Abstract
Although NMDA receptor (NMDAR)-dependent long-term potentiation (LTP) and long-term depression (LTD) of glutamatergic transmission are candidate mechanisms for long-term spatial memory, the precise contributions of LTP and LTD remain poorly understood. Here, we report that LTP and LTD in the hippocampal CA1 region of freely moving adult rats were prevented by NMDAR 2A (GluN2A) and 2B subunit (GluN2B) preferential antagonists, respectively. These results strongly suggest that NMDAR subtype preferential antagonists are appropriate tools to probe the roles of LTP and LTD in spatial memory. Using a Morris water maze task, the LTP-blocking GluN2A antagonist had no significant effect on any aspect of performance, whereas the LTD-blocking GluN2B antagonist impaired spatial memory consolidation. Moreover, similar spatial memory deficits were induced by inhibiting the expression of LTD with intrahippocampal infusion of a short peptide that specifically interferes with AMPA receptor endocytosis. Taken together, our findings support a functional requirement of hippocampal CA1 LTD in the consolidation of long-term spatial memory.
Keywords: hippocampus, learning and memory, long-term potentiation, AMPA receptor endocytosis, Morris water maze
The hippocampus plays crucial roles in encoding and consolidating memory (1, 2). Activity-dependent plasticity of hippocampal glutamatergic synapses, particularly NMDA receptor (NMDAR)-dependent long-term potentiation (LTP) and long-term depression (LTD), has been proposed as the primary cellular substrate for fulfilling these cognitive functions (3, 4). Indeed, formation of long-term spatial memory in the Morris water maze (MWM) can be impaired by preventing NMDAR activation using either pharmacological or genetic approaches (5–7). However, blocking NMDARs affects both LTP and LTD (8, 9), making it hard to attribute the observed spatial memory deficits to selective disruption of either LTP or LTD. Recent attempts using transgenic mice with deficits in either LTP (10–12) or LTD (13–15) have achieved some success in delineating the contribution of these two opposing forms of plasticity in memory formation. However, results obtained from transgenic studies are equivocal, perhaps because of structural alterations and/or functional compensatory changes at synapses that often arise after prolonged genetic alterations (14). Thus, determining the exact roles of hippocampal LTP and/or LTD in spatial memory requires new experimental approaches that enable acute, selective inhibition of LTP or LTD in freely moving animals.
Evidence accumulated from recent studies suggests that GluN2A- and GluN2B-containing NMDARs preferentially contribute to the induction of hippocampal LTP and LTD in vitro (12, 16, 17) and in vivo (18). For example, the GluN2A preferential antagonist NVP-AAM077 (NVP) (19) and the GluN2B-specific antagonist Ro25-6981 (Ro) (20) selectively inhibit LTP and LTD, respectively, in anesthetized rats (18, 21). If such GluN2 subunit-selective requirements for LTP and LTD can be shown in freely moving animals, these subunit-preferential antagonists may be useful in delineating the roles of LTP and LTD in spatial memory. Recent confirmation of the involvement of regulated AMPA receptor (AMPAR) exocytosis and endocytosis in the expression of LTP and LTD, respectively, and consequent development of reagents to disrupt these intricate molecular events (22, 23) provides a complementary strategy for direct examination of the roles of LTP and LTD in vivo (24).
In this study, we show that GluN2 subunit-preferential antagonists separately block hippocampal LTP or LTD in freely moving adult rats. Using the MWM, we find that although selectively blocking LTP with NVP leaves spatial memory intact, preventing LTD with Ro impairs spatial memory consolidation. The importance of LTD in memory consolidation is corroborated further by bilateral intrahippocampal injection of a membrane-permeable Tat-GluA23Y peptide, which prevents LTD expression by inhibiting regulated AMPAR endocytosis (23). These findings reveal a critical role for hippocampal LTD in mediating the consolidation of long-term spatial memory.
Results
Effects of NMDAR Subunit-Preferential Antagonists on the Induction of Hippocampal CA1 LTP and LTD in Freely Moving Adult Rats.
