Abstract
Mutations in PARK2/Parkin, which encodes a ubiquitin E3 ligase, cause autosomal recessive Parkinson disease (PD). Here we show that the nonreceptor tyrosine kinase c-Abl phosphorylates tyrosine 143 of parkin, inhibiting parkin's ubiquitin E3 ligase activity and protective function. c-Abl is activated by dopaminergic stress and by dopaminergic neurotoxins, 1-methyl-4-phenylpyridinium (MPP+) in vitro and in vivo by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), leading to parkin inactivation, accumulation of the parkin substrates aminoacyl-tRNA synthetase-interacting multifunctional protein type 2 (AIMP2) (p38/JTV-1) and fuse-binding protein 1 (FBP1), and cell death. STI-571, a c-Abl-family kinase inhibitor, prevents the phosphorylation of parkin, maintaining parkin in a catalytically active and protective state. STI-571’s protective effects require parkin, as shRNA knockdown of parkin prevents STI-571 protection. Conditional knockout of c-Abl in the nervous system also prevents the phosphorylation of parkin, the accumulation of its substrates, and subsequent neurotoxicity in response to MPTP intoxication. In human postmortem PD brain, c-Abl is active, parkin is tyrosine-phosphorylated, and AIMP2 and FBP1 accumulate in the substantia nigra and striatum. Thus, tyrosine phosphorylation of parkin by c-Abl is a major posttranslational modification that inhibits parkin function, possibly contributing to pathogenesis of sporadic PD. Moreover, inhibition of c-Abl may be a neuroprotective approach in the treatment of PD.
Keywords: AIMP2, Parkinson disease, STI-571, ubiquitin
Parkinson disease (PD) is a common neurodegenerative disorder characterized by the loss of dopamine (DA) neurons and protein accumulation in intracellular inclusions designated as Lewy bodies and Lewy neurites (1). Although the majority of PD is sporadic in nature, rare familial mutations are providing insight into this chronic, progressive neurodegenerative disease. Mutations in α-synuclein and LRRK2 cause autosomal-dominant PD, whereas mutations in DJ-1, PINK1, and parkin result in autosomal-recessive PD (2). Parkin mutations are the most common cause of autosomal-recessive PD and, for the most part, PD due to parkin mutations is indistinguishable from sporadic PD (3). Parkin is a ubiquitin E3 ligase, and familial mutations are thought to impair the E3 ligase activity of parkin (4, 5).
Parkin ubiquitinates proteins via monoubiquitination or polyubiquitination using either lysine 48 (K48) or lysine 63 (K63). Monoubiquitination by parkin is thought to regulate receptor trafficking (6). Polyubiquitination by parkin via K48 is thought to mediate proteasomal degradation (7, 8), whereas polyubiquitination by K63 may be involved in inclusion formation (9). Parkin's differential ubiquitination properties are likely to be regulated by different ubiquitin-conjugating E2s and other associated proteins or regulatory processes (3). A number of putative parkin substrates have been identified (for a review, see ref. 3). Aminoacyl-tRNA synthetase-interacting multifunctional protein type 2 (AIMP2) (p38/JTV-1) and fuse-binding protein 1 (FBP1) are parkin substrates that appear to be regulated by K48 ubiquitination and proteasomal degradation because they accumulate in parkin exon 7 knockout (KO) mice and in autosomal-recessive PD brains due to parkin mutations (8). AIMP2 may play a pathogenic role, as it is selectively toxic to DA neurons (8). Parkin may also regulate the biologic function of other substrates (3).
The nonreceptor tyrosine kinase c-Abl localizes to the nucleus and cytoplasm and is activated by cellular stress (10). c-Abl plays a prominent role in tumorigenesis, as c-Abl is a homolog of the transforming element of the Abelson murine leukemia virus (11). In brain, c-Abl is involved in neuronal plasticity, neurite outgrowth, and neurogenesis (12). Activation of c-Abl may also play a role in neurologic disorders such as Alzheimer's disease (13) and Niemann–Pick type-2 disease (14) through aberrant activation.
Parkin's E3 ligase activity and protective function are regulated by posttranslational modifications including S-nitrosylation (15), phosphorylation (16, 17), and dopamine conjugation (18). Here we report functional regulation of parkin activity by phosphorylation of tyrosine 143 by c-Abl. Phosphorylation of parkin by c-Abl inhibits parkin's E3 ligase activity and protective function in both in vitro and in vivo models of PD. In human postmortem PD brain, c-Abl is active, parkin is tyrosine-phosphorylated, and AIMP2 and FBP1 accumulate, suggesting a pathophysiologic regulation of parkin by c-Abl in sporadic PD.
Results
Parkin Interacts with c-Abl.
