Abstract
Recent functional imaging studies demonstrated that both the left and right supramarginal gyri (SMG) are activated when healthy right-handed subjects make phonological word decisions. However, lesion studies typically report difficulties with phonological processing after left rather than right hemisphere damage. Here, we used a unique dual-site transcranial magnetic stimulation (TMS) approach to test whether the SMG in the right hemisphere contributes to modality-independent (i.e., auditory and visual) phonological decisions. To test task-specificity, we compared the effect of real or sham TMS during phonological, semantic, and perceptual decisions. To test laterality and anatomical specificity, we compared the effect of TMS over the left, right, or bilateral SMG and angular gyri. The accuracy and reaction times of phonological decisions were selectively disrupted relative to semantic and perceptual decisions when real TMS was applied over the left, right, or bilateral SMG. These effects were not observed for TMS over the angular gyri. A follow-up experiment indicated that the threshold-intensity for inducing a disruptive effect on phonological decisions was identical for unilateral TMS over the right or left SMG. Taken together, these findings provide converging evidence that the right SMG contributes to accurate and efficient phonological decisions in the healthy brain, with no evidence that the left and right SMG can compensate for one another during TMS. Our findings motivate detailed studies of phonological processing in patients with acute or long-term damage of the right SMG.
Keywords: human brain, language lateralization, compensation, semantic, transcranial magnetic stimulation
Many previous functional imaging studies have shown that the left and right supramarginal gyri (SMG) are activated when right-handed participants make decisions about the sounds of words (i.e., their phonology) compared with decisions about their meanings (i.e., their semantics) (1–4). However, the functional significance of right SMG activation is unclear because lesion studies have reported phonological difficulties following left rather than right temporo-parietal lesions (5–8). Consequently, anatomical models of phonological processing have included left but not right parietal cortex (9, 10). The present study was designed to address the discrepancy between functional imaging and lesion studies. More specifically, we examined how “online” transcranial magnetic stimulation (i.e., TMS during a task) over the left and right SMG influences phonological word processing in healthy subjects (Fig. 1). We used the neurodisruptive effect of TMS to distinguish between three alternative hypotheses to explain right SMG activation with phonological processing.
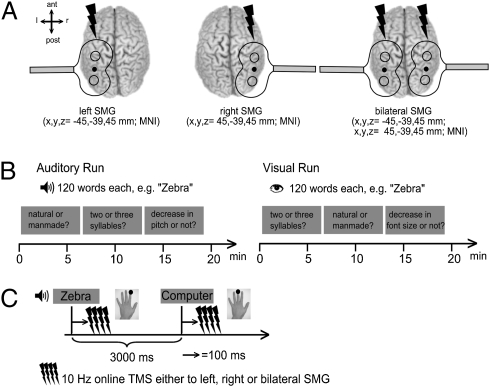
Fig. 1.
Experimental design. (A) Stimulation sites over the supramarginal gyri (SMG). ant, anterior; post, posterior; l, left; r, right. (B) Auditory and visual run of the three blocked tasks. (C) Each trial had a duration of 3,000 ms. A four-pulse train of 10 Hz TMS was applied 100 ms after word onset over left, right, or bilateral SMG. Subjects responded with their left index or middle finger.
Hypothesis 1 is that right SMG only contributes to the speed but not the accuracy of phonological decisions. Consequently, right SMG lesions have a subtle effect on phonological processing that might be missed unless reaction times were measured. In this case, we expect a selective effect of right SMG TMS on reaction times in the healthy brain without affecting error rates.
Hypothesis 2 is that right SMG is necessary for accurate and efficient phonological decisions in the healthy brain, but following right SMG lesions, the function of right SMG can be supported by alternative brain regions. Consequently, right SMG lesions may temporarily impair phonological decision performance in the acute phase after brain damage, but this lesion effect will not be apparent after functional reorganization. In this case, we expect a significant effect of right SMG TMS on both the reaction times and accuracy of phonological decisions in the healthy brain.
Hypothesis 3 is that right SMG is not necessary for accurate and efficient phonological decisions but is activated in functional MRI (fMRI) studies of the healthy brain because it is involved in task-related activation that is incidental to performance [i.e., redundant processing (11)]. In this case, neither right SMG lesions nor right SMG TMS will influence phonological decision performance.
