Abstract
Modification of the number of GABAA receptors (GABAARs) clustered at inhibitory synapses can regulate inhibitory synapse strength with important implications for information processing and nervous system plasticity and pathology. Currently, however, the mechanisms that regulate the number of GABAARs at synapses remain poorly understood. By imaging superecliptic pHluorin tagged GABAAR subunits we show that synaptic GABAAR clusters are normally stable, but that increased neuronal activity upon glutamate receptor (GluR) activation results in their rapid and reversible dispersal. This dispersal correlates with increases in the mobility of single GABAARs within the clusters as determined using single-particle tracking of GABAARs labeled with quantum dots. GluR-dependent dispersal of GABAAR clusters requires Ca2+ influx via NMDA receptors (NMDARs) and activation of the phosphatase calcineurin. Moreover, the dispersal of GABAAR clusters and increased mobility of individual GABAARs are dependent on serine 327 within the intracellular loop of the GABAAR γ2 subunit. Thus, NMDAR signaling, via calcineurin and a key GABAAR phosphorylation site, controls the stability of synaptic GABAARs, with important implications for activity-dependent control of synaptic inhibition and neuronal plasticity.
Keywords: ion channels, plasticity, trafficking, diffusion, calcineurin
Synaptic inhibition plays a critical role in regulating neuronal excitability and information processing in the brain. The number of GABAA receptors (GABAARs) in the surface membrane and at synaptic sites is an important determinant of inhibitory synapse strength (1), but the mechanisms that rapidly control synaptic GABAAR number and stability remain poorly understood. Activation of Ca2+-permeable ionotropic glutamate receptors (GluRs) during plasticity and in pathology can result in down-modulation of inhibitory synapse strength and GABAAR function (2–5) but the molecular and cellular mechanisms underlying GluR-dependent changes in the strength of GABAergic inhibition remain unclear.
A major mechanism for modulating GABAAR activity is the direct phosphorylation of residues within the intracellular loops of GABAAR subunits, which can regulate synaptic inhibition, GABAAR channel kinetics, and trafficking (6–9). The rapid movement of neurotransmitter receptors (including GABAARs) (10–12) into and out of synapses has also recently emerged as an important mechanism for regulating synaptic strength (13). However, whether GABAAR phosphorylation can directly regulate the synaptic stability of GABAARs and their lateral diffusion and movement into and out of synapses is unknown.
Here, by live cell imaging of surface GABAAR clusters with pH-sensitive superecliptic pHluorin (SEP) and single GABAARs with quantum dots (QDs), we investigate the mechanisms that regulate activity-dependent control of the lateral diffusion, clustering, and stability of GABAARs at inhibitory synapses. We find that Ca2+ entry through NMDA receptors (NMDARs) leads to a reversible dispersal of GABAAR clusters and an increase in GABAAR lateral mobility. This mechanism can also allow for a localized GABAAR dispersal in dendrites when only a few excitatory synapses are activated. The dispersal of GABAARs from synapses requires activation of the phosphatase calcineurin, and is dependent on serine 327, a key phosphorylation site in the GABAAR γ2 subunit (14). Thus, activity-dependent control of phospho-dependent signaling can rapidly regulate the number of synaptic GABAARs with important implications for inhibitory synaptic plasticity, pathology, and information processing in the brain.
Results
GABAAR Clusters Are Stable Under Basal Conditions, Although Single GABAARs Can Be Highly Mobile.