To determine if NVP and Ro are suitable for probing the functional roles of LTP and LTD in spatial memory formation, an in vivo model of synaptic plasticity in freely moving adult rats was established. Field excitatory postsynaptic potentials (fEPSPs) induced by Schaffer collateral stimulation were recorded from the CA1 region. LTP was induced by high-frequency stimulation (HFS, 100 Hz, 100 pulses) (Fig. 1A), whereas LTD was induced using a paired-burst (PB) LTD protocol (200 pairs of two-pulse bursts, one pair per second, 2.5-ms interpulse interval, 10-ms interburst interval) (Fig. 1B) (25). Both LTP and LTD were NMDAR-dependent because they were blocked by injection of the subunit-nonspecific NMDAR antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) (10 mg/kg, i.p., 1 h before induction) (Fig. 1A).
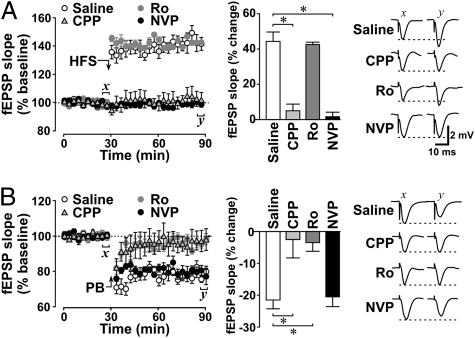
Fig. 1.
GluN2A and GluN2B subunit-preferential antagonists prevent the formation of hippocampal CA1 LTP and LTD in freely moving adult rats. (A) HFS (100 Hz, 100 pulses) triggered LTP of fEPSPs in freely moving rats. (Left) The plot of normalized slopes of fEPSPs shows the effects on hippocampal CA1 LTP induction of an i.p. injection of saline (n = 8), non–subunit-selective NMDAR antagonist CPP (10 mg/kg; n = 5), GluN2A antagonist NVP (1.2 mg/kg; n = 7), or GluN2B antagonist Ro (6 mg/kg; n = 4). Drugs were injected 30 min before plasticity induction. Note that HFS failed to induce LTP in CPP- and NVP-treated rats. (Center) Histogram summarizes the average percent change of fEPSP slope before (x) and after HFS (y). (Right) Representative fEPSP traces. (B) PB stimulation (200 paired bursts at 1 Hz; interpulse interval = 2.5 ms; interburst interval = 10 ms) induced LTD of fEPSP in freely moving rats. The effects of different drug treatments on LTD are summarized in the scatter plot (Left) and histogram (Center). LTD was induced in control rats (n = 6) and NVP-treated rats (n = 5), whereas CPP (n = 4) and Ro (n = 7) blocked LTD formation. Representative traces are shown on the right. *P < 0.05 vs. control, post hoc Fisher's test after ANOVA.
Next we determined if GluN2A- and GluN2B-containing NMDARs are necessary for LTP and LTD induction (Fig. 1A). We found that i.p. injection of NVP (1.2 mg/kg) 30 min before HFS prevented LTP without altering PB-induced LTD (Fig. 1B). Importantly, Ro (6 mg/kg, i.p. injected 30 min before PB) failed to affect LTP but prevented LTD. Note that both NVP and Ro did not affect basal synaptic transmission (Fig. S1). These results reveal that hippocampal CA1 LTP and LTD in freely moving adult rats were blocked by systemic injection of NVP and Ro, respectively.
Effects of GluN2 Subunit Antagonists on Long-Term Spatial Memory Formation.
NVP and Ro were used to examine the relative contributions of LTP and LTD to spatial memory formation in an MWM task. We used a well-characterized 1-d MWM training protocol (Fig. 2A) (21, 26) with eight training trials that can be completed within 30 min. This short protocol has the advantage of clearly delineating the acquisition and consolidation phases, better differentiating relative contributions of synaptic plasticity to learning. One day after training, a 60-s probe test with the platform removed was performed to examine long-term spatial memory retrieval.
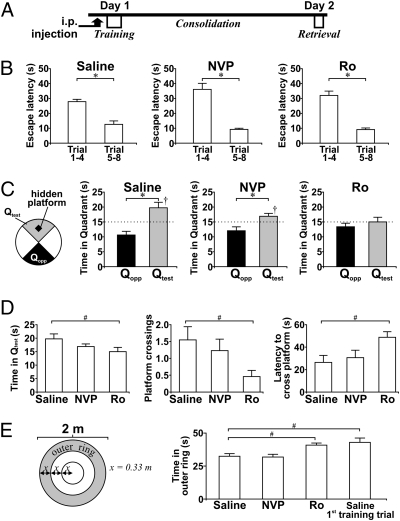
Fig. 2.