There are three unique features of our study relative to previous online TMS studies of phonological processing (12, 13). First, we investigated the effect of TMS to the right SMG. Second, we compared unilateral TMS over the right SMG to unilateral TMS to the left SMG and dual-site TMS over left and right SMG simultaneously. This manipulation allowed us to test whether impaired unilateral SMG function was supported by the contralateral hemisphere. If so, then the effect of dual-site TMS to both the left and right SMG should be greater than the effect of TMS to either the left or right SMG alone (11). Third, we compared the effect of TMS on phonological decisions to words presented in the auditory as well as visual modality, whereas previous studies investigated the effect of online TMS to left SMG with visually presented words only (12, 13). This process enabled us to assess whether the expected TMS effects were dependent or independent of stimulus modality. To test the functional and anatomical specificity of our effects, we also investigated how online TMS affected semantic or perceptual decisions on the same sets of stimuli, and whether the effect of TMS on phonological decisions was greater when TMS was over the SMG than over a neighboring parietal area in the angular gyrus (ANG).
Results
Reaction Times.
Effect of real vs. sham TMS over left, right, and bilateral SMG.
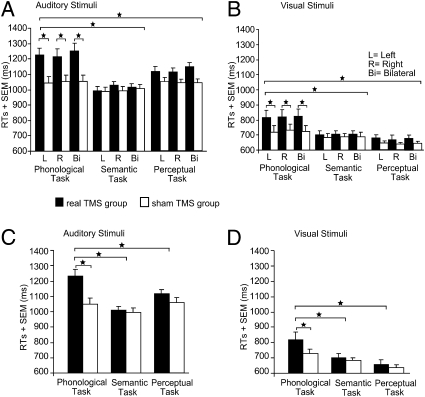
Subjects’ mean reaction times (RTs) (Table S1) were analyzed with a four-way repeated-measures ANOVA. The four factors were: group (14 subjects with real TMS vs. 14 subjects with sham TMS), task (phonological, semantic, perceptual), TMS laterality (left, right, bilateral) and modality (auditory vs. visual). A main effect of group showed increased RTs for real TMS relative to sham TMS (F1,25 = 4.27, P = 0.049) (Table 1). However, this group effect interacted with task (F2,50 = 5.82, P = 0.005) (Fig. 2). Across modalities and laterality sites, the disruptive effect of real TMS on RTs was greater on phonological compared with semantic (t27 = 5.89, P = 0.0001; posthoc paired t-test) or perceptual decisions (t27 = 5.45, P = 0.0001). There were no significant differences between real vs. sham TMS on the perceptual and semantic tasks, and no task effects in the sham TMS group (all P > 0.12). Further t-tests confirmed that real TMS compared with sham TMS increased RTs in the phonological task (t27 = 2.12, P = 0.039) but not in the semantic (P = 0.75) or perceptual (P = 0.42) tasks. The task-specific delay of phonological decisions with TMS over SMG was independent of TMS laterality as there was no task-by-group-by-laterality interaction (P = 0.35). Effects that did not interact with TMS group (i.e., real vs. sham TMS) can be found in the SI Results.
Table 1.
Results from the ANOVA comparing the effect of real vs. sham TMS over left, right, and bilateral SMG
| Effect | F | df | P |
| Main effect | |||
| Task | 16.39 | 1.34, 33.41 | 0.0001 |
| TMS laterality | 5.74 | 2, 50 | 0.006 |
| Modality | 586.97 | 1, 25 | 0.0001 |
| Group | 4.27 | 1, 25 | 0.049 |
| Interaction | |||
| Task × group | 5.82 | 2, 50 | 0.005 |
| Task × modality | 13.17 | 1.54, 38.52 | 0.0001 |
df, degrees of freedom; P < 0.05 was considered significant.
Fig. 2.
Mean RTs for the effect of real vs. sham TMS over left, right, and bilateral SMG. For illustrating purposes, responses for auditorily and visually presented stimuli are displayed in different panels (A–D). All panels depict the significant two-way interaction between the factors task and group. Note that the three different TMS laterality sites (left, right, bilateral) are displayed separately for illustrating purposes in A and B, although the interaction was pooled across the factors TMS laterality site and modality. Error bars represent 1-fold SEM; *P < 0.05; two-tailed.
Effect of real TMS over SMG vs. ANG.
