can be blocked by a dominant negative (DN) of the transcriptional coactivator Mastermind (MAML1) (18). We cloned DN-MAML1 in an HIV-based lentiviral vector containing an internal ribosome entry-site GFP, and high-titer virus was used to transduce ATL-derived HTLV-I–transformed cells. Transduction of DN-MAML1 (19) drastically reduced the transcriptional function of ICN1, as shown by an 80% reduction in Hes 1 expression 72 h after transduction (Fig. 3 C and D). Importantly, a 50–70% reduction in HTLV-I–transformed cell survival also was observed in transduced cells when Notch signaling was inhibited (Fig. 3D). However, in contrast to T-ALL, ATL-derived HTLV-I–transformed cells were arrested in all phases of the cell cycle when treated with GSI for 8 d. To confirm these observations further, we investigated the effect of GSI on the proliferation of ATL-derived HTLV-I–transformed cells by BrdU incorporation (Fig. 3 F and G). Jurkat T-ALL cells were used as a control, because previous reports suggested that these cells are resistant to GSI (20). Our experiments demonstrated that ATL-derived HTLV-I–transformed cell lines (21) ceased to proliferate when cultured in the presence of 1 μM of GSI for 6 d (Fig. 3 F and G), a dose previously used in numerous T-ALL studies (22, 23). Consistent with these observations, we found an increase in the cyclin-dependent kinase inhibitors (CDKI) p21WAF and p27KIP and decreased expression of cyclin E, preventing cells from progressing from G1 to S (Fig. 3H). In addition, increased expression of cyclin B was detected in ATL-derived HTLV-I–transformed cells treated with GSI (Fig. 3H). Because degradation of cyclin B is required for the mitosis exit, GSI also blocked treated cells in G2/M. These results are consistent with cell-cycle data presented in Fig. 3E.
Fig. 3.
Inhibition of Notch signaling reduces proliferation and survival of ATL cells in vitro. (A) Expression of ICN1 in ATL-derived cells compared with normal PBMC. (B) Relative expression of hes1 correlates with ICN1 expression in ATL cells. Relative expression of Hes1 in T-ALL Jurkat and ATL-derived transformed cell lines [TL-Om1 and ED40515(−)] quantified by real-time quantitative RT-PCR. (C) Inhibition of Hes1 expression in ATL cells transduced with a lentiviral vector expressing DN-MAML1. (D) Inhibition of Notch signaling by DN-MAML1 results in 50–70% reduction in ATL tumor cell survival. (E) GSI-induced cell-cycle arrest in all phases of the cell cycle in ATL-derived transformed cells. For ATL-derived TL-Om1–transformed cells without GSI (TL −GSI), G1: 68.1%; S: 8.5%; G2/M: 17.9%; and for ATL-derived TL-Om1–transformed cells with GSI (TL +GSI), G1: 67.7%; S: 7.7%; G2/M: 17.2%). For ATL-derived ED40515(−)-transformed cells without GSI (ED −GSI). G1:73%; S: 11.8%; G2/M: 10.2%; and for ATL-derived ED40515(−)-transformed cells with GSI (ED +GSI), G1: 61.2%; S: 11.5%; G2/M: 12.3%. (F and G) Proliferation of ATL cells in vitro was inhibited by GSI treatment and was shown by BrdU incorporation. Quantification results represent the percentage of cells that incorporated BrdU. (H) Western blot analysis of ATL cells before and after treatment with GSI. Increased expression of CDKI p21WAF and p27KIP and cyclin B with concomitant reduction in cyclin E levels was detected after GSI-mediated inhibition of cell proliferation. Actin was used as loading control.
GSI-Mediated Inhibition of Notch Signaling Reduces ATL Tumor Formation in Vivo.