To determine whether parkin directly interacts with c-Abl, a glutathione S-transferase (GST) pull-down assay was performed with recombinant GST-parkin and recombinant c-Abl (Fig. 1A). c-Abl interacts with GST-parkin as determined by immunoblot with an anti-c-Abl antibody, whereas c-Abl does not interact with GST alone (Fig. 1A). Immunoprecipitation of parkin from mouse brain coimmunoprecipitates c-Abl, indicating that c-Abl and parkin interact in vivo. c-Abl fails to coimmunoprecipitate with mouse IgG, indicating that the interaction between parkin and c-Abl is specific (Fig. 1B). To further determine the specificity of the interaction, immunoprecipitiation was compared between wild-type (WT) and parkin KO mice. Antibodies to parkin coimmunoprecipitate c-Abl from WT brain but not parkin KO brain (Fig. S1A). Cotransfection experiments in SH-SY5Y cells indicate that V5-tagged parkin (V5-parkin) interacts with GFP-tagged c-Abl (GFP-c-Abl) via coimmunoprecipitation of either V5 or GFP (Fig. 1 C and D). Interestingly, a kinase-dead (KD) (lysine 290 arginine) version of c-Abl (19) fails to interact with parkin (Fig. 1 C and D). To determine the domain of c-Abl that interacts with parkin, different domains of c-Abl were cotransfected with V5-parkin. Constructs of c-Abl that contain the SH3 domain interact with parkin, whereas c-Abl constructs lacking the SH3 domain fail to interact with parkin (Fig. 1E and Fig. S1B). Thus, the c-Abl SH3 domain is required for the interaction with parkin. To ascertain the domain of parkin that interacts with c-Abl, different myc-tagged domains of parkin were cotransfected with GFP-c-Abl. c-Abl interacts with the RING finger and in-between RING finger domains of parkin (Fig. 1F).
Fig. 1.
Parkin interacts with c-Abl. (A) GST-parkin immobilized on glutathione-Sepharose beads pulls down recombinant c-Abl. Pull downs were immunoblotted with anti-c-Abl. (B) Mouse brain lysates were immunoprecipitated with anti-parkin and anti-IgG, followed by immunoblot with anti-parkin or anti-c-Abl antibodies. (C) Lysates from SH-SY5Y cells transfected with GFP-c-Abl or a kinase-dead (KD) (lysine 290 arginine) version of c-Abl (GFP-c-Abl-KD) and V5-parkin were subjected to immunoprecipitation (IP) with anti-GFP, followed by anti-V5 immunoblotting (Middle) or with anti-GFP antibody (Bottom) to show an equivalent amount of immunoprecipitated c-Abl. (D) Lysates from SH-SY5Y cells transfected with V5-parkin and GFP-c-Abl and GFP-c-Abl-KD subjected to IP with anti-V5, followed by anti-GFP (Middle) or anti-V5 (Bottom) immunoblotting to show an equivalent amount of immunoprecipitated parkin. (E) Lysates from SH-SY5Y cells transfected with V5-parkin and c-Abl domain constructs were subjected to IP with anti-V5, followed by anti-c-Abl (Middle) or anti-V5 (Bottom) immunoblotting. The arrow highlights the F3 domain of c-Abl. The deletion domains of c-Abl used are shown at the bottom of the panel. (F) Lysates from SH-SY5Y cells transfected with GFP-c-Abl and myc-tagged fragments of parkin subjected to IP with anti-myc antibodies, followed by anti-GFP (Middle) or anti-myc (Bottom) immunoblotting. A schematic representation of the different parkin fragments used is shown. All experiments were repeated at least three times and representative images of the immunoblots are shown.
c-Abl Tyrosine Phosphorylates Parkin.
An in vitro kinase assay was performed using recombinant c-Abl and recombinant His6-tagged parkin to determine whether c-Abl is able to phosphorylate parkin. Phosphorylation was monitored with an anti-phosphotyrosine (anti-p-tyrosine) antibody. c-Abl tyrosine-phosphorylates parkin, and the c-Abl-family kinase inhibitor STI-571 prevents the tyrosine phosphorylation of parkin by c-Abl (Fig. 2A). To determine the site of phosphorylation of parkin by c-Abl, two-dimensional (2-DE) gel electrophoresis was used to separate phosphorylated from nonphosphorylated parkin (Fig. 2B). c-Abl-phosphorylated parkin was submitted for mass spectrometry for determination of the phosphorylation site. Mass spectrometric analysis provided 96.5% sequence coverage of parkin, and all tyrosine residues were investigated for phosphorylation status. Only tyrosine 143 is phosphorylated, whereas none of the other tyrosines are phosphorylated (Fig. 2B). To confirm that tyrosine 143 was the sole site of phosphorylation by c-Abl, tyrosine 143 in parkin was mutated to phenylalanine to create a Y143F parkin mutant. Cotransfection experiments in SH-SY5Y cells indicate that WT parkin is phosphorylated by c-Abl but not c-Abl-KD. c-Abl is not able to tyrosine-phosphorylate parkin Y143F (Fig. 2C). These results taken together indicate that c-Abl phosphorylates parkin on tyrosine 143.
Fig. 2.