GluN2B subunit antagonist impairs the formation of long-term spatial memory in MWM. (A) Schematic of the MWM protocol. On day 1, rats received i.p. injection of drugs 30 min before eight training trials. Probe test without the hidden platform (retrieval) was performed on day 2. (B) Histograms display the average escape latencies of rats for the first four training trials (trials 1–4) and last four training trials (trials 5–8) on day 1 (saline: n = 13; NVP: n = 13; Ro: n = 13). (C) Histograms show probe-test performance of rats from different treatment groups on day 2. Long-term spatial memory of the location of the hidden platform is indicated by preference for Qtest over Qopp. The schematic (Left) shows the location of test and opposite quadrants and the hidden platform. Moreover, trained rats spent significantly longer than chance (15 s, dotted lines) in Qtest. (D) Histograms summarize the effect of Ro and NVP on probe-test performances such as swimming time in Qtest (Left), hidden-platform crossings (Center), latency of first platform crossing (Right), and (E) thigmotaxic behavior defined as swimming in the perimeter (i.e., the outer ring) of the pool. †P < 0.05, Qtest vs. chance (15 s), paired t test; *P < 0.05, Qtest vs. Qopp, paired t test; #P < 0.05 vs. control, post hoc Tukey's test after ANOVA.
Injecting rats with either NVP or Ro 30 min before MWM training did not affect spatial learning, as exhibited by a decreased latency to find the hidden platform across the eight training trials (Fig. 2B). During the probe test, saline-treated rats spent significantly longer than chance (15 s) in the test quadrant (Qtest) where the hidden platform was located (P < 0.01). Moreover, they spent significantly longer in Qtest than in the opposite quadrant (Qopp) (Qtest vs. Qopp, P < 0.01) (Fig. 2C), confirming the establishment of long-term memory. Surprisingly, NVP, which prevents LTP formation in freely moving rats, did not affect Qtest preference (Qtest vs. Qopp, P < 0.01) (Fig. 2C), and NVP-treated rats spent significantly longer than chance in Qtest (P = 0.04). In contrast, the preference for Qtest was abolished in Ro-treated rats (P > 0.05 in all tests) (Fig. 2C).
ANOVA was used to examine between-group differences on several probe-test performance indices (Fig. 2E). Compared with other groups, Ro-treated rats spent significantly less time in Qtest [F(2,36) = 3.60; P = 0.04; post hoc: saline vs. NVP, P = 0.22; saline vs. Ro, P = 0.03], crossed the location of the hidden platform fewer times [F(2,36) = 4.28, P = 0.02; post hoc: saline vs. NVP, P = 0.36; saline vs. Ro, P = 0.02], and exhibited longer latencies to cross the location of the hidden platform [F(2,36) = 4.66, P = 0.02; post hoc: saline vs. NVP, P = 0.79; saline vs. Ro, P = 0.02]. Moreover, Ro-treated rats displayed more thigmotaxic behavior in the pool perimeter, remarkably similar to the behavior of naive saline-treated rats during the first training trial [F(2,36) = 7.93, P < 0.01; post hoc: saline vs. NVP, P = 1.00; saline vs. Ro, P < 0.01] (Fig. 2E, Right). Because all rats learned the location of the hidden platform during training, the results strongly suggest that Ro-treated rats could not remember the training a day later and reverted to thigmotaxic behavior which usually is observed only in untrained rats.
The compromised probe-test performance of Ro-treated rats was not the result of changes in swimming speed (Table S1). Moreover, Ro did not affect rats’ performance in a visible-platform version of the MWM (Fig. S2). Although NMDAR antagonists have been shown to inhibit spatial memory retrieval in a state-dependent manner [i.e., the presence of antagonists during learning creates a chemical state that must be reinstated for memory retrieval (27)], state-dependent learning cannot explain the present findings with Ro because the presence of Ro during probe tests failed to rescue the impairment of spatial memory retrieval caused by pretraining Ro injection (Fig. S3). Together, these results strongly suggest that pretraining application of Ro, but not of NVP, impairs the formation of long-term spatial memory.
Effects of Blocking Hippocampal LTD Expression on Long-Term Spatial Memory.