To image surface GABAAR clusters in the dendrites of live cultured hippocampal neurons, we expressed SEP-tagged GABAAR α2 subunits (α2SEP-GABAARs) (Fig. S1A) (15), the majority of which formed bright fluorescent clusters along the neuronal dendrites in addition to lower levels of diffuse staining (Fig. 1A). Heterologously expressed α2-SEP subunits could not access the cell surface without the presence of coexpressed GABAAR β- and γ2 subunits (Fig. S1 B and C) and thus cannot form homomeric surface GABAARs. In agreement with this, immunoprecipitating native GABAARs from α2-SEP subunit transfected neurons followed by Western blotting with GFP antibodies confirmed that α2-SEP subunits assembled with endogenous GABAAR subunits, and the lack of any cleavage products confirmed that the fluorescence from α2SEP-GABAARs was reporting only full-length α2-SEP subunits (Fig. S1D). α2SEP-GABAAR fluorescence was rapidly and reversibly eclipsed by transient exposure to low extracellular pH buffer, which completely eclipsed the signal (Fig. S1 E–H), confirming surface expression of the clusters. A large fraction of α2SEP-GABAAR clusters (84.3 ± 1.8%) were localized to inhibitory synapses marked by GAD-6 staining (Fig. S1I) and 65.3 ± 5.2% were apposed to FM4-64 labeled presynaptic terminals (Fig. S1J, in agreement with previous reports for the proportion of native GABAAR clusters found opposite active FM dye-labeled presynaptic terminals) (16, 17). However, only 9.1 ± 1.3% were found apposed to excitatory synapses labeled with homer1 (Fig. S1K). Thus, α2-SEP fluorescence reports surface heteromeric GABAARs that accumulate in clusters at inhibitory synapses.
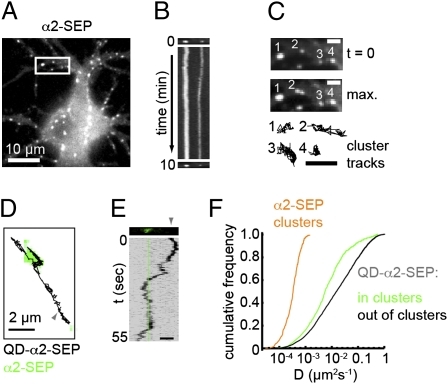
Fig. 1.
Surface GABAAR clustering under basal conditions. (A) α2SEP-GABAARs expressed in cultured hippocampal neurons. (B) Kymograph (a line scan vertically projected over time) showing stability of α2SEP-GABAAR clusters in boxed region in A over the movie (duration 10 min). (C) Region boxed in A shown at t = 0 (Top), as a maximum projection over time (Middle) and with cluster trajectories shown (Bottom) (3× zoom). (Scale bars, 2 μm.) (D) Trajectory of a QD-tagged α2SEP-GABAAR (black, origin marked by gray arrowhead) undergoing lateral diffusion into and out of an α2SEP-GABAAR cluster (green). (E) Kymograph for trajectory in D. Note the period of low horizontal displacement corresponding to confined motion within cluster (position marked by green line). (Scale bar, 2 μm.) (F) α2SEP-GABAAR cluster diffusion coefficients (orange) and instantaneous diffusion coefficients for QD-α2SEP-GABAARs inside (green) and outside of clusters (black). Single GABAARs are less mobile when within GABAAR clusters [P = 4 × 10−13, Kolmogorov–Smirnov (K-S) test, nin= 598, nout= 3665].
Under resting conditions, continuous imaging revealed that the intensities and locations of α2SEP-GABAAR clusters were stable for periods of 10 min (Fig. 1 A–C) although interestingly, some clusters exhibited small, asynchronous lateral movements (Fig. 1 B and C). Tracking the mass centers of individual clusters gave trajectories that were used to determine their diffusivities. The low mean diffusivity of α2SEP-GABAAR clusters (6 × 10−4 μm2·s−1, Fig. 1F and Movie S1) is similar to that previously reported for clusters of the inhibitory postsynaptic scaffold gephyrin (18), suggesting that α2SEP-GABAAR cluster movement may represent movement of the entire inhibitory postsynaptic apparatus.