Parkin is tyrosine-phosphorylated by c-Abl. (A) Samples were separated by SDS/PAGE and immunoblotted with anti-phosphotyrosine antibody to show activated c-Abl (third panel) and phosphorylated parkin (first panel) and with an anti-parkin antibody (second panel) and an anti-c-Abl antibody (fourth panel) to show equal amounts of proteins were used. WB, Western blot. (B) Parkin is phosphorylated by c-Abl at tyrosine (Y) 143. Mass spectrometric analysis reveals 96.5% sequence coverage of parkin, showing that all tyrosine residues were investigated for phosphorylation status. Phosphorylated Y143 is indicated in red; other tyrosines are indicated in green (Top). Phosphorylated parkin by c-Abl was separated by 2-DE followed by immunoblot (left panel on bottom). The red asterisks indicate tyrosine phosphorylation of parkin. Both nonphosphorylated and phosphorylated parkin were subjected to LC-MS/MS to identify the phosphorylation site (right panel on bottom). LC-MS/MS spectra of the nonphosphorylated peptide (SIYNSFYVYCK) and the phosphorylated peptide (SIpYNSFYVYCK) are compared, demonstrating that there is the 80-Da shift for the Y143 ion containing the phosphate moiety. The phosphorylated amino acid is preceded by a “p” and is highlighted in red. (C) V5-parkin WT or V5-parkin Y143F were coexpressed with GFP-c-Abl or GFP-c-Abl-KD in SH-SY5Y cells. Anti-V5 immunoprecipitates were subjected to SDS/PAGE followed by immunoblotting with an anti-phosphotyrosine antibody (third panel), with anti-GFP (second panel), and with anti-V5 to show an equivalent amount of immunoprecipitated parkin (fourth panel). Inputs were immunoblotted with antibodies to GFP to show equivalent expression and loading. All immunoblot experiments were repeated at least three times and representative images of the immunoblots are shown.
Tyrosine Phosphorylation of Parkin Inhibits Its Ubiquitin E3 Ligase Activity.
To determine whether c-Abl phosphorylation of parkin modulates parkin's ubiquitination activity, autoubiquitination of parkin was monitored in the presence of kinase-active c-Abl (c-Abl-KA) or c-Abl-KD. SH-SY5Y cells were cotransfected with V5-tagged parkin and HA-tagged ubiquitin followed by immunoprecipitation of parkin. Parkin is autoubiquitinated, as detected by anti-HA immunoreactivity as previously reported (Fig. 3A) (4, 5). Kinase-active c-Abl, which tyrosine-phosphorylates parkin, almost completely inhibits the autoubiquitination of parkin, whereas c-Abl-KD has no effect (Fig. 3A). To ascertain whether tyrosine 143 is required for the c-Abl-mediated inhibition of parkin autoubiquitination, WT parkin and Y143F parkin autoubiquitination were monitored in the presence of c-Abl-KA or c-Abl-KD. WT parkin ubiquitination is inhibited by c-Abl-KA, whereas it has no effect on parkin Y143F ubiquitination (Fig. 3B). c-Abl-KD has no effect on WT parkin or Y143F parkin autoubiquitination (Fig. 3B). Next, the effects of c-Abl on parkin ubiquitination of AIMP2 were monitored. Parkin ubiquitinates AIMP2 as previously described (8, 20) and Y143F parkin also ubiquitinates AIMP2 (Fig. 3C). c-Abl-KA impairs WT parkin ubiquitination of AIMP2, but c-Abl-KD has no effect (Fig. 3C). Y143F parkin ubiquitination of AIMP2 is not affected by either c-Abl-KA or c-Abl-KD (Fig. 3C). To pharmacologically confirm the role of c-Abl in tyrosine-phosphorylating parkin and inhibiting its ubiquitination function, AIMP2 ubiquitination by parkin was evaluated in the presence and absence of STI-571, a c-Abl-family kinase inhibitor. The c-Abl-mediated tyrosine phosphorylation of parkin and the inhibition of parkin's ubiquitination of AIMP2 are prevented by STI-571 (Fig. 3D). To determine whether c-Abl affects parkin's ubiquitination of other substrates, the ubiquitination of FBP1 was monitored. FBP1 ubiquitination by parkin is inhibited by c-Abl-KA, and STI-571 inhibits the c-Abl-mediated tyrosine phosphorylation of parkin and restores the ubiquitination of FBP1 (Fig. S2).
Fig. 3.
