Abstract
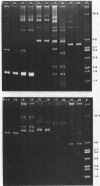
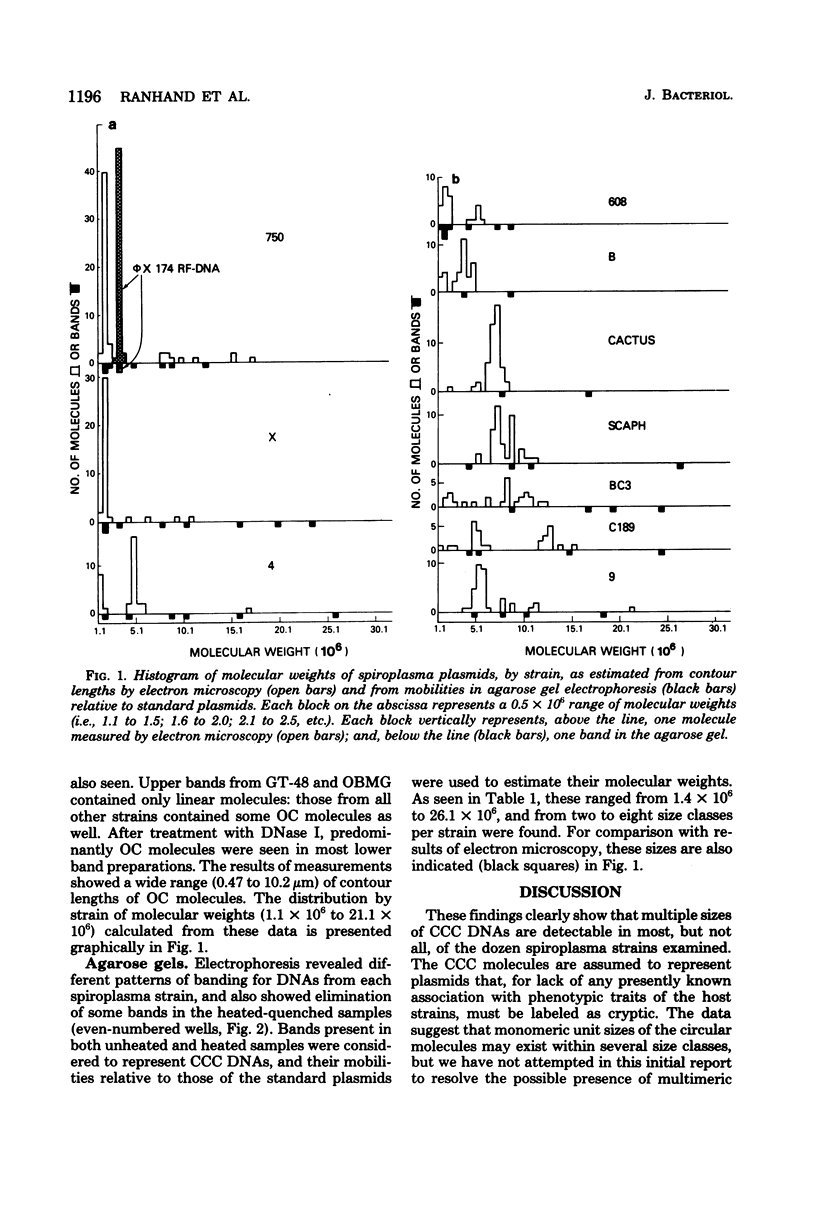
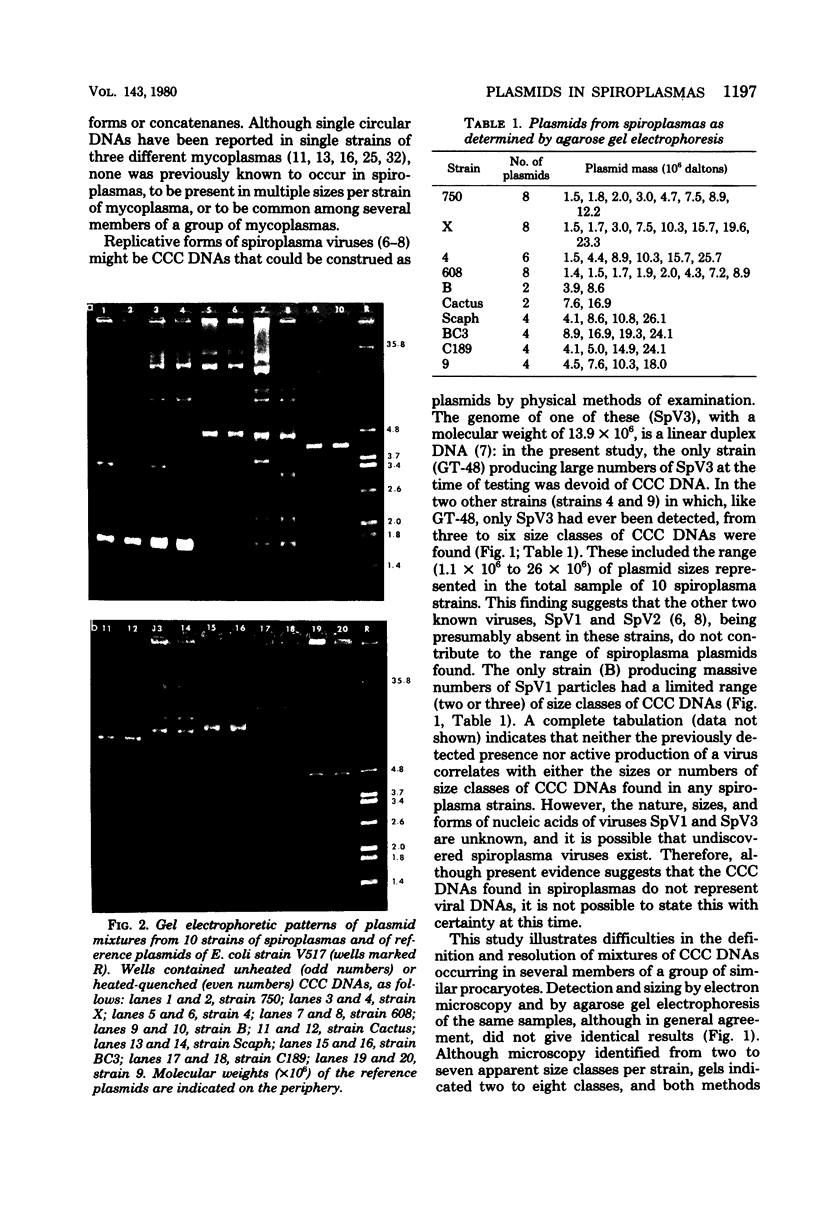
Ten of twelve spiroplasma strains from different sources carried multiple covalently closed circular duplex deoxyribonucleic acid molecules, as shown by ethidium bromide-cesium chloride gradient centrifugation of cell lysates and examination of resulting bands by electron microscopy and agarose gel electrophoresis. Two to eight size classes per strain, comprising molecules of masses from 1 X 10(6) to 26 X 10(6), were detected. Several size classes of molecules were found in common in different spiroplasma strains. The amount of covalently closed circular deoxyribonucleic acid per strain was as much as 12% of total cellular deoxyribonucleic acid. The presence of sizes of the circular molecules appeared unrelated to either carriage or active production of known spiroplasma viruses, and it is tentatively concluded that they are plasmids rather than genomes or replicative forms of viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Mitchell W. O., Garon C. F. Spiroplasmavirus citri 3: propagation, purification, proteins, and nucleic acid. Science. 1977 Dec 23;198(4323):1262–1263. doi: 10.1126/science.929198. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Das J., Maniloff J., Bhattacharjee S. B. Dark and light repair in ultraviolet-irradiated Acholeplasma laidlawii. Biochim Biophys Acta. 1972 Jan 31;259(2):189–197. doi: 10.1016/0005-2787(72)90058-5. [DOI] [PubMed] [Google Scholar]