Although imaging of α2SEP-GABAARs allowed the behavior of synaptic GABAAR clusters to be followed, it could not resolve the behavior of individual GABAARs. To simultaneously visualize synaptic GABAAR clusters and single GABAARs, we combined SEP tagging with QD tracking (13, 19, 20). Single α2SEP-GABAARs were labeled using a low concentration of GFP antibody to recognize the extracellular SEP tag on the α2 subunit in conjunction with an anti-mouse secondary coupled to 605-nm QDs (Fig. S2A). In contrast to the relative immobility of α2SEP-GABAAR clusters, single QD-labeled α2SEP-GABAARs could be observed to rapidly diffuse into and out of GABAAR clusters (Fig. 1 D and E and Movie S2). QD-labeled α2SEP-GABAARs were less mobile when inside GABAAR clusters (median Din = 0.0078 μm2·s−1, median Dout = 0.0192 μm2·s−1, Fig. 1F) and exhibited confined motion within clusters (Fig. S2 B and C), presumably due to interactions with the scaffold gephyrin. α2SEP-GABAAR mobility and residency time were similar inside clusters whether or not these were apposed to FM 4-64 positive puncta (Fig. S2 D–F). Native GABAARs imaged by QD tracking could also be found either confined at inhibitory postsynaptic domains labeled by expression of GFP-tagged gephyrin or seen to rapidly diffuse into and out of these structures (Fig. S2 G–J). Thus, while clusters of synaptic GABAARs remain stable over time, single receptors can rapidly exchange between synaptic and extrasynaptic locations, providing a potential mechanism for rapid regulation of synaptic GABAAR number.
Ca2+ Influx Through NMDARs Causes Rapid Dispersal of Surface GABAAR Clusters.
To directly examine the influence of neuronal activity on GABAAR clustering in live neurons we investigated the effect of altering excitatory synaptic activity by activating GluRs. Perfusion of 30 μM glutamate and 1 μM glycine for 4 min caused a rapid dispersal of surface α2SEP-GABAAR clusters (Fig. 2 A–D and Movie S3). At t = 9 min, α2SEP-GABAAR cluster intensities were significantly decreased [fluorescence in clusters at t = 9 min normalized to t = 0 (cluster F/F0); control, 0.95 ± 0.02; glut/gly, 0.65 ± 0.05, P = 0.0001]. Loss of clustered α2SEP-GABAAR fluorescence on GluR activation was significantly reduced in zero extracellular Ca2+ (cluster F/F0: 0.87 ± 0.03, P = 0.005) or in the presence of the NMDAR antagonist APV (cluster F/F0: 0.86 ± 0.02, P = 0.0003), confirming that synaptic α2SEP-GABAAR cluster dispersal driven by GluR activation was dependent on extracellular Ca2+ entry via NMDARs (Fig. 2 A–C). Interestingly, GABAAR activation was not required for GluR-dependent α2SEP-GABAAR cluster dispersal, which was also seen in the presence of the GABAAR antagonist bicuculline (100 μM) (cluster F/F0: 0.74 ± 0.04, P = 0.001, Fig. 2C).
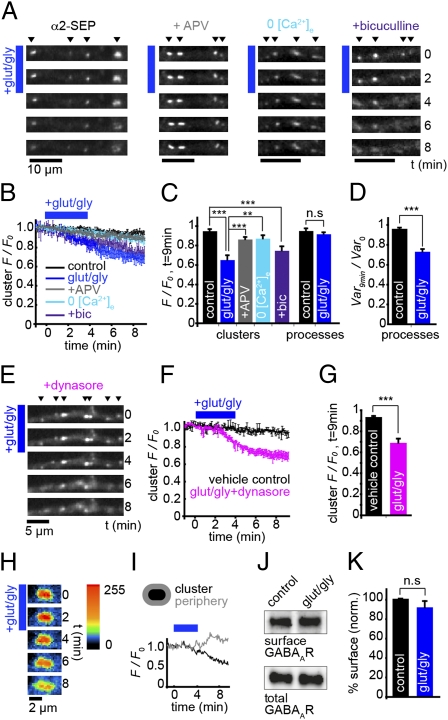
Fig. 2.