Tyrosine phosphorylation of parkin by c-Abl inhibits its E3 ubiquitin ligase activity. (A) SH-SY5Y cells were transfected with V5-WT parkin, HA-ubiquitin (Ub), and kinase-active (KA) or kinase-dead (KD) GFP-c-Abl. Cells were lysed and immunoprecipitated with an anti-V5 antibody. Immunoblotting with an anti-HA antibody shows autoubiquitination of parkin, an anti-V5 antibody shows equivalent immunoprecipitated parkin, and an anti-phosphotyrosine shows parkin phosphorylation. Input samples were immunoblotted with anti-HA and anti-GFP antibodies to show equivalent expression. (B) SH-SY5Y cells were transfected with either V5-WT-parkin or V5-Y143F-parkin along with HA-ubiquitin and GFP-c-Abl-KA or GFP-c-Abl-KD. Autoubiquitination of parkin was visualized through immunoblotting with an anti-HA antibody after immunoprecipitation with the V5 antibody. Immunoblotting with anti-V5 shows an equivalent amount of immunoprecipitated parkin and anti-phosphotyrosine shows parkin phosphorylation. Input samples were immunoblotted with anti-HA and anti-GFP antibodies to show equivalent expression. (C) SH-SY5Y cells were transfected with FLAG-AIMP2, V5-WT-parkin, or V5-Y143F-parkin, HA-ubiquitin and GFP-c-Abl-KA, or GFP-c-Abl-KD. Immunoblotting of anti-FLAG immunoprecipitates with anti-HA shows ubiquitination of AIMP2 and anti-FLAG shows equivalent levels of immunoprecipitated AIMP2. Immunoprecipitation with anti-V5 and immunoblotting with anti-phosphotyrosine antibody shows parkin phosphorylation and with anti-V5 shows equivalent amounts of immunoprecipitated parkin. Inputs were immunoblotted with anti-HA and anti-GFP to show equivalent expression. (D) SH-SY5Y cells were transfected with FLAG-AIMP2, V5-WT-parkin, HA-ubiquitin, and GFP-c-Abl-KA in the presence or absence of 10 μM STI-571. Anti-FLAG immunoprecipitates were probed with anti-HA to show the amount of ubiquitination of AIMP2 and anti-FLAG to show an equivalent amount of immunoprecipitated AIMP2. Immunoprecipitation with anti-V5 and immunoblotting with anti-phosphotyrosine antibody shows parkin phosphorylation and anti-V5 shows an equivalent amount of immunoprecipitated parkin. Inputs were immunoblotted with anti-HA and anti-GFP to show equivalent expression. All experiments were repeated at least three times and representative images of the immunoblots are shown.
Dopaminergic Stress Activates c-Abl Inhibiting Parkin's E3 Ligase Activity and Protective Function.
Different types of genotoxic stress are known to activate c-Abl (21). To determine whether PD-associated stressors activate c-Abl, we monitored c-Abl activation with an anti-p-tyrosine antibody to tyrosine 245 of c-Abl in SH-SY5Y cells transfected with V5-parkin. The DA neurotoxin 1-methyl-4-phenylpyridinium (MPP+) (250 μM) or DA (250 μM) activates c-Abl, as determined by the anti-p-tyrosine 245 c-Abl antibody (Fig. 4A). c-Abl activation by MPP+ or DA is attenuated by the c-Abl-family kinase inhibitor STI-571 (Fig. 4A). Accompanying the activation of c-Abl by MPP+ or DA is tyrosine phosphorylation of parkin, as detected with an anti-p-tyrosine antibody against immunoprecipitated parkin. The tyrosine phosphorylation of parkin is completely attenuated by STI-571 (Fig. 4A) or suppression of c-Abl expression with siRNA (Fig. S3A). MPP+ or DA also increases the level of the parkin substrate AIMP2, and this increase is blocked by STI-571 (Fig. 4A) or suppression of c-Abl expression with siRNA (Fig. S3A). To determine whether activation of c-Abl by MPP+ or DA impairs parkin's ubiquitination activity, parkin autoubiquitination was monitored in SH-SY5Y cells transfected with V5-parkin. Both MPP+ and DA reduce parkin autoubiquitination, which is attenuated by STI-571 (Fig. 4B) or knockdown of c-Abl expression with siRNA (Fig. S3B).
Fig. 4.