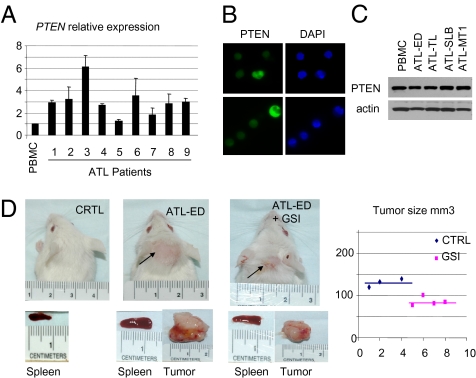
Resistance of T-ALL cells, such as Jurkat, to GSI has been attributed recently to mutational loss of phosphatase and tensin homolog (PTEN) (20). In contrast, PTEN mRNA expression was not altered in freshly isolated, uncultured ATL samples (Fig. 4A). We sequenced the PTEN gene in eight ATL patients and found only silent mutations. We also confirmed that PTEN was correctly localized in the cytoplasm of freshly isolated, uncultured ATL cells (Fig. 4B). Finally, Western blot analyses showed that several ATL-derived HTLV-I–transformed cell lines all express PTEN comparable to its expression in PBMC (Fig. 4C), suggesting that these ATL-derived HTLV-I–transformed cells may be more sensitive than T-ALL cells to GSI treatment. To investigate this possibility, we engrafted Central Institute for Experimental Animals (CIEA) NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac (NOG) mice with ATL-derived HTLV-I–transformed cells. These mice are very permissive to ATL cell engraftment, and 100% of the animals developed tumors (24). Starting 3 d after injection of ATL-derived HTLV-I–transformed cells, GSI or vehicle only was administrated by oral gavage once every 3 d to avoid gastrointestinal side effects (25). After 28 d, the animals were killed, and an external examination suggested a significant reduction in tumor size in the animals that received GSI (Fig. 4D). In fact, animals that received GSI had a significantly reduced splenomegaly and more than a 50% reduction in tumor size (Fig. 4D). Recent studies suggest that GSI and rapamycin treatment inhibit T-ALL growth and extend survival in a mouse xenograft model (26) and that GSI significantly improves the cytotoxicity of the chemotherapeutic drugs doxorubicin and melphalan (27). Along these lines, our results suggest that constitutive activation of Notch signaling in ATL is a promising target for a combination therapy, especially because ATL has a dismal prognosis and currently no favorable therapeutic options (28).
Fig. 4.
GSI-mediated inhibition of Notch signaling reduces ATL tumor formation in vivo. (A) Relative expression of PTEN by quantitative RT-PCR in samples from patients with acute ATL. (B) Immunodetection of cytoplasmic expression of PTEN protein in freshly isolated, uncultured ATL samples from two patients. (C) Western blot analyses showing similar expression of PTEN protein in normal PBMC and four ATL-derived transformed cells. (D) Inhibition of Notch signaling in vivo by GSI in NOG mice engrafted with ATL tumor cells. Four animals were assigned randomly to each group (not injected with ATL cells and receiving DAPT; injected with ATL cells and receiving vehicle only; or injected with ATL cells and receiving DAPT treatment). DAPT (GSI) or vehicle only was administered by oral gavage every 3 d for 28 d. One animal in group 2 injected with ATL cells and receiving vehicle only died during the 28-d period. External tumors are shown by arrows. Splenomegaly was significantly reduced in animals that received DAPT as compared with the control group. Tumors from animals were removed and measured. An ~50% reduction in tumor size was observed in animals that were treated with DAPT (mean, 78 mm3 versus 128 mm3; P < 0.05).
Discussion
In this study we found a high rate of activating mutations in the Notch gene and the constitutive activation of Notch signaling in a high percentage of patients with HTLV-I–associated ATL. Surprisingly, in contrast to T-ALL, mutations in ATL were never found in the HD domain of ICN1. These results suggest that activation of Notch differs in HTLV-I–infected cells and occurs mainly through normal receptor processing by the γ-secretase complex. In support of this notion, our results show that inhibition of γ-secretase abrogates ICN1 accumulation in ATL-derived HTLV-I–transformed cells and in C8166 HTLV-I–transformed cells in vitro. Our results may suggest that, in HTLV-I–infected cells, cell–cell contact is important during activation of Notch. It is possible that in HTLV-I cells additional signaling pathways are required for efficient stabilization of ICN1 after its cleavage. In all, we found mutations in Notch in approximately half the ATL patients tested. This result does not mean that Notch is not important or is not stabilized through alternative mechanisms in ATL samples for which no mutation was found. In fact, the analyses of ICN1 expression in ATL presented in Fig. 1A indicate that a majority of HTLV-I patients have increased intracellular Notch expression at the protein level. Clearly additional studies are needed to investigate whether the levels of Notch correlate with disease prognosis or progression and whether ICN1 can be stabilized in the absence of genetic mutations. Another striking observation is that, among the ATL patients with mutations in the PEST domain of Notch, 70% of these mutations were missense amino acid substitutions rather than stop codons. Point mutations in the PEST domain that are not frameshift or nonsense mutations are known to be very infrequent in ALL (8, 29, 30). Early termination of the PEST domain prevents proteasome-mediated degradation and increases expression of ICN1. Interestingly, ICN1 PEST mutants found in ATL patients had an increased half-life, and, in spite of their interaction with CDC4/FBW7, these mutants were resistant to CDC4/FBW7-mediated ubiquitination and degradation. These data demonstrate an unusual mechanism for reduced turnover of ICN1 and increased activation of Notch signaling and warrant further studies.