GABAAR clusters disperse on GluR activation. (A) α2SEP-GABAAR clusters disperse on GluR activation (blue bar), which requires Ca2+ influx through NMDA receptors but not GABAAR activity. (B) Time course of α2SEP-GABAAR cluster F/F0: control, black, n = 6 cells; glut/gly, dark blue, n = 6; in presence of APV, gray, n = 8; in 0 [Ca2+]e, light blue, n = 5; in presence of bicuculline, purple, n = 4. (C) Summary of cluster F/F0 at t = 9 min after initial GluR activation. Loss of fluorescence in α2SEP-GABAAR clusters on GluR activation is significant compared with control (P = 0.0001) and is significantly blocked in 0 [Ca2+]e (P = 0.005) and by APV (P = 0.0003), but not by bicuculline (P = 0.001 compared with control). Overall fluorescence in processes is not altered by GluR activation (P > 0.05). (D) Variance normalized to t = 0 (Var/Var0) in processes at t = 9 min after initial GluR activation. Variance in pixel intensity is decreased significantly by GluR activation (P = 0.001). (E) α2SEP-GABAAR clusters disperse on GluR activation when endocytosis is blocked. (F) Time course of α2SEP-GABAAR cluster F/F0: vehicle, black, n = 7 cells; glut/gly after pretreatment with dynasore, pink, n = 7. (G) Cluster F/F0 at t = 9 min. Loss of fluorescence in α2SEP-GABAAR clusters is significant compared with vehicle control (P = 3 × 10−5). (H) Close-up of α2SEP-GABAAR dispersal on GluR activation (blue bar). Intensity is represented by a custom look-up table. (I) F/F0 in perisynaptic region surrounding cluster (gray) increases as F/F0 in cluster (black) decreases, indicating receptor dispersal. (J) Blot showing surface biotinylated GABAAR γ2 subunit and total γ2 subunit for control and glut/gly treatment. (K) Surface GABAAR-γ2 fraction is unaffected by glut/gly treatment (91.2 ± 6.2%, P > 0.05, paired t test, n = 4).
In contrast to the decrease in fluorescence of α2SEP-GABAAR clusters, the total fluorescence in dendrites remained unaltered (Fig. 2C, F/F0, control, 0.96 ± 0.01; glut/gly, 0.92 ± 0.01, P > 0.05), suggesting a redistribution of GABAARs at the plasma membrane (i.e., out of clusters and into extrasynaptic regions), rather than their removal from the cell surface. Dynamin and AP2-dependent endocytosis can rapidly modify the number of synaptic GABAARs (21–23) and occurs predominantly at extrasynaptic sites (24). Pretreatment of neurons with the dynamin inhibitor dynasore (10 min, 80 μM) (25, 26) did not prevent the loss of clustered α2SEP-GABAAR fluorescence (Fig. 2 E–G, cluster F/F0: vehicle, 0.93 ± 0.01; dynasore + glut/gly, 0.69 ± 0.04, P = 3 × 10−5), confirming that the observed loss of GABAAR clusters is due to cell surface dispersal of α2SEP-GABAARs and not endocytosis. In agreement with this, α2SEP-GABAAR fluorescence in perisynaptic regions surrounding clusters could be seen to transiently increase on GluR activation (Fig. 2 H and I), and the variance in the distribution of α2SEP-GABAAR fluorescence intensities in neuronal processes at t = 9 min was significantly decreased by GluR activation (normalized to variance at t = 0: control, 0.97 ± 0.03; glut/gly, 0.73 ± 0.03, P = 0.001, Fig. 2D), suggesting a more diffuse GABAAR distribution (cluster dispersal). The above results were confirmed for native receptors as GluR activation did not change the total intensity of native surface GABAARs in dendrites but significantly decreased the variance of pixel intensities in dendrites, suggesting that surface clusters of native GABAARs are also dispersed upon GluR activation (Fig. S3). Moreover, surface biotinylation to directly quantify the effect of GluR activation on native GABAAR levels revealed no change in the surface fraction of the γ2 subunit (glut/gly: 92 ± 6.2% of control, P > 0.05, paired t test, Fig. 2 J and K). These data suggest that GABAAR cluster dispersal upon GluR activation is due to lateral movement of GABAARs out of synaptic clusters and into the extrasynaptic plasma membrane.
Activity-Dependent GABAAR Cluster Dispersal Is Reversible and Can Be Induced by Local, Synaptic Glutamate Release.