Parkin is tyrosine-phosphorylated after dopaminergic stress. (A) SH-SY5Y cells were transiently transfected with V5-parkin and treated with MPP+ (250 μM) or dopamine (DA) (250 μM) for 24 h in the presence or absence of STI-571 (10 μM). Cell lysates were immunoprecipitated with an anti-V5 antibody and immunoblotted with anti-phosphotyrosine to show phosphorylated parkin, and anti-parkin to show equivalent IP. Inputs were immunoblotted with anti-phospho-c-Abl to show activated c-Abl, and with anti-c-Abl, anti-AIMP2, or anti-actin antibodies. Data were repeated with similar results. (B) SH-SY5Y cells were transiently transfected with V5-parkin and HA-ubiquitin and treated with MPP+ (250 μM) or DA (250 μM) for 24 h in the presence or absence of STI-571 (10 μM). Cell lysates were immunoprecipitated with an anti-V5 antibody and immunoblotted with anti-HA to monitor ubiquitination of parkin, anti-phosphotyrosine to show phosphorylated parkin, and anti-V5 to show equivalent levels of immunoprecipitated parkin. Inputs were immunoblotted with anti-phospho-c-Abl to show activated c-Abl, and with anti-c-Abl or anti-HA to show equivalent expression. Data were repeated with similar results. (C) PC12-AIMP2-inducible cells were transfected with V5-WT-parkin and V5-Y143F-parkin in the presence or absence of MPP+ (200 μM) for 24 h. Some samples were incubated with 10 μM STI-571. Cell death was assessed by trypan blue exclusion. Both STI-571 and transfection with phosphorylation-resistant Y143F-parkin protect against AIMP2-induced cell death and after MPP+ treatment. Doxycycline (Dox) (+) prevents the induction of AIMP2. Dox withdrawal (−) induces the expression of AIMP2 (Fig. S3). Data are mean ± SEM for three separate experiments performed in duplicate. Statistical significance was determined by one-way ANOVA and Tukey's post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001; ***1: DMSO versus MPP+–Dox (+); **2: MPP+ versus STI+MPP+–Dox (+); ***3: DMSO–Dox (+) versus AIMP2–Dox (−); ***4: DMSO/AIMP2–Dox (−) versus AIMP2–Dox (−) + WT-parkin; *5: DMSO/AIMP2-Dox (−) versus MPP+/AIMP2–Dox (−) + WT-parkin; ***6: MPP++/AIMP2–Dox (−) + WT-parkin versus STI + MPP+/AIMP2–Dox (−) + WT-parkin; **7: DMSO/AIMP2–Dox (−) versus AIMP2–Dox (−) + Y143F parkin; ***8: MPP+/AIMP2–Dox (−) versus MPP+/AIMP2–Dox (−) + Y143F parkin. (D and E) The protective effect of STI-571 requires parkin. (D) SH-SY5Y cells were transfected with either parkin-shRNA or GFP-shRNA in the presence or absence of MPP+ (250 μM) for 24 h. Some samples were incubated with 10 μM STI-571. Cell lysates were subjected to immunoprecipitation with an anti-parkin antibody. The parkin immunoprecipitates were immunoblotted with anti-phosphotyrosine to monitor tyrosine phosphorylation of parkin and anti-parkin to monitor the levels of immunoprecipitated parkin. Total lysates were immunoblotted with anti-phospho-c-Abl to show tyrosine-phosphorylated c-Abl, anti-actin as a loading control, and with an AIMP2 antibody to monitor the levels of AIMP2 and an anti-parkin antibody to confirm the parkin knockdown by parkin-shRNA. Data were repeated with similar results. (E) Trypan blue cell death assessments in SH-SY5Y neuroblastoma cells that were transiently transfected with parkin-shRNA or GFP-shRNA and treated with MPP+ (200 μM). Some samples were incubated with 10 μM STI-571 as indicated. Data are mean ± SEM for three separate experiments performed in duplicate. Statistical significance was determined by one-way ANOVA and Tukey's post hoc test. ***P < 0.001.
The susceptibility of PC-12 cells to AIMP2-induced cell death was used to determine whether tyrosine phosphorylation of parkin by c-Abl regulates parkin's protective function. A Tet-off–inducible PC-12 cell line that overexpresses AIMP2 in the absence of doxycycline was developed to monitor AIMP2 toxicity (Fig. S3C). In the absence of doxycycline, AIMP2 is substantially induced, leading to a greater than twofold increase in cell death as monitored by trypan blue exclusion (Fig. 4C). In noninduced cells, MPP+ treatment leads to a greater than threefold increase in cell death that is significantly inhibited by STI-571 (Fig. 4C). AIMP2 toxicity is unaffected by STI-571, and STI-571 treatment alone has no substantial effect on cell viability in noninduced cells (Fig. 4C). MPP+ treatment enhances AIMP2 toxicity, and this enhancement is inhibited by STI-571 (Fig. 4C). Parkin overexpression attenuates AIMP2 toxicity, but MPP+ treatment prevents parkin's protective effects against AIMP2 toxicity. Coadministration of STI-571 restores parkin's protective effects against AIMP2 toxicity in the setting of MPP+ treatment (Fig. 4C). Y143F parkin protects against AIMP2 toxicity and MPP+ treatment is incapable of attenuating Y143F parkin's protective properties. Similar results were observed with DA stress-induced toxicity (Fig. S3D). These results taken together indicate that parkin's protective function is impaired by the PD-associated stressors MPP+ and DA through c-Abl-mediated phosphorylation of parkin at tyrosine 143.
To determine whether the protective effects of STI-571 require parkin, shRNA was used to knock down parkin and the ability of STI-571 to protect against MPP+ toxicity was monitored. shRNA to GFP was used as a control. Knockdown of parkin leads to an up-regulation of AIMP2 (Fig. 4D). MPP+ treatment leads to tyrosine phosphorylation of c-Abl and parkin, which further increases AIMP2 levels. STI-571, which prevents the tyrosine phosphorylation of parkin by c-Abl, attenuates the increase in AIMP2 levels. In the setting of parkin knockdown, c-Abl inhibition by STI-571 has no effect on AIMP2 levels (Fig. 4D). MPP+ treatment of SH-SY5Y cells leads to a twofold increase in toxicity as assessed by trypan blue exclusion, which is prevented by STI-571 (Fig. 4E) or suppression of c-Abl expression with siRNA (Fig. S3E). Parkin knockdown attenuates the ability of STI-571 (Fig. 4E) or knockdown of c-Abl (Fig. S3E) to protect against MPP+ toxicity. Taken together, these results indicate that STI-571 protective effects require parkin.
c-Abl Deficiency Protects Against MPTP-Induced DA Neuron Cell Death In Vivo.