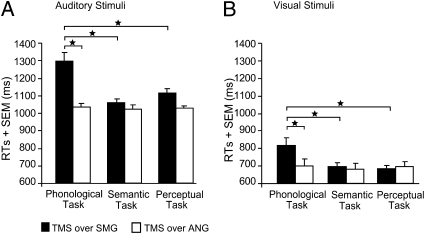
A four-way repeated measures ANOVA (subset of 10 subjects) investigated the effect of real TMS on region (SMG vs. ANG), task (phonological, semantic, perceptual), TMS laterality (left, right, bilateral) and modality (auditory, visual). The results demonstrate that the task-specific effect of TMS over the SMG (i.e., delayed responses for phonological relative to semantic or perceptual decisions) was not observed with TMS over ANG (P > 0.41 for all comparisons). This finding was confirmed by a two-way interaction between region (SMG vs. ANG) and task (F2,18 = 8.37, P = 0.003) (Fig.3), which arose because the effect of region (slower RTs for TMS over SMG than ANG) was greater during the phonological than semantic (t19 = 5.02, P = 0.0001) or perceptual (t19 = 4.43, P = 0.0001) tasks. Post hoc tests confirmed significant longer RTs for TMS over SMG than ANG with the phonological task (t19 = 5.65, P = 0.001) but not for the semantic (P = 0.30) or the perceptual tasks (P = 0.12). These effects did not interact with modality (P = 0.43) or TMS laterality (P = 0.51).
Fig. 3.
Mean RTs for the effect of real TMS over SMG vs. ANG. (A and B) The significant two-way interaction between the factors region and task is shown. For illustrating purposes, responses for auditorily and visually presented stimuli are displayed in different panels, although the interaction was pooled across modality. *P < 0.05 two-tailed.
Error Rates.
Effect of real vs. sham TMS over left, right, and bilateral SMG.
There were no significant differences between the three TMS laterality sites (left, right, or bilateral TMS) in any of the tasks (all P > 0.32) (Table S1). Consequently, error rates (ERs) were pooled across the factor TMS laterality to reduce the number of necessary comparisons.
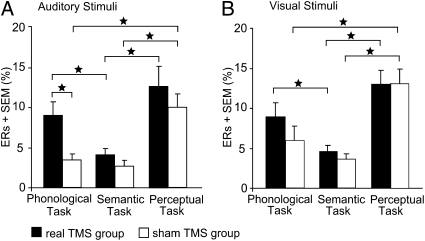
In both the auditory (Fig. 4A) and visual modalities (Fig. 4B), ERs were higher for phonological than semantic decisions during real TMS (Z = 2.73, P = 0.004 in the auditory; and Z = 2.67, P = 0.004 in the visual modality) but not during sham TMS (P = 0.39 for auditory and 0.23 for visual stimuli). The task effect of real vs. sham TMS was significant for phonological decisions in the auditory (Z = 2.72, P = 0.006) but not the visual modality (P = 0.11), and not during semantic decisions in either the auditory (P = 0.14) or visual (P = 0.45) modalities. There were no significant differences between phonological and perceptual errors in the real TMS group (P = 0.30 and P = 0.18 for auditory and visual stimuli, respectively); however, the sham group showed decreased errors for phonological compared with perceptual decisions (Z = 3.05, P = 0.001 in the auditory; Z = 3.18, P = 0.001 in the visual modality). The latter is likely to be the consequence of a speed-accuracy tradeoff. For both TMS groups, errors were higher during the perceptual than semantic task in both modalities (Z = 2.72, P = 0.004 for real TMS in the auditory; Z = 3.23, P = 0.001 for real TMS in the visual modality; Z = 3.04, P = 0.001 for sham TMS in the auditory; and Z = 3.18, P = 0.001 for sham TMS in the visual modality).
Fig. 4.
Mean error rates (ERs) for the effect of real vs. sham TMS over left, right, and bilateral SMG. (A and B) ERs are pooled across the factor TMS laterality as there were no differences between left, right, and bilateral TMS. Error bars represent 1-fold SEM; *P < 0.05; two-tailed.
Effect of real TMS over SMG vs. ANG.
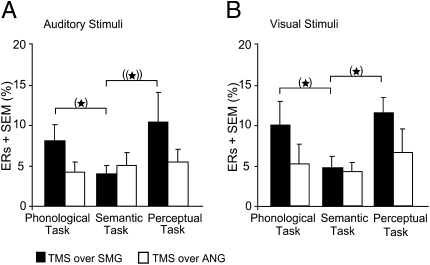
With only 10 subjects, we did not find significant differences in ER for TMS over SMG versus ANG that survived a Bonferroni-Holm correction for multiple comparisons (P < 0.004). However, there were trends toward increased ER for phonological relative to semantic decisions with TMS to SMG but not ANG (Fig. 5).