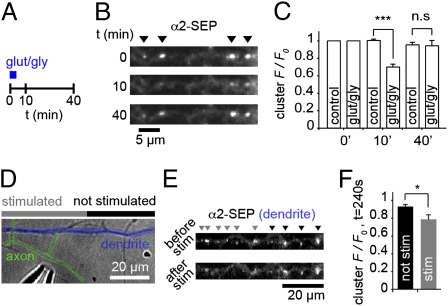
The activity-induced movement of GABAARs out of synapses and into the extrasynaptic space suggests a pool of redistributed surface receptors available for eventual replenishment of synaptic GABAAR content. To further investigate this possibility we imaged α2SEP-GABAAR cluster fluorescence for longer periods after GluR activation to determine whether GABAAR clusters could recover from dispersal (Fig. 3 A–C). GABAAR clusters were dispersed at t = 10 min after initial drug application (cluster F/F0, control, 1.00 ± 0.01; glut/gly, 0.70 ± 0.03, P = 0.001), but 40 min after GluR activation, α2SEP-GABAAR clustering had fully recovered (cluster F/F0, control, 0.95 ± 0.03; glut/gly, 0.94 ± 0.06, P > 0.05, Fig. 3C), suggesting a return of α2SEP-GABAARs back to synapses on longer timescales. Cluster recovery was not due to recycling of receptors that had been internalized upon GluR stimulation, as dynasore treatment did not block the recovery from dispersal (Fig. S4). To explore the possibility that synaptic activation could cause local GABAAR dispersal, we used a patch pipette to locally stimulate axons crossing dendrites expressing α2SEP-GABAARs (Fig. 3D). By maximally stimulating single axons, we found that fluorescence from clusters in stimulated regions (<15 μm from stimulated synapses) was rapidly decreased (at t = 240 s, cluster F/F0 was 0.94 ± 0.02 in unstimulated regions and 0.79 ± 0.05 in stimulated regions, P = 0.014, Fig. 3 E and F, time course in Fig. S5), dependent on action potential firing and GluR activation (Fig. S5). Thus, GABAAR dispersal is reversible and can be triggered by glutamate released from single or a few synapses with important implications for plasticity and control of local dendritic excitability.
Fig. 3.
GABAAR cluster dispersal is reversible and can be induced by local glutamate release. (A) Schematic of recovery experiment. Images were taken at 0, 10, and 40 min. (B) α2SEP-GABAAR clustering can recover after dispersal induced by GluR activation. (C) Cluster F/F0 at t = 0, 10, and 40 min. Loss of α2SEP-GABAAR cluster fluorescence is significant at t = 10 min (P = 0.001) but is not significantly different at t = 40 min (P > 0.05), ncontrol = 5 cells, nglut/gly = 5 cells. (D) DIC image showing local stimulation of axon (colored green) crossing α2SEP-GABAAR containing dendrites (blue), using a glass microelectrode (lower left). (E) α2SEP-GABAAR fluorescence on dendrite in D before and after electrical stimulus (20 Hz, 1 min). Clusters are marked by gray arrows in stimulated regions (within 15 μm of apparent axonal contacts) and by black arrows in unstimulated regions. (F) F/F0 at t = 240 s is significantly reduced in stimulated regions compared with unstimulated regions (P = 0.014, n = 8 cells).
GluR Activation Increases GABAAR Lateral Mobility.