Because Ro may affect neuronal functions mediated by GluN2B-containing receptors other than LTD, we used an LTD-specific inhibitor that differed from Ro in both chemical structure and mechanism of action to confirm further the role of LTD in spatial memory formation. The membrane-permeable GluA2-derived Tat-GluA23Y peptide prevented LTD expression by blocking regulated AMPAR endocytosis (23). We first examined whether the Tat-GluA23Y peptide could inhibit hippocampal LTD selectively in freely moving rats. Systemic injection (3 μmol/kg i.p.) of Tat-GluA23Y peptide 30 min before PB prevented LTD formation (Fig. 3A). In contrast, a control scrambled peptide with an intact Tat domain but scrambled GluRA23Y sequence did not influence LTD. Finally, neither Tat-GluA23Y nor scrambled peptide affected LTP and basal synaptic transmission (Fig. S1). Thus, systemic injection of Tat-GluA23Y peptide specifically blocks LTD in freely moving rats.
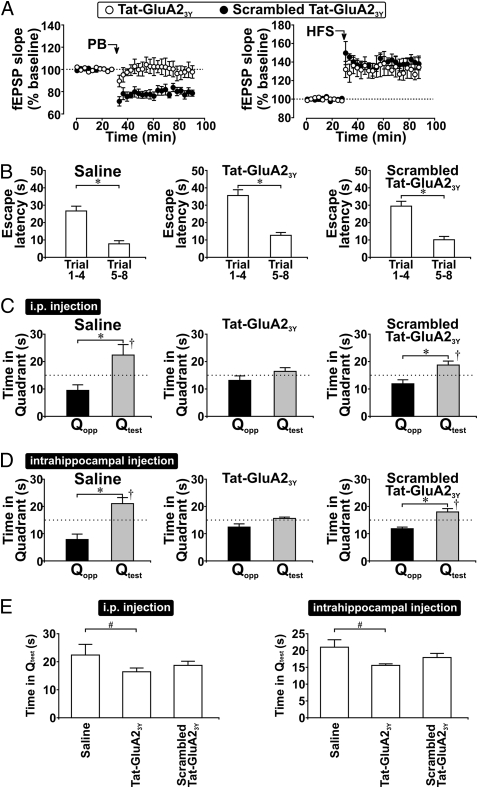
Fig. 3.
LTD expression is required for the formation of long-term spatial memory in MWM. (A) Scatter plots show the specific prevention of hippocampal LTD (n = 6) but not LTP (n = 6) by Tat-GluA23Y peptide in freely moving rats. Note that scrambled control peptide did not affect LTP (n = 5) or LTD (n = 5). Peptide (3 μmol/kg i.p.) was applied 30 min before stimulation. (B) Histograms show that i.p. injection (30 min before training) of saline (n = 8), Tat-GluA23Y (n = 14), or scrambled Tat-GluA23Y peptide (n = 15) did not affect the decrease of average escape latencies during MWM training trials. (C) Histograms show rats’ probe-test performance on day 2. Note that the Qtest preference was abolished by treatment with Tat-GluA23Y peptide. (D) Histograms show that intrahippocampal infusion of Tat-GluA23Y before training impaired spatial memory formation. Saline (n = 6), Tat-GluA23Y (30 pmol, n = 10;), or scrambled Tat-GluA23Y peptide (30 pmol, n = 6;) was injected bilaterally (1 μL) into dorsal hippocampi 15 min before training. Note that the Qtest preference was abolished only by Tat-GluA23Y peptide. (E) Histograms show the effect of i.p. (Left) or intrahippocampal (Right) injection of saline or peptides on rats’ swimming time in Qtest. Only rats treated withTat-GluA23Y peptide spent significantly less time in Qtest than control rats. †P < 0.05, Qtest vs. chance (15 s), paired t test; *P < 0.05, Qtest vs. Qopp, paired t test; #P < 0.05 vs. control, post hoc Tukey's test after ANOVA.
In the MWM task, we found i.p. injection (3 μmol/kg) of either control or active peptides 30 min before MWM training had no effect on learning the location of the hidden platform (Fig. 3B). However, pretraining injection of Tat-GluA23Y, but not the scrambled peptide, disrupted long-term memory retrieval as evidenced by lack of Qtest preference in probe tests performed 24 h after training (Fig. 3C). Moreover, Tat-GluA23Y–treated rats spent significantly less time in Qtest than control rats [F(2,34) = 3.63, P = 0.04; post hoc: saline vs. TatGluA23Y, P = 0.03; saline vs. scrambled peptide, P = 0.23] (Fig. 3E). Like Ro, pretraining application of Tat-GluA23Y peptide was sufficient to impair the formation of long-term spatial memory.