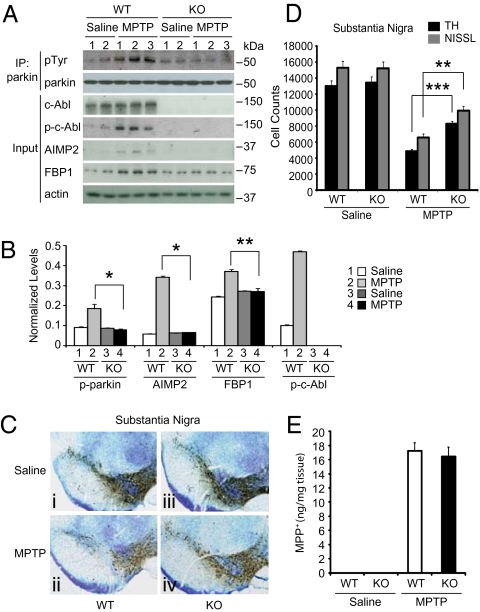
Germ-line deletion of c-Abl leads to elevated perinatal lethality (22, 23). Accordingly, conditional c-Abl KO mice (24) were used to test the role of c-Abl in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD. c-Ablflox/flox mice were bred to Nestin-Cre transgenic mice to generate conditional KO of c-Abl in neurons (Fig. S4). PCR and immunoblot analysis were used to determine the genotype of c-Abl KO (c-AblKO/flox/nestin Cre+) and WT mice (c-Ablflox/flox/nestin Cre−) (Fig. S4 C and D). For all studies, age-matched WT and c-Abl KO littermates were used and treated with either saline or MPTP (four i.p. injections, 20 mg/kg, at 2-h intervals). MPTP activates c-Abl in WT mice as assessed 7 d after the last MPTP injection as determined by the anti-p-tyrosine 245 c-Abl antibody, leading to a fivefold increase in phospho-c-Abl (Fig. 5A). There is a twofold increase in tyrosine phosphorylation of parkin, a threefold increase in AIMP2, and a moderate increase in FBP1 that accompanies the activation of c-Abl. In c-Abl KO mice, c-Abl is not detectable, as expected (Fig. 5A). Following MPTP intoxication, there is no increase in tyrosine phosphorylation of parkin and no increase in AIMP2 and FBP1 levels (Fig. 5 A and B). DA cell death in response to MPTP toxicity was monitored through stereologic measurement of tyrosine hydroxylase (TH) immunoreactivity and Nissl staining. MPTP treatment leads to a 60% reduction in TH-positive neurons and a similar loss of Nissl-positive neurons (Fig. 5 C and D). c-Abl KO significantly prevents the loss of TH-positive and Nissl-positive neurons following MPTP intoxication (Fig. 5 C and D). Equivalent levels of MPP+, the active metabolite of MPTP, are observed in WT and c-Abl KO animals, indicating that the protective effect observed in c-Abl KO mice is not due to altered bioavailability of MPP+ (Fig. 5E). Striatal TH-positive fiber density was assessed in WT and c-Abl KO mice after MPTP administration. MPTP leads to a 50% reduction in TH-positive fiber density in the striatum of WT mice. KO of c-Abl significantly protects against the loss of striatal TH-positive fibers (Fig. S5 A and B). Taken together, these data indicate that c-Abl activation plays a role in DA cell loss following MPTP intoxication.
Fig. 5.
c-Abl knockout (KO) protects against MPTP-induced loss of DA neurons. (A) c-Abl KO prevents parkin tyrosine phosphorylation and the accumulation of AIMP2 and FBP1 in the striatum of MPTP-treated mice. Immunoblots of Parkin immunoprecipitation samples from WT and c-Abl KO littermates treated with saline or MPTP (four i.p. injections. 20 mg/kg, at 2-h intervals). Immunoblotting with an anti-phosphotyrosine antibody shows tyrosine-phosphorylated parkin and an anti-parkin antibody shows immunoprecipitated parkin. Brain lysates were immunoblotted with anti-phospho-c-Abl to show tyrosine-phosphorylated c-Abl, an anti-actin antibody was used as a loading control, and AIMP2 and FBP1 antibodies were used to monitor their levels. (B) Normalized levels of tyrosine-phosphorylated parkin (p-parkin), AIMP2, FBP1, and phospho-c-Abl (p-c-Abl). Data are expressed as mean ± SEM and were evaluated for statistical significance by one-way ANOVA and Tukey's post hoc test. *P < 0.05, **P < 0.01. (C) Photomicrographs of tyrosine hydroxylase (TH)-immunostained sections in the substantia nigra of WT (a and b) and c-Abl KO (c and d) littermate mice treated with vehicle (a and c) and MPTP (b and d). (D) Number of TH- and Nissl-positive neurons in the substantia nigra of WT and c-Abl KO littermate mice treated with PBS vehicle and MPTP, determined by stereological counting. The data are the mean ± SEM (n = 6). Statistical significance was determined by one-way ANOVA and Tukey's post hoc test. **P < 0.01, ***P < 0.001. (E) Levels of MPP+ in the striatum of WT and c-Abl KO mice treated with PBS vehicle and MPTP (four i.p. injections. 20 mg/kg, at 2-h intervals) 90 min after final injection. All experiments were repeated three times and representative images of the immunoblots are shown.