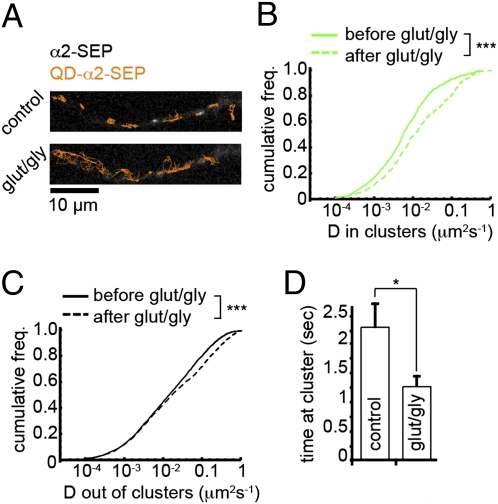
To further explore GluR-driven modulation of GABAAR surface movement we examined the dynamics of single QD-tagged α2SEP-GABAARs, which allowed us to directly monitor the behavior of single GABAARs with respect to the positions of the synaptically localized GABAAR clusters. GluR activation resulted in a significant increase in the surface mobility of GABAARs both inside and outside of α2SEP-GABAAR clusters [median Din increased 1.87-fold from 0.0078 to 0.0146 μm2·s−1, P = 8 × 10−8; median Dout increased 1.16-fold from 0.0192 to 0.0219 μm2·s−1, P = 4 × 10−10, Kolmogorov–Smirnov (K-S) test, Fig. 4 A–C and Movie S4]. Although the mobility of both receptor pools increased, the difference in mobilities of single α2SEP-GABAARs was more striking for receptors within clusters, suggesting that the effect of GluR activation on GABAAR mobility was strongest for receptors at GABAAR cluster locations (inhibitory synapses). Furthermore, GluR activation strongly decreased the average time spent by single QD-labeled GABAARs at GABAAR clusters (control, 2.35 ± 0.41 s; glut/gly, 1.30 ± 0.17 s, P = 0.035, Fig. 4D). Using QD tracking to follow the behavior of single native GABAARs labeled with antibodies to either α2 or β2/β3 GABAAR subunits and 605 nm QDs we found that GABAARs were notably more dynamic after GluR activation (Fig. S6). Median diffusivity increased 1.20-fold for the α2 subunit and 1.27-fold for the β3 subunit (both P < 1 × 10−10, K-S test). These results describe an effect of GluR activation on individual GABAAR mobilities consistent with that of GABAAR dispersal from synaptic clusters.
Fig. 4.
GluR activation increases GABAAR lateral mobility and decreases synaptic residency time. (A) QD-tagged α2SEP-GABAAR trajectories (orange) before and after GluR activation (t = 9 min) overlaid on α2SEP-GABAAR image. (B and C) Instantaneous diffusion coefficients for QD-tagged α2SEP-GABAARs before (solid line) and after (dashed line) GluR activation inside (B) and outside of clusters (C). Mobilities of both pools increased significantly: median Din increased 1.87-fold (nbefore = 598, nafter = 436, P = 8 × 10−8, K-S test); median Dout increased 1.16-fold (nbefore = 3,665, nafter = 2,719, P = 4 × 10−10, K-S test). (D) Mean residency time of QD-tagged α2SEP-GABAARs at clusters is significantly lower after GluR activation. Control, n = 10 cells; glut/gly, n = 6 cells, P = 0.035.
GABAAR Cluster Dispersal and Increased Lateral Diffusion Depend on Activation of Calcineurin and Serine 327 in the GABAAR γ2 Subunit.
To investigate the signaling mechanisms involved in NMDAR-mediated GABAAR cluster dispersal, we asked whether Ca2+ influx through NMDARs could activate the phosphatase calcineurin, which has previously been implicated in activity-dependent down-modulation of synaptic inhibition (3, 27). We found that treating cells with a calcineurin autoinhibitory peptide (50 μM, 30 min) or cyclosporin A (20 μM, 10 min, also added in perfusate) did not affect α2SEP-GABAAR cluster intensity (Fig. S7 A–D), suggesting that calcineurin has no effect on GABAAR clustering under basal conditions on this timescale. However, blocking calcineurin activity with the autoinhibitory peptide inhibited α2SEP-GABAAR cluster dispersal on GluR activation [cluster F/F0, control, 0.92 ± 0.01; glut/gly, 0.71 ± 0.03 (P = 5 × 10−5); treatment, 0.86 ± 0.04, P = 0.018 compared with glut/gly, Fig. 5 A–C] as did cyclosporin A treatment (Fig. S7 E–G). This result suggests that calcineurin activation upon Ca2+influx through NMDARs is directly involved in GABAAR dispersal.
Fig. 5.