- Dugle D. L., Dugle J. R. Presence of two DNA populations in Mycoplasma laidlawii. Can J Microbiol. 1971 Mar;17(3):433–434. doi: 10.1139/m71-072. [DOI] [PubMed] [Google Scholar]
- Furness G., Cerone A. M. Preparation of competent single-cell suspensions of Mycoplasma hominis tets and Mycoplasma salivarium tets for genetic transformation to tetracycline resistance by DNA extracted from Mycoplamsa hominis tetr. J Infect Dis. 1979 Apr;139(4):444–451. doi: 10.1093/infdis/139.4.444. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Grossman L. I. Electrophoresis of DNA in agarose gels. Optimizing separations of conformational isomers of double- and single-stranded DNAs. Biochemistry. 1977 Sep 20;16(19):4217–4225. doi: 10.1021/bi00638a014. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Transfection mediated by Mycoplasmatales viral DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3423–3427. doi: 10.1073/pnas.69.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Das J., Christensen J. R. Viruses of mycoplasmas and spiroplasmas. Adv Virus Res. 1977;21:343–380. doi: 10.1016/s0065-3527(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Williamson D. L., Whitcomb R. F. Plant mycoplasmas: a cultivable spiroplasma causes corn stunt disease. Science. 1975 Jun 6;188(4192):1018–1020. doi: 10.1126/science.188.4192.1018. [DOI] [PubMed] [Google Scholar]
- Zouzias D., Mazaitis A. J., Simberkoff M., Rush M. Extrachromosomal DNA of Mycoplasma hominis. Biochim Biophys Acta. 1973 Jul 13;312(3):484–491. doi: 10.1016/0005-2787(73)90446-2. [DOI] [PubMed] [Google Scholar]