Activation of c-Abl in Sporadic PD.
The potential pathophysiologic relevance of c-Abl activation and tyrosine phosphorylation of parkin was monitored in human postmortem brain tissue from PD patients and age-matched controls (Tables S1 and S2). Cell lysates were prepared from substantia nigra, striatum, and cortex. In substantia nigra and striatum from PD patient samples, there is a significant increase in tyrosine-phosphorylated c-Abl over age-matched control tissue (Fig. 6 A and B). There is also a significant increase in tyrosine-phosphorylated parkin and a significant increase in AIMP2 and FBP1 levels in PD substantia nigra and striatum. There is a strong positive correlation between phosphorylated c-Abl and phosphorylated parkin and between phosphorylated parkin and levels of AIMP2 and FBP1 in substantia nigra and striatum (Fig. S6). There is no significant difference between the levels of tyrosine-phosphorylated c-Abl, tyrosine-phosphorylated parkin, AIMP2, or FBP1 in cortex, a region relatively unaffected in PD (Fig. 6C). To determine whether other c-Abl substrates are tyrosine-phosphorylated in PD, tyrosine phosphorylation of cyclin-dependent kinase 5 (CDK5), a well-known c-Abl kinase substrate, was monitored (25). There is a significant increase in tyrosine-phosphorylated CDK5 in PD substantia nigra and striatum compared with controls (Fig. S7). Taken together, these results indicate that c-Abl activation and tyrosine phosphorylation of parkin and the accumulation of the parkin substrates AIMP2 and FBP1 occur in PD and appear to be specific to the nigrostriatal system.
Fig. 6.
Parkin is tyrosine-phosphorylated in the striatum and substantia nigra of PD patients. Anti-parkin IP samples from substantia nigra (A), striatum (B), and cortex lysates (C) were immunoblotted with anti-phosphotyrosine antibodies to monitor tyrosine-phosphorylated parkin, anti-parkin antibodies to show immunoprecipitated parkin, anti-c-Abl antibodies to show coimmunoprecipitated c-Abl, and anti-ubiquitin to show ubiquitinated parkin. Brain lysates were immunoblotted with anti-AIMP2, anti-phospho-c-Abl, anti-c-Abl, and anti-FBP1 antibodies to monitor their levels and an anti-actin antibody was used as a loading control. Relative phospho-parkin levels normalized to immunoprecipitated parkin, relative phospho-c-Abl levels normalized to c-Abl, and relative AIMP2 and FBP1 levels normalized to β-actin are indicated in D (substantia nigra), E (striatum), and F (cortex). The data are the mean ± SEM. Statistical significance was determined by applying the unpaired two-tailed Student's t test. *P < 0.05, **P < 0.01, ***P < 0.001. All experiments were repeated at least three times and representative images of the immunoblots are shown.
Discussion
The major finding of this study is that c-Abl is a major regulator of parkin function. c-Abl phosphorylates parkin on tyrosine 143. This phosphorylation inhibits parkin's E3 ubiquitin ligase activity, leading to accumulation of AIMP2 and FBP1 and loss of parkin's cytoprotective function and cell death. The c-Abl-family kinase inhibitor STI-571 maintains parkin in a catalytically active and neuroprotective state by preventing the tyrosine phosphorylation of parkin. shRNA knockdown of parkin attenuates STI-571 protection, indicating that the protective effect of STI-571 is parkin-dependent. c-Abl is active in sporadic PD substantia nigra and striatum, correlating with the increase in phosphorylated parkin and accumulation of AIMP2 and FBP1. These data implicate c-Abl as an important regulator of parkin's E3 ligase activity and cytoprotective function and identify c-Abl as a potentially important therapeutic target for the treatment of PD.
Our findings provide further support to the idea that parkin is inactivated in sporadic PD. Previous studies suggest that parkin is inactivated in sporadic PD through S-nitrosylation (15, 26), oxidative stress (27), and dopamine conjugation (18). Here we describe another posttranslational modification of parkin that is present in sporadic PD. Thus, parkin inactivation is emerging as a common event in sporadic PD. Maintaining parkin function through inhibition of S-nitrosylation, dopamine conjugation, or phosphorylation by c-Abl is an attractive therapeutic target. Parkin inactivation by c-Abl may be a dominant pathway, because inhibition of c-Abl with STI-571 or knockdown and knockout of c-Abl prevents the inactivation of parkin by MPTP and DA.