The biological function of activated Notch in HTLV-I was investigated also. First, we found that activated Notch is critical for the proliferation and survival of ATL-derived HTLV-I–transformed cell lines in vitro. Inhibition of Notch signaling using a DN-MAML1 effectively prevented proliferation of ATL-derived HTLV-I–transformed cells. In addition, expression of p21, p27, and cyclin B1 was increased, whereas cyclin E expression was down-regulated, consistent with cell-cycle arrest in G1 and G2/M. Although resistance of T-ALL cells to GSI has been attributed to mutational loss of PTEN, we found only silent mutations in the PTEN gene in ATL patients. These ATL patients also showed normal PTEN expression and localization, and PTEN protein expression was normal in ATL-derived HTLV-I–transformed cells. These results suggested that ATL cells may be sensitive to GSI therapy. To test this hypothesis, we injected CIEA NOG mice with ATL-derived HTLV-I–transformed cells [ED40515(−)] and treated them with gamma secretase inhibitor DAPT [N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester] or vehicle only for 3 wk. Our results showed that inhibition of Notch signaling by GSI was associated with significantly reduced splenomegaly and tumor formation in mice engrafted with ATL-derived HTLV-I–transformed cells. In conclusion, our study supports the notion that activated Notch is important for HTLV-I–associated ATL. A high rate of activating mutations was found in ATL patients. However, in contrast to T-ALL (5), mutation of the HD domain of Notch was never observed in ATL patients. Consistent with these observations, ICN1 was generated by γ-secretase–mediated processing of the Notch receptor in ATL cells. In fact GSI was efficient in preventing growth of the ATL-derived HTLV-I–transformed cell line ED40515(−) in vitro and in vivo. These data identify Notch as a potential therapeutic target in HTLV-I–associated ATL.
Materials and Methods
Patients and Cell Lines.
Samples from 32 ATL patients were obtained after informed consent in a study approved by the Institutional Review Board to the National Institutes of Health and the Necker Hospital. C8166, Jurkat, and ATL-derived HTLV-I–transformed cell lines SLB1, TL-Om1, ED40515(−), and MT 1 were grown in RPMI-1640 complete medium. U2OS cells were cultured in DMEM (Invitrogen) 10% FBS (Atlanta Biologicals).
Plasmids and Transfections.
Transfections were carried out in 293T or U2OS2 cells using PolyFect (Qiagen) and Effectene (Qiagen) reagents, respectively. ICN1 point mutants were generated by site-directed mutagenesis using cMyc-ICNI vectors as templates. A modified human retrovirus cytomegolavirus HIV-based lentiviral vector was used to generate high-titer pseudotype DN-MAML1 virus particles as previously reported (31).
Western Blots, Immunofluorescence, Cell Cycle, and Real-Time Quantitative RT-PCR.
Anti-Myc (9E10; Roche), p21, p27, cyclin E, cyclin B, and anti-actin (Santa Cruz Biotechnology), anti-Flag (Sigma), anti-HA (3F10) (Roche), and anti-cleaved Notch 1 (Val1744) (Cell Signaling) were used. For coimmunoprecipitation, 293T cells were cotransfected with the indicated Myc-ICN1 and Flag-CDC4 constructs for 24 h using PolyFect, harvested with RIPA (Tris-HCl: 50 mM, pH 7.4, NaCl 150 mM, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], EDTA 1 mM, 10 mg/ml phenylmethylsulfonyl fluoride, aprotinin [2 μg/mL], and 100 mM sodium orthovanadate), and immunoprecipitated and immunoblotted as indicated. For cell-cycle analyses, cells were mock-treated or treated with GSI at 1 μM for 8 d, collected, stained with propidium iodide, and analyzed by FACS. For quantitative real-time RT-PCR, total RNA was isolated by TRIzol (Invitrogen), treated with DnaseI, and reverse transcribed. The resulting cDNAs were analyzed by real-time PCR using Sybr Green Master Mix (Applied Biosystems).
Ubiquitination Assays.
U2OS cells were transfected with 0.1 μg of c-Myc ICNI constructs (Effectene; Qiagen), 0.2 μg Flag-CDC4, and 0.2 μg HA-tagged ubiquitin (HA-Ub). After 24 h, cells were treated with 10 μM MG132. Cells were lysed in RIPA containing N-ethylmaleimide, iodoacetamide, and EDTA, were immunoprecipitated with 9E10 Ab (Roche), and were analyzed by Western blotting as indicated.