Because systemically injected peptides could affect other brain regions, we ascertained the need for hippocampal LTD in spatial memory formation using bilateral intrahippocampal injection of peptide (30 μM, 1 μL, 15 min before training). Rats that received saline displayed normal Qtest preference in probe tests (Fig. 3D); however, rats given intrahippocampal Tat-GluA23Y peptide exhibited no Qtest preference. Intrahippocampal scrambled peptide had no effect. Finally, only rats receiving intrahippocampal injection of Tat-GluA23Y spent significantly less time in Qtest than control rats [F(2,19) = 3.83, P = 0.04; post hoc: saline vs. Tat-GluA23Y, P = 0.03; saline vs. scrambled peptide, P = 0.37] (Fig. 3E). Note that direct intrahippocampal Tat-GluA23Y peptide did not affect rats’ performance in a visible-platform task (Fig. S4), showing that the injection procedure did not affect rats’ ability to perform the nonspatial aspects of this task. Ro and Tat-GluA23Y peptide affected probe-test performance in a similar manner, thus, our findings strongly suggest that hippocampal LTD, a common central process that is targeted by these two chemically distinct drugs, is required for the formation of long-term spatial memory.
Hippocampal LTD Is Necessary for Consolidation of Long-Term Spatial Memory.
Finally, we examined the possible contribution of LTD to each of three distinct phases of spatial memory formation: acquisition, consolidation, and retrieval. Pretraining injection of LTD inhibitors did not affect MWM training (Figs. 2B and 3B), suggesting that LTD is not required for spatial memory acquisition. To test this possibility further, we performed a probe test 30 s after the last training trial (Fig. 4A) and found that the Qtest preference in the posttraining probe test was well preserved in both Ro- and Tat-GluA23Y peptide–treated rats. These results strongly argue against a critical role of LTD in spatial memory acquisition.
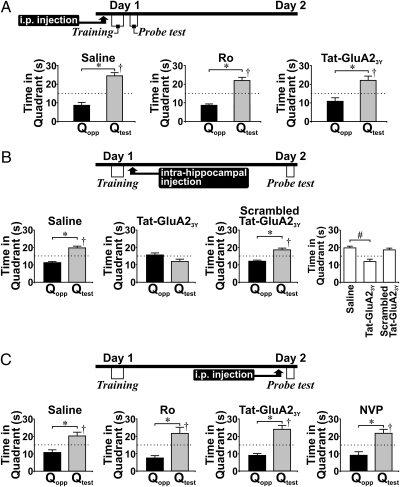
Fig. 4.
Hippocampal LTD is specifically required for spatial memory consolidation. (A) Histograms show probe-test performance of rats that received i.p. injection of saline (n = 6), Ro (6 mg/kg; n = 6), or Tat-GluA23Y peptide (3 μmol/kg; n = 6) 30 min before training and were given a single probe test 30 s after the last training trial. These drugs did not affect the Qtest preference of rats. (B) The three histograms on the left show probe-test performance (conducted 24 h after training) of rats that received bilateral intrahippocampal injection of Tat-GluA23Y peptide (n = 7) or scrambled peptide (n = 7) at 30 μM, 1 μL, or saline (1 μL; n = 6) within 5 min of the last trial. The histogram on the right summarizes the effect of drugs on rats’ swimming time in Qtest. Note that Tat-GluA23Y significantly impaired the Qtest preference of rats. (C) Histograms summarize probe-test performance in rats that received i.p. injection of saline (n = 6), Ro (n = 6), Tat-GluA23Y peptide (n = 8), or NVP (n = 8) 30 min before probe tests (day 2). No treatment affected Qtest preference in probe tests. †P < 0.05, Qtest vs. chance (15 s), paired t test; *P < 0.05, Qtest vs. Qopp, paired t test; #P < 0.05 vs. control, post hoc Tukey's test after ANOVA.