The mechanisms underlying c-Abl activation in sporadic PD await further characterization. One of the possible mechanisms, however, can be partially explained by oxidative stress, which is prevalent in sporadic PD brain, because various oxidative stressors activate c-Abl (28). In addition, as shown here, dopaminergic stress also increases c-Abl activity. In sporadic PD, c-Abl activation and parkin inactivation and accumulation of parkin substrates appear to be limited to the striatum and substantia nigra, regions of the brain that are primarily affected in PD, as these changes are not observed in the cerebral cortex of PD patients. Thus, activation of c-Abl, tyrosine phosphorylation of parkin, and accumulation of parkin substrates may contribute to the pathogenesis of sporadic PD.
c-Abl inhibitors are widely used in the treatment of chronic myeloid leukemia. Because the c-Abl inhibitor maintains parkin in a catalytically active neuroprotective state and KO of c-Abl protects against dopaminergic cell loss following MPTP intoxication, the testing and use of brain-permeable c-Abl inhibitors as a neuroprotective treatment for PD are reasonable and potentially exciting objectives.
Materials and Methods
All methods used in this article are routinely used in our laboratories and are thus referenced (7, 8, 24, 29) and are described in SI Materials and Methods. For protein interactions, standard coimmunoprecipitation experiments and ubiquitination assays were used as previously described (7, 8). For LC-MS/MS, excised 2-DE spots were subjected to a modified in-gel trypsin digestion procedure. Protein identity was determined by the software program Sequest (Thermo Finnigan). Conditional c-Abl knockouts were used, and MPTP intoxication studies and assessments in mice were performed as previously described (24, 29).
Supplementary Material
Acknowledgments
This work was supported by NIH/NINDS Grants NS38377, NS048206, NS051764, and NS39475, and the Bachmann Strauss Dystonia and Parkinson's Disease Foundation. Y.L. is supported by the Samsung Scholarship Foundation. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006083107/-/DCSupplemental.
References
- 1.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasser T. Molecular pathogenesis of Parkinson disease: Insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 3.Dawson TM, Dawson VL. The role of parkin in familial and sporadic Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S32–S39. doi: 10.1002/mds.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Parkin functions as an E2-dependent ubiquitin-protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci USA. 2000;97:13354–13359. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallon L, et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol. 2006;8:834–842. doi: 10.1038/ncb1441. [DOI] [PubMed] [Google Scholar]
- 7.Ko HS, Kim SW, Sriram SR, Dawson VL, Dawson TM. Identification of far upstream element-binding protein-1 as an authentic Parkin substrate. J Biol Chem. 2006;281:16193–16196. doi: 10.1074/jbc.C600041200. [DOI] [PubMed] [Google Scholar]
- 8.Ko HS, et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci. 2005;25:7968–7978. doi: 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim KL, et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: Implications for Lewy body formation. J Neurosci. 2005;25:2002–2009. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 11.Reddy EP, Smith MJ, Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: Structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci USA. 1983;80:3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moresco EM, Koleske AJ. Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr Opin Neurobiol. 2003;13:535–544. doi: 10.1016/j.conb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez AR, Sandoval PC, Leal NR, Castro PU, Kosik KS. Activation of the neuronal c-Abl tyrosine kinase by amyloid-β-peptide and reactive oxygen species. Neurobiol Dis. 2004;17:326–336. doi: 10.1016/j.nbd.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez AR, et al. Imatinib therapy blocks cerebellar apoptosis and improves neurological symptoms in a mouse model of Niemann-Pick type C disease. FASEB J. 2008;22:3617–3627. doi: 10.1096/fj.07-102715. [DOI] [PubMed] [Google Scholar]
- 15.Chung KK, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 16.Avraham E, Rott R, Liani E, Szargel R, Engelender S. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- 17.Rubio de la Torre E, et al. Combined kinase inhibition modulates parkin inactivation. Hum Mol Genet. 2009;18:809–823. doi: 10.1093/hmg/ddn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 19.Kawai H, Nie L, Yuan ZM. Inactivation of NF-κB-dependent cell survival, a novel mechanism for the proapoptotic function of c-Abl. Mol Cell Biol. 2002;22:6079–6088. doi: 10.1128/MCB.22.17.6079-6088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti O, et al. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: Linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. doi: 10.1093/hmg/ddg159. [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda S, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 22.Schwartzberg PL, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 23.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 24.Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zukerberg LR, et al. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000;26:633–646. doi: 10.1016/s0896-6273(00)81200-3. [DOI] [PubMed] [Google Scholar]
- 26.Yao D, et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, et al. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem. 2000;275:17237–17240. doi: 10.1074/jbc.C000099200. [DOI] [PubMed] [Google Scholar]
- 29.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.