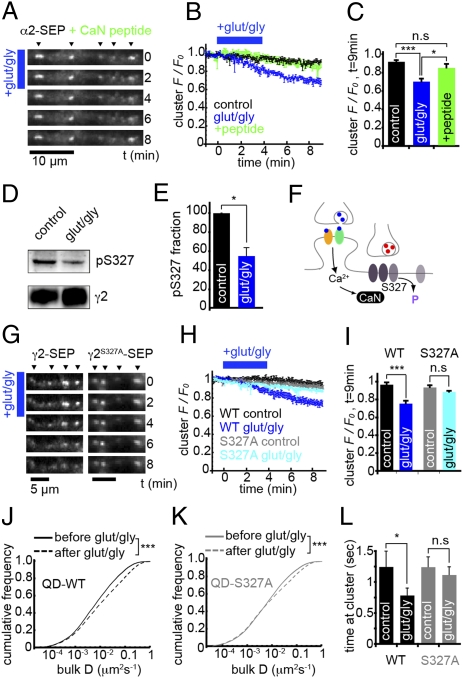
GABAAR dispersal is dependent on calcineurin activity and serine 327 in the γ2 subunit. (A) α2SEP-GABAAR fluorescence on GluR activation after pretreatment with calcineurin autoinhibitory peptide (CaN peptide). (B) Time course of cluster F/F0; control, black, n = 6 cells; vehicle + glut/gly, blue, n = 5; glut/gly + CaN block, green, n = 6. (C) F/F0 in α2SEP-GABAAR clusters at t = 9 min. GABAAR dispersal seen under vehicle + glut/gly (P = 5 × 10−5) was blocked by calcineurin inhibitory peptide (P > 0.05 vs. control, P = 0.018 vs. vehicle + glut/gly). (D) Example blot with pS327 and γ2 antibodies from control and glut/gly conditions. Experiments were performed on hippocampal neurons. (E) Normalized to control, the ratio of pS327/γ2 decreased to 0.55 ± 0.09 on GluR activation; P = 0.035, n = 3. (F) Schematic of hypothesized mechanism of GluR-mediated changes in GABAAR clustering and lateral mobility: dephosphorylation of S327 by calcineurin. (G) WTγ2-SEP and S327Aγ2-SEP fluorescence on GluR activation (blue bar). (H) Time course of cluster F/F0; WT control, black, n = 6 cells; WT glut/gly, blue, n = 8; S327A control, gray, n = 7; S327A glut/gly, light blue, n = 7. (I) Cluster F/F0 at t = 9 min. WTγ2-SEP clusters disperse on GluR activation (P = 6 × 10−5). However, S327Aγ2-SEP clusters were stable on GluR activation (P > 0.05). (J) Instantaneous diffusion coefficients for QD-tagged GABAARs containing WTγ2-SEP before (solid line) and after (dashed line) GluR activation. Median D increased 1.43-fold (P = 2 × 10−16, nbefore = 8,085, nafter = 6,316, K-S test). (K) As in J, for QD-tagged S327Aγ2SEP-GABAARs. Median D increased 1.06-fold (P = 2 × 10−16, nbefore = 8,868, nafter = 7,327, K-S test). (L) Mean residency time of WTγ2SEP-GABAARs at clusters is significantly lower after GluR activation (n = 7 cells, P = 0.04), but not significantly different for S327Aγ2SEP-GABAARs (n = 7 cells, P > 0.05).