Pretraining application of LTD inhibitors may have disrupted probe-test performance by affecting consolidation and/or retrieval of spatial memory. To test this possibility directly, we examined the effect of posttraining (within 5 min of the last training trial) bilateral intrahippocampal injection of Tat-GluA23Y peptide (30 μM, 1 μL) on probe-test performance (Fig. 4B). Direct intrahippocampal injection of peptide results in a faster blockade of LTD than is achieved with i.p. injection, allowing examination of the potential requirement of LTD in rapid consolidation of spatial memory. Although the Qtest preference of saline-treated rats remained intact, no Qtest preference was observed in Tat-GluA23Y peptide–treated rats. Note that Qtest preference in probe tests remained intact in rats receiving scrambled peptide. Moreover, rats receiving posttraining intrahippocampal injection of Tat-GluA23Y spent significantly less time in Qtest than control rats [F(2,17) = 13.60, P < 0.01; post hoc: saline vs. TatGluA23Y, P < 0.01; saline vs. scrambled peptide, P = 0.78] (Fig. 3E). Our data strongly suggest that hippocampal LTD is required for spatial memory consolidation or retrieval, or both.
To differentiate the role of LTD in memory consolidation or retrieval, we applied either Ro or Tat-GluA23Y peptide 30 min before the probe test and found that the Qtest preference was well preserved (Fig. 4C). Thus, hippocampal LTD probably is not required for spatial memory retrieval; rather, our results strongly suggest that the disruption of probe-test performances by LTD inhibition either before or immediately after training is a result of compromised consolidation of spatial memory. Note that injection of NVP before the probe test also did not affect retrieval of long-term spatial memory. Together with findings that pretraining application of NVP failed to affect probe-test performance (Fig. 2), our data do not support a critical involvement of hippocampal LTP, which is blocked by NVP (Fig. 1), in the formation of long-term spatial memory.
Discussion
This study used a pharmacological approach to ascertain the contribution of LTP and LTD to the formation of long-term spatial memory. First, we confirmed that the induction of LTP and LTD in the hippocampal CA1 region can be selectively disrupted pharmacologically in freely moving rats. Specifically, the GluN2A-preferential antagonist NVP prevented LTP without affecting LTD. In contrast, LTD was blocked by either the GluN2B antagonist Ro (induction inhibitor) or the Tat-GluA23Y peptide (expression disruptor). Importantly, neither Ro nor Tat-GluA23Y affected LTP. Examining the putative roles of LTP and LTD in spatial memory formation, we observed no effect of NVP on performance in the MWM. In marked contrast, the present experiments suggest that both Ro and Tat-GluA23Y peptide impair spatial memory by disrupting its consolidation.
Previous studies using pharmacological and genetic approaches in rodents established the essential role of NMDAR activation in spatial memory formation (6, 28). Because both LTP and LTD were abolished in these studies, the exact roles of these two opposing form of synaptic plasticity in spatial memory remained elusive. Recent findings that LTP and LTD are mediated by activation of GluN2A- and GluN2B-containing NMDARs (17, 18) suggested the potential utility of GluN2-preferential antagonists to probe the functional roles of LTP and LTD in spatial memory. This possibility is supported further by transgenic data showing that selective knockout of the GluN2A subunit or deletion of its carboxyl tail impairs LTP (12, 29), whereas knocking out the GluN2B subunit produces a deficit in LTD (14, 30). Nonetheless, contradictory results challenge these findings (31, 32). These discrepancies may be accounted for by pharmacological specificity of subunit-preferential antagonists (33), developmental stages of the subjects (34), specific brain areas investigated (35), and other differences in experimental conditions (36). Given this controversy, determining if the subunit-specific requirements for LTP and LTD observed in slices and anesthetized animals could be extended to freely moving animals is essential. Importantly, the present results show clearly that NVP and Ro selectively inhibit LTP and LTD, respectively, in freely moving rats. Regardless of the subunit selectivity of NVP and Ro toward native NMDARs in freely moving rats, our findings that they selectively blocked the induction of LTP or LTD validates their utility as specific and functional inhibitors to probe the roles of bidirectional synaptic plasticity in long-term spatial memory.