Fig. 5.
Mean ERs for the effect of real TMS over SMG versus ANG. (A and B) ERs are pooled across the factor stimulation site as there were no differences between left, right and bilateral TMS. (*): does not survive the Bonferroni-Holm correction (P > 0.004); ((*))P < 0.10.
Effect of TMS intensity over left vs. right SMG.
The above results indicated comparable effects for unilateral TMS over left and right SMG during phonological decisions. In a follow-up experiment 2 mo after the main experiment, we compared the intensity-dependence of the “lesion” effect induced by unilateral TMS to the left or right SMG. We thus wanted to investigate whether the TMS-intensity-effect size curves for left vs. right SMG were different. This process enabled us to test if left SMG TMS disrupted phonological processing at lower intensities than right SMG TMS. All subjects from the real TMS group (n = 14) performed two sessions of the phonological task again while receiving TMS over left (session one) or right SMG (session two). Both sessions consisted of four blocks of different TMS intensities. Each block included 30 trials of the phonological task and was separated by a 5-min rest to prevent carry-over effects. TMS was applied at four different intensities (55, 60, 75, and 90% of individual resting motor threshold [RMT]). The order of sessions was counterbalanced across subjects. In all other aspects, the follow-up experiment was identical to the main experiment.
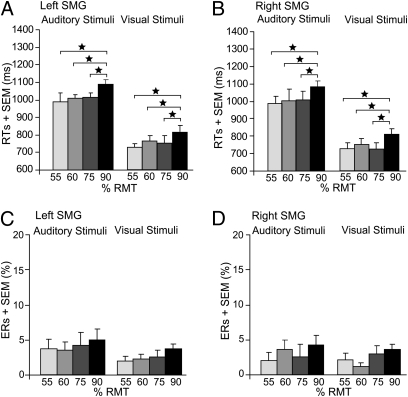
Repeated-measures ANOVA revealed a main effect of intensity on RTs (F3,39 = 15.34, P = 0.0001) (Fig. 6 A and B). Only the highest intensity increased RTs of phonological judgments (post hoc t tests: t13 = 5.38, P = 0.0001; t13 = 4.03, P = 0.0001; t13 = 5.24, P = 0.0001; for 90 vs. 55, 60, and 75%; respectively). This intensity effect was comparable for left and right SMG TMS (P = 0.88). ERs were not significantly different between the different intensities (all P > 0.25) (Fig. 6 C and D), but RTs were longer in the auditory than visual conditions (F1,13 = 72.31, P = 0.0001).
Fig. 6.
Mean RTs (A and B) and ERs (C and D) for the phonological task in the follow-up experiment (TMS at different intensities over the left and right SMG). In A and B, the main effect of intensity on RTs is displayed. The two different TMS laterality sites (left and right SMG) and the two modalities are displayed here separately for illustrating purposes, although the main effect was pooled across the factors TMS laterality and modality. Error bars represent 1-fold SEM; RMT, resting motor threshold. *P < 0.05; two-tailed.
A comparison with the results of the sham group revealed significant differences for the highest intensity only (independent-samples t tests: t27 = 2.39, P = 0.034; t27 = 2.09, P = 0.041 for left-hemisphere TMS in the auditory and visual modality, respectively, and t27 = 2.42, P = 0.032; t27 = 2.06, P = 0.044 for right-hemisphere TMS) but neither for the three lower intensities (all P > 0.13), nor for the error data (all P > 0.20).
Please refer to SI Results for the results of the unpleasantness scores.
Discussion
We used a unique dual-site TMS approach to compare the disruptive effects of online TMS over the left, right, and bilateral SMG during phonological word decisions. This design allowed us to test three different explanations for why previous fMRI studies have shown bilateral SMG activation during phonological decisions in healthy subjects but lesion studies emphasize the importance of left rather than right hemisphere damage in aphasia. Our finding that reaction times and errors increased following TMS to right SMG as well as left SMG indicates that unperturbed right SMG activation is necessary for accurate and efficient phonological decisions in the healthy brain. Moreover, our finding that phonological decision performance was not worse for bilateral SMG TMS than unilateral SMG TMS provides no evidence that the left and right SMG can acutely compensate for one another: If phonological decisions are possible with either the left or right SMG, then dual-site TMS over the left and right SMG should produce a greater lesion effect than TMS over left or right SMG alone (11). In contrast, both the main experiment and the follow-up experiment manipulating TMS intensity indicated that the lesion effect of unilateral TMS to the right SMG was comparable to the lesion effect induced by unilateral TMS to the left SMG or bilateral TMS to the right and left SMG. Furthermore, the disruptive effect was independent of the stimulus modality. Moreover, the TMS-induced lesion effect was both functionally and anatomically specific: We found a selective impairment in modality-independent phonological decisions but not semantic nor perceptual decisions when TMS was given over the SMG, and these effects were not observed when TMS targeted the ANG.
When interpreting TMS-induced effects, one should bear in mind that TMS causes a synchronized discharge in a relatively large population of neurons that is terminated by a long lasting GABAergic inhibition (14). On the one hand, TMS suppresses ongoing processing by silencing neurons. On the other hand, TMS adds extra “noisy” activity to ongoing processing (14). Both mechanisms adversely affect the neuronal activity in the stimulated area for a limited period. At a behavioral level, the neurodisruptive effects of TMS may increase reaction times or errors (15). In previous TMS studies of language, TMS usually affected either RTs or errors (1, 16, 17). In our experiments, TMS increased both RTs and errors during phonological decisions, providing evidence for a strong “virtual lesion” effect independent of the laterality of TMS. The concurrent increase in RTs and errors also excludes a nonspecific speed-accuracy tradeoff during phonological decisions.
The critical contribution of the left SMG to phonological decisions has been demonstrated previously in TMS studies of healthy volunteers (12, 13) and is consistent with functional imaging studies showing greater SMG activation during phonological than semantic decisions (3, 18). The precise role of the SMG in phonological decisions is unclear: It may be directly involved in phonological processing (e.g., subvocal articulation) or it may be involved in executive processing that is particularly important for phonological decisions. For example, Romero et al. (12) found that 5 Hz TMS to the left SMG significantly disrupted judgments on visually presented words in a variety of tasks, providing evidence for the involvement of the left SMG in short-term retention of verbal material as well as phonological judgments (12). Nevertheless, we can exclude explanations in terms of general executive processing in our study because the effect of TMS was not related to task difficulty, as measured by accuracy and response times in the sham TMS group. In contrast, task difficulty was greatest during the auditory perceptual task, with no significant differences between the linguistic tasks, whereas the effect of effective TMS was greatest in the phonological task, with only a nonsignificant trend during the auditory perceptual task. It is also possible that the trend towards increased RTs in the auditory perceptual task after effective SMG TMS reflects the demands on acoustic-phonetic processing (19) or phonological short-term memory (20). Although future studies are required to understand the exact computations of the left and right SMG, our study is unique in demonstrating that the right SMG contributes to phonological decisions, and that both SMG are important for phonological decisions on auditorily as well as visually presented words.
Our finding that the right SMG contributes to phonological decisions in healthy participants may appear to be in discordance with the existing literature on phonological processing in patients with focal brain lesions. Damage to the right hemisphere is not typically associated with deficits in phonological processing (7), although there is a lack of studies directly comparing phonological deficits after left vs. right supramarginal lesions. Although recent studies indicate that the (temporary) recruitment of homolog right-hemisphere areas after left-hemisphere stroke may be adaptive, longer-term language improvement is associated with left-hemisphere language function (21, 22). For example, Winhuisen et al. (22) argue that restoration of the left-hemisphere network seems to be more effective for recovery after stroke, but in some cases, right-hemisphere areas are integrated successfully. Our TMS results contribute by showing that the involvement of right-hemisphere language areas is not limited to recovery after stroke but is also essential for phonological processing in healthy subjects.
The discrepancy between our study and previous patient data may be because of differences in the time scale of functional reorganization. In our study, TMS was applied during task performance, leaving the language system no time to develop adaptive plasticity. This finding may be different in patients with chronic structural lesions where massive reshaping of the language network occurs during recovery (21). The neurodisruptive effects of TMS over right SMG on phonological decisions in healthy subjects call for a reevaluation of phonological deficits in patients with right-hemisphere inferior parietal lesions. As emphasized by Seghier et al. (23), further investigation of patients with right-hemisphere lesions is necessary to fully understand the causal basis of aphasia. Prospective longitudinal studies might demonstrate that the right SMG may be more functionally relevant in the acute phase after stroke than in the chronic phase when reorganization of the language networks has occurred (21).
Alternatively, unilateral TMS of right SMG might have produced its detrimental effect on phonological processing not by disrupting neuronal processing in the stimulated SMG but by activating transcallosal inputs from the right to the left SMG (14). These transcallosal inputs might have activated inhibitory circuits or added noisy activity in the left SMG, and thereby interfered with phonological processing in the left SMG. This interpretation would be in line with previous studies demonstrating significant, acute remote effects of TMS in contralateral homotopic areas (24, 25). For example, it has been shown that TMS over the motor cortex can change the metabolic rate in contralateral motor areas and may lead to behavioral or functional effects ipsilateral to the side of stimulation (26). Although we cannot discard the “transcallosal” hypothesis, several considerations render this explanation unlikely. Previous neurophysiological studies showed that TMS of the ipsilateral motor hand area has much stronger excitatory and longer-lasting inhibitory effects on regional excitability, as opposed to the transcallosally induced effects induced by TMS of the contralateral motor area (27). The threshold for inducing transcallosal inhibitory effects is also considerably higher than for inducing intracortical inhibition with the coil over the motor cortex (28, 29). Therefore, the effect size of a lesion effect should be stronger and the threshold for inducing a lesion effect should be lower with ipsilateral than contralateral TMS using the same stimulation intensity. This result was not the case in the present study. The threshold, as well as the magnitude, of the disruptive effect on phonological decisions was comparable with TMS to both hemispheres. Furthermore, there is little evidence from previous studies that transcallosal excitation spread to the homolog parietal area makes a substantial contribution to the behavioral effects obtained with TMS. Indeed, many studies found a specific deterioration in performance with unilateral TMS over one hemisphere but not over the contralateral homolog area (30–32). The fact that most previous studies revealed a clear asymmetric sensitivity of the right and left parietal cortex to TMS lesions argues against a significant contribution of transcallosal excitation of the homolog area to the TMS-induced effects. It should be noted, however, that a direct comparison of the RTs for different intensities in the effective TMS and sham groups revealed significant differences for the highest intensity only. It might have been more informative to use 80 and 85% of RMT for the intensity experiment to investigate more subtle differences between the two hemispheres. However, in the absence of any a priori predictions of the most sensitive threshold, we chose 15% steps from 90% downward to test for marked differences. Smaller steps were not included because this would have required either the inclusion of additional stimuli (to avoid stimulus repetition across intensities) or the use of considerably fewer trials per condition. The impact of reducing trials per condition on the power of the experiment is illustrated by noting that the effect sizes in the intensity experiment were smaller than those in the main experiment, which included more stimuli per condition.
The absence of any disruptive effect of TMS over the ANG during semantic decisions is at odds with functional imaging findings that the ANG is involved in semantic relative to phonological processing in healthy subjects (1, 3, 18, 33). Because we are not aware of any previous study having targeted the ANG with TMS during language processing, we think that our null-finding (the absence of any disruptive effect of ANG TMS on semantic processing) should be interpreted with caution. It is possible that targeting another subregion within the ANG or using higher intensities would have interfered with semantic processing. For example, previous TMS studies of semantic processing (1, 16, 34) used considerably higher stimulation intensities, ranging from 100 to 110% RMT or 60% total stimulator output compared with 90% RMT (approximately corresponding to 40–50% total stimulator output) in the present study. Our stimulation intensities might therefore have been too low to effectively disrupt semantic processing in the ANG, although they were sufficient to disrupt phonological processing in the SMG. Thus, further TMS research is needed to investigate the critical involvement of the ANG in semantic processing.
In conclusion, our study highlights the importance of the right SMG in phonological decisions. This finding strongly motivates the investigation of phonological processing abilities in patients with acute right SMG damage. According to our results, we would predict that these patients have some degree of phonological processing impairment, irrespective of whether words are presented in the auditory or visual modality.
Materials and Methods
Subjects.
For examining the effect of real vs. sham TMS, 28 native German speakers with no history of neurological disorders or head injury were randomly assigned to the real TMS group (n = 14, 8 females, 20–28 y old, mean: 24) or sham TMS group (n = 14, 8 females, 22–32 y old, mean: 25). All subjects were right-handed (laterality index >95%) according to the Edinburgh Handedness Inventory (35) and were naive to TMS. Written informed consent was obtained before the experiment. The study was performed according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty of the Christian-Albrechts-University of Kiel. For comparing the effect of real TMS over SMG versus ANG, 10 subjects from the real TMS group were reexamined after 6 mo to minimize repetition and familiarity effects.
Experimental Design.
The main experiment compared the effect of real versus sham TMS over left, right and bilateral SMG. It entailed a 2 × 3 × 3 × 2 design with two groups (real TMS vs. sham TMS), three different tasks (phonological, semantic, and perceptual), three different TMS laterality sites (left, right, and bilateral stimulation over the SMG) in two modalities (auditory and visual). An identical set of 120 stimuli (SI Materials and Methods) were presented across tasks and modalities. This resulted in six repetitions of the same words with the effect of repetition controlled across tasks. To keep the repetition of identical stimuli per subject at a minimum, we decided against the inclusion of real vs. sham TMS as within-subject factor and thus included the sham TMS group. The factorial design enabled us to test for task, laterality, and group-specific modality-independent effects while controlling for stimulus and repetition effects. For comparing the effect of real TMS over SMG vs. ANG, 10 of the subjects in the real TMS group participated in the same experiment, with the exception that TMS was over left, right, or bilateral ANG. To avoid repetition and familiarity with the stimuli, this experiment was conducted 6 mo after the SMG experiment.
Tasks.
Subjects performed three tasks on the same set of visual or auditory stimuli. In the phonological task, subjects categorized the items as having two or three syllables. The semantic task consisted of deciding whether a word represented a natural or man-made item. A perceptual task was included as a baseline. Thus, subjects decided whether or not there had been a decrease in pitch toward the end of the word (auditory task) and whether or not font size had decreased toward the end of the word (visual task). Tasks were blocked to ensure a constant cognitive set. Subjects were instructed to respond as quickly and accurately as possible by pressing a button with the left index or middle finger (Fig. 1).
Procedure.
After a training session (SI Materials and Methods), the TMS coils were positioned over the left and right SMG (Fig. 1A). Neuronavigated TMS was used to guide the placement of the coil and to monitor the correct coil position throughout the experiments. Subjects received three test bursts of 10 Hz TMS over left, right, and bilateral SMG each and judged them on a four-point scale (1 = neutral, 4 = highly unpleasant). The experiment consisted of an auditory and a visual run for each subject (Fig. 1B). During each run, the three blocked tasks were presented. The order of runs and blocks was counterbalanced across subjects. Each task started with a verbal or written instruction and consisted of 120 trials for each condition, with a trial-duration of 3 s (Fig. 1C). Presentation of visual words was matched to the mean duration of the auditory stimuli (range = 0.74–0.87s) and followed by a fixation cross to complete the 3-s trial. The fixation cross stayed on the screen for the whole auditory run. Having completed all conditions, subjects again rated the unpleasantness of the TMS sites. The sham TMS group underwent exactly the same procedure. Stimulus presentation and response recording was obtained using E-PRIME (Psychology Software Tools Inc., version 1.1).
Transcranial Magnetic Stimulation.
Neuronavigated TMS was performed by using the mean Montreal Neurological Institute (MNI) coordinates across previous studies (1–3) (Fig. 1A) (SI Materials and Methods). Stimulation intensity was set to 90% of individual RMT of the left motor hand area and was corrected for the difference in the scalp-cortex distance between the motor cortex (mean coordinates taken from Mayka et al. [36]) and the SMG (37) (SI Materials and Methods). During each experimental trial, a four-pulse train of biphasic stimuli was applied at a rate of 10 Hz over left, right, or bilateral SMG 100 ms after word onset (Fig. 1C). Trials with left, right and bilateral TMS (40 each) were pseudorandomly intermingled. Our TMS protocol (i.e., 10-Hz bursts starting 100 ms after stimulus onset) was motivated by previous TMS studies of semantic and phonological processing with visually presented words (1, 16). To our best knowledge, our study is unique in auditory word-stimuli design. However, the similar effects of TMS that we observed across modalities demonstrate that these parameters are appropriate for both visual and auditory modalities. The overall application of TMS was well within safety limits (38).
Data Analysis.
Repeated-measures ANOVAs were used to investigate the effects of TMS on reaction times in all experiments (SI Materials and Methods). Posthoc paired t-tests or independent samples t-tests further explored differences among conditions within or between groups, respectively. An α level of 0.05 (two-tailed) was considered significant for all comparisons. To analyze errors, we used Bonferroni-Holm corrected nonparametric Wilcoxon signed-rank tests and Mann-Whitney U tests because Kolmogorov-Smirnov tests had indicated that these data were not normally distributed.
Supplementary Material
Acknowledgments
This work was supported by the Bundesministerium für Bildung und Forschung Grant 01GW0663 and the Volkswagenstiftung Grant I/79-932.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008121107/-/DCSupplemental.
References
- 1.Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- 2.McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 3.Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. J Cogn Neurosci. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- 4.Buchsbaum BR, D'Esposito M. Repetition suppression and reactivation in auditory-verbal short-term recognition memory. Cereb Cortex. 2009;19:1474–1485. doi: 10.1093/cercor/bhn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandervliet EJ, et al. fMRI findings in an aphasic patient with reversed cerebral dominance for language. Acta Neurol Belg. 2008;108:161–166. [PubMed] [Google Scholar]
- 6.Sakurai Y, et al. Mechanism of short-term memory and repetition in conduction aphasia and related cognitive disorders: A neuropsychological, audiological and neuroimaging study. J Neurol Sci. 1998;154:182–193. doi: 10.1016/s0022-510x(97)00227-x. [DOI] [PubMed] [Google Scholar]
- 7.Wilde MC. Lesion location and repeatable battery for the assessment of neuropsychological status performance in acute ischemic stroke. Clin Neuropsychol. 2010;24:57–69. doi: 10.1080/13854040902984505. [DOI] [PubMed] [Google Scholar]
- 8.Dewarrat GM, et al. Acute aphasia after right hemisphere stroke. J Neurol. 2009;256:1461–1467. doi: 10.1007/s00415-009-5137-z. [DOI] [PubMed] [Google Scholar]
- 9.Shalom DB, Poeppel D. Functional anatomic models of language: Assembling the pieces. Neuroscientist. 2008;14:119–127. doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- 10.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 12.Romero L, Walsh V, Papagno C. The neural correlates of phonological short-term memory: A repetitive transcranial magnetic stimulation study. J Cogn Neurosci. 2006;18:1147–1155. doi: 10.1162/jocn.2006.18.7.1147. [DOI] [PubMed] [Google Scholar]
- 13.Stoeckel C, Gough PM, Watkins KE, Devlin JT. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45:1091–1096. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009;45:1035–1042. doi: 10.1016/j.cortex.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37:125–135. [PubMed] [Google Scholar]
- 16.Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- 18.Démonet JF, Price C, Wise R, Frackowiak RS. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: A positron-emission tomography study in normal human subjects. Neurosci Lett. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 19.Sharp DJ, et al. The neural response to changing semantic and perceptual complexity during language processing. Hum Brain Mapp. 2010;31:365–377. doi: 10.1002/hbm.20871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquemot C, Scott SK. What is the relationship between phonological short-term memory and speech processing? Trends Cogn Sci. 2006;10:480–486. doi: 10.1016/j.tics.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Saur D, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 22.Winhuisen L, et al. The right inferior frontal gyrus and poststroke aphasia: A follow-up investigation. Stroke. 2007;38:1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- 23.Seghier M, et al. Language representation in a patient with a dominant right hemisphere: fMRI evidence for an intrahemispheric reorganisation. Neuroreport. 2001;12:2785–2790. doi: 10.1097/00001756-200109170-00007. [DOI] [PubMed] [Google Scholar]
- 24.Irlbacher K, Voss M, Meyer BU, Rothwell JC. Influence of ipsilateral transcranial magnetic stimulation on the triphasic EMG pattern accompanying fast ballistic movements in humans. J Physiol. 2006;574:917–928. doi: 10.1113/jphysiol.2006.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiel A, et al. Direct demonstration of transcallosal disinhibition in language networks. J Cereb Blood Flow Metab. 2006;26:1122–1127. doi: 10.1038/sj.jcbfm.9600350. [DOI] [PubMed] [Google Scholar]
- 26.Paus T, et al. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79:1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 28.Kujirai T, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferbert A, et al. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Göbell SM, Rushworth MF, Walsh V. Inferior parietal rtms affects performance in an addition task. Cortex. 2006;42:774–781. doi: 10.1016/s0010-9452(08)70416-7. [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo Z, Silvanto J, Pascual-Leone A, Battelli L. The role of the angular gyrus in the modulation of visuospatial attention by the mental number line. Neuroimage. 2009;44:563–568. doi: 10.1016/j.neuroimage.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sack AT, et al. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex. 2007;17:2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- 33.Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 34.Köhler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J Cogn Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes MG, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94:4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- 38.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.