A major target site for calcineurin-mediated regulation of GABAARs is serine 327 (S327) on the γ2 subunit, dephosphorylation of which has been previously shown to lead to a reduction in inhibitory postsynaptic current amplitudes (27). We therefore tested whether S327 is important for NMDAR-mediated GABAAR cluster dispersal. By Western blotting with a phospho-specific pS327 antibody (27) we found that GluR activation of hippocampal neurons caused a significant decrease in phosphorylation at S327 (normalized to control, pS327/γ2 ratio = 0.55 ± 0.09, P = 0.035, paired t test, Fig. 5 D and E). Using α2-SEP to report GABAARs as above, we then cotransfected a myc-tagged γ2 GABAAR subunit, either with its S327 phosphorylation site intact (γ2-myc) or with S327 mutated to alanine (γ2S327A-myc). Cotransfection efficiency was found to be ∼90% (Fig. S8 A–C). Neurons cotransfected with γ2-myc exhibited robust α2SEP-GABAAR dispersal on GluR activation, but coexpression of γ2S327A-myc subunits partially blocked cluster dispersal (Fig. S8 D–H). Incomplete blockade of cluster dispersal was likely due to α2-SEP subunits being able to assemble with the pool of endogenous γ2 subunits (with their S327 sites intact); therefore, to directly test the influence of S327 on GABAAR dispersal we imaged GABAAR clusters with wild-type or mutant SEP-tagged γ2 subunits. S327A mutation did not affect the efficient targeting of S327Aγ2SEP-GABAARs to synapses, cluster intensity, or cluster mobility compared with wild type (Fig. S9). However, whereas GABAAR clusters containing WTγ2-SEP subunits were dispersed by GluR activation (cluster F/F0, control, 0.96 ± 0.03; glut/gly, 0.74 ± 0.03, P = 6 × 10−5), GABAAR clusters containing S327Aγ2-SEP subunits remained stable upon GluR activation (cluster F/F0, control, 0.93 ± 0.02; glut/gly, 0.88 ± 0.02, P > 0.05) (Fig. 5 G–I).
We then examined the role of S327 in the γ2 subunit on GABAAR diffusion dynamics in response to increased neuronal activity. By labeling γ2-SEP subunits with quantum dots via a GFP antibody, we were able to track single GABAARs containing either WTγ2-SEP or S327Aγ2-SEP subunits. Under control conditions, GABAARs containing S327Aγ2-SEP subunits were marginally more mobile than WTγ2-SEP containing receptors (median DWT = 0.0161 μm2·s−1, median DS327A = 0.0187 μm2·s−1). On GluR activation, mobilities of WTγ2SEP-GABAARs were much more increased compared with their S327Aγ2SEP-GABAAR mutant counterparts (Fig. 5 J and K; median DS327A increased only 1.06-fold from 0.0187 to 0.0199 μm2·s−1, compared with median DWT that increased 1.43-fold from 0.0161 to 0.0231 μm2·s−1, P = 2 × 10−16, K-S test), suggesting that S327A mutation blocked an activity-induced increase in GABAAR lateral diffusion. Similarly, the residency time of single WTγ2SEP-GABAARs at clusters was significantly decreased by GluR activation (control, 2.29 ± 0.41 s; glut/gly, 1.55 ± 0.20 s, P = 0.04), whereas no significant change was seen for S327Aγ2SEP-GABAARs (control, 2.16 ± 0.51 s; glut/gly, 2.19 ± 0.36 s, P > 0.05) (Fig. 5L). Together with the results above, this finding suggests that calcineurin-dependent dephosphorylation of S327 on the γ2 subunit controls activity-dependent changes in the lateral diffusion and clustering of surface GABAARs.
Discussion
We have investigated the surface behavior of single GABAARs and GABAAR clusters under resting conditions and during increased neuronal activity. Our work provides several important insights into the molecular mechanisms that underlie activity dependence of GABAAR lateral diffusion and clustering. We show that synaptic GABAAR clusters are stable under resting conditions whereas single GABAARs can be highly mobile. NMDAR activation can cause a rapid increase in surface GABAAR mobility and a reversible and local dispersal of synaptic GABAARs into the extrasynaptic membrane. This mechanism for fast modulation of surface GABAAR mobility depends on the activity of the phosphatase calcineurin and S327 within the intracellular loop of the γ2 subunit. Our results suggest a cellular mechanism through which GluR signaling can regulate synaptic GABAAR accumulation on rapid timescales.
Imaging endogenous QD-labeled GABAARs does not allow simultaneous visualization of receptor clusters at synapses, and direct imaging of GABAAR clusters and single receptors has not yet been reported. Simultaneously visualizing α2SEP-GABAAR clusters via SEP fluorescence and single α2SEP-GABAARs labeled with QDs allowed us to image the behavior of individual receptors compared with synaptic GABAAR clusters. Using this approach we found that single α2SEP-GABAARs can be highly dynamic, both inside and outside clusters, and can also rapidly exchange into and out of