Although NVP blocked hippocampal CA1 LTP, NVP did not affect either acquisition or retrieval of spatial memory when it was applied before either training (Fig. 2) or probe test (Fig. 4). These data strongly suggest that hippocampal CA1 LTP is not essential for the formation of long-term spatial memory. These results are interesting in the context of similar findings from several previous studies in which LTP was disrupted selectively. For example, knocking out AMPAR subunit GluR1 (GluA1) expression abolishes LTP but not spatial memory formation (11). Similarly, both GluN2A knockout and GluN2A carboxyl-tail deletion mutant mice exhibit impaired LTP (5, 12) but no major spatial memory deficits (37). Notably, hippocampal LTP may be involved in the formation of spatial working memory, which is affected in LTP-disrupted mice (37, 38). LTP also could be important for other hippocampal-dependent cognitive functions, such as the formation of inhibitory avoidance (39).
The failure to confirm a role for NMDAR-dependent LTP in spatial memory formation raises the distinct possibility that impaired LTD could be responsible for the spatial memory deficit following hippocampal NMDAR blockade (6). Although the involvement of LTD in spatial memory consolidation is strongly supported by our Ro data (Fig. 2), it is interesting that pretraining administration of GluN2B antagonist CP-101,606 (40) or Ro63-1908 (41) did not impair spatial memory during a multiday training protocol. An important factor in explaining these contradictory findings may be the concentrations of GluN2B antagonist used to prevent the induction of either hippocampal LTP or LTD, because in vivo recordings of LTD were not performed. Notably, we found that blocking LTD expression via either i.p. or intrahippocampal injection of the Tat-GluA23Y peptide, which prevents regulated AMPAR endocytosis, produced a spatial memory deficit nearly identical to that observed in Ro-treated rats (Figs. 3 and 4). Given that Ro and Tat-GluA23Y prevent LTD via distinct mechanisms, our results strongly support the involvement of NMDAR-dependent LTD in this particular form of spatial memory. Moreover, our finding that LTD inhibition affects probe-test performance but not spatial learning, combined with similar effects of LTD inhibitors when administered before or immediately after training but not when administered before probe test, strongly suggests that NMDAR-dependent LTD is involved specifically in the consolidation, but not in the acquisition or retrieval, of long-term spatial memory.
Hippocampal LTD has been implicated in forms of learning and memory other than spatial memory. For example, LTD induction in behaving animals can be facilitated by exposure to novel objects (42). Moreover, novelty exposure could reverse LTP in the hippocampus (43). These findings suggest a correlation between LTD and novelty detection during learning. Notably, LTD-null mice lacking serum response factor (SRF) failed to habituate to novel objects in an object-recognition task (44). It is interesting that SRF-knockout mice also display poor spatial memory in MWM, paralleling the presently observed effect of acute LTD blockade on spatial memory consolidation.
The manner in which hippocampal LTD contributes to spatial memory consolidation requires further clarification. Although LTD reduces the strength of glutamate synapses, it also shares several properties with LTP, including input specificity, cooperativity, and associativity (8), which make LTD a potential Hebbian mechanism for information storage. Because hippocampal synapses are spontaneously active, information could be consolidated by persistently depressing some active synapses through LTD. Indeed, in vivo findings that LTD and LTP are facilitated by exposing rats to novel objects and empty space, respectively, suggested that LTD and LTP encode different types of information during spatial learning (45). Blocking LTD by either Ro or Tat-GluA23Y peptide therefore could disturb the storage of spatial information and lead to memory deficit.
Diverse roles for LTD in numerous brain areas have been demonstrated recently (24), and mechanisms consistent with LTD also are involved in other aspects of memory regulation. For example, the disruptive effects of acute stress on memory retrieval are prevented by systemic administration of Ro in spatial and recognition memory paradigms (21, 46), and the Tat-GluA23Y peptide also blocks the disruptive effects of acute stress on memory retrieval in MWM (21). Thus, the timing of GluN2B-containing NMDAR activation and AMPAR endocytosis may be critical in determining their specific effects on memory.
In conclusion, our data support the importance of hippocampal CA1 LTD in the formation of long-term spatial memory during MWM tasks in freely moving adult rats. Moreover, the specific inhibitors used to manipulate hippocampal plasticity may provide important tools for dissecting the contribution of LTP and LTD to other aspects of cognitive function and other forms of hippocampal-mediated behavior.
Materials and Methods
Methods are described in detail in SI Materials and Methods.
Recording of Hippocampal LTP and LTD in Freely Moving Rats.