ATL-Engrafted NOG Mice and GSI Treatment.
NOG mice distributed by Taconic were used. For each animal, 5.10^7 ED40515(−) ATL-derived HTLV-I–transformed cells were injected subcutaneously as previously reported (23). DAPT (GSI) (Calbiochem) was resuspended in a 0.5% methyl cellulose suspension and administrated by oral gavage every 3 d for 3 wk. Vehicle only was administrated as control. All animals were killed at day 28, and tumors were removed and measured. The study was approved by the Animal Care and Use Committee of Advanced Bioscience Laboratories, Inc.
Acknowledgments
We thank Dr. J. Aster (Brigham and Women's Hospital) for providing the MIG-ICN and MIGR1-MAML1 dominant-negative construct. ATL-derived HTLV-I–transformed cells MT1, TL-Om1, and ED40515(−) were provided by Dr. Michiyuki Maeda (University Sakyo-ku). SLB1 ATL-derived HTLV-I–transformed cells were provided by Dr. G. Feuer (State University of New York Upstate Medical University). The authors thank Elizabeth Jenkins for editorial assistance. This work was supported by Grants CA106258 and CA115398 from the National Cancer Institute (to C.N.) and by funds from the University of Kansas Medical Center Research Institute, Inc. (to C.N.).
Footnotes
The authors declare no conflict of interest.
References
- 1.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 3.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 4.Aifantis I, Raetz E, Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. 2008;8:380–390. doi: 10.1038/nri2304. [DOI] [PubMed] [Google Scholar]
- 5.Grabher C, von Boehmer H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6:347–359. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- 6.Gupta-Rossi N, et al. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J Biol Chem. 2001;276:34371–34378. doi: 10.1074/jbc.M101343200. [DOI] [PubMed] [Google Scholar]
- 7.Minella AC, Clurman BE. Mechanisms of tumor suppression by the SCF(Fbw7) Cell Cycle. 2005;4:1356–1359. doi: 10.4161/cc.4.10.2058. [DOI] [PubMed] [Google Scholar]
- 8.Weng AP, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 9.Poiesz BJ, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migone TS, et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 12.Curry CL, et al. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi's sarcoma tumor cells. Oncogene. 2005;24:6333–6344. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 13.Welcker M, Clurman BE. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 14.O'Neil J, Look AT. Mechanisms of transcription factor deregulation in lymphoid cell transformation. Oncogene. 2007;26:6838–6849. doi: 10.1038/sj.onc.1210766. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Irizarry C, et al. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol. 2004;24:9265–9273. doi: 10.1128/MCB.24.21.9265-9273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Neil J, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204:1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002;1:337–341. [PubMed] [Google Scholar]
- 18.Maillard I, et al. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, et al. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 20.Palomero T, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda M, et al. Origin of human T-lymphotrophic virus I-positive T cell lines in adult T cell leukemia. Analysis of T cell receptor gene rearrangement. J Exp Med. 1985;162:2169–2174. doi: 10.1084/jem.162.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang MY, et al. Leukemia-associated NOTCH1 alleles are weak tumor initiators but accelerate K-ras-initiated leukemia. J Clin Invest. 2008;118:3181–3194. doi: 10.1172/JCI35090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullion K, et al. Targeting the Notch1 and mTOR pathways in a mouse T-ALL model. Blood. 2009;113:6172–6181. doi: 10.1182/blood-2008-02-136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takajo I, et al. Engraftment of peripheral blood mononuclear cells from human T-lymphotropic virus type 1 carriers in NOD/SCID/gammac(null) (NOG) mice. Int J Cancer. 2007;121:2205–2211. doi: 10.1002/ijc.22972. [DOI] [PubMed] [Google Scholar]
- 25.Real PJ, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 28.Tsukasaki K, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breit S, et al. Activating NOTCH1 mutations predict favorable early treatment response and long-term outcome in childhood precursor T-cell lymphoblastic leukemia. Blood. 2006;108:1151–1157. doi: 10.1182/blood-2005-12-4956. [DOI] [PubMed] [Google Scholar]
- 30.Zhu YM, et al. NOTCH1 mutations in T-cell acute lymphoblastic leukemia: Prognostic significance and implication in multifactorial leukemogenesis. Clin Cancer Res. 2006;12:3043–3049. doi: 10.1158/1078-0432.CCR-05-2832. [DOI] [PubMed] [Google Scholar]
- 31.Nicot C, et al. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]