Electrodes were implanted into the CA1 of anesthetized male adult Sprague–Dawley rats (300–400 g) as described (47). In vivo recording of fEPSPs was performed in a recording chamber (40 × 40 × 60 cm). To allow movement during recording, implanted electrodes were connected by a flexible cable and swivel commutator. fEPSPs were evoked at 0.033 Hz at 50% of the maximal size.
Morris Water Maze.
Rats were trained in eight consecutive trials (intertrial interval = 30 s) to find a hidden platform as described (21). Probe tests (60 s) with the platform removed were performed 24 h after training. All trials were recorded and analyzed offline. The experimenter was blinded to drug treatment in all experiments.
Bilateral Hippocampal Injection.
Rats were implanted with guide cannulae for bilateral intrahippocampal injection as described (21). After recovery, rats were habituated to the mock-injection procedures three or four times in the week before MWM training. Drugs were injected with a syringe pump (0.5 μL/min for 2 min) through 11-mm injection needles extending 1 mm beyond guide cannulae. Needles remained in place for 1 min to allow diffusion of drug. Rats were killed for verification of injection sites.
Statistical Analysis.
Data are presented as mean ± SEM. The effect of drugs on probe-test performance indices was analyzed by ANOVA and post hoc Tukey's test. Paired Student's t tests were used for within-subjects comparisons [swimming time in Qtest vs. chance (15 s) and in Qtest vs. Qopp]. fEPSPs were analyzed by ANOVA and post hoc Fisher's tests.
Supplementary Material
Acknowledgments
We thank Dr. Loren Oschipok for editorial assistance. This work was supported by the Canadian Institutes of Health Research Grant M0P-38090 (to Y.T.W.), Natural Sciences and Engineering Research Council of Canada Grants 214991 (to T.P.W.) and 7808 (to A.G.P.), and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 (to Y.G. and Y.T.W). Y.G. was supported by a Doctoral Award from the Canadian Institutes of Health Research, and Z.D. was the recipient of a Fellowship from the Heart and Stroke Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008200107/-/DCSupplemental.
References
- 1.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Sakimura K, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 6.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 7.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 8.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abel T, et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 11.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 12.Köhr G, et al. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng H, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 14.Brigman JL, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls RE, et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 18.Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 2006;16:907–915. doi: 10.1002/hipo.20230. [DOI] [PubMed] [Google Scholar]
- 19.Auberson YP, et al. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- 20.Fischer G, et al. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- 21.Wong TP, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadian G, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 25.Thiels E, Xie X, Yeckel MF, Barrionuevo G, Berger TW. NMDA receptor-dependent LTD in different subfields of hippocampus in vivo and in vitro. Hippocampus. 1996;6:43–51. doi: 10.1002/(SICI)1098-1063(1996)6:1<43::AID-HIPO8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 27.Jackson A, Koek W, Colpaert FC. NMDA antagonists make learning and recall state-dependent. Behav Pharmacol. 1992;3:415–421. doi: 10.1097/00008877-199208000-00018. [DOI] [PubMed] [Google Scholar]
- 28.McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao JP, Constantine-Paton M. NR2A-/- mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci. 2007;27:13649–13654. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutsuwada T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 31.Berberich S, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morishita W, et al. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: Limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartlett TE, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Weitlauf C, et al. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett TE, Wang YT. Regulated coupling of NMDA receptors to plasticity signaling pathways. Abstr Soc Neurosci. 2009 40.5, C55. [Google Scholar]
- 37.Bannerman DM, et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisel D, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 39.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 40.Guscott MR, et al. The effect of (+/-)-CP-101,606, an NMDA receptor NR2B subunit selective antagonist, in the Morris watermaze. Eur J Pharmacol. 2003;476:193–199. doi: 10.1016/s0014-2999(03)02182-4. [DOI] [PubMed] [Google Scholar]
- 41.Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R. Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: Evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology. 2003;44:324–341. doi: 10.1016/s0028-3908(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 42.Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 44.Etkin A, et al. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol Learn Mem. 2010;93:261–267. doi: 10.1016/j.nlm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Dong Z, Han H, Cao J, Zhang X, Xu L. Coincident activity of converging pathways enables simultaneous long-term potentiation and long-term depression in hippocampal CA1 network in vivo. PLoS ONE. 2008;3:e2848. doi: 10.1371/journal.pone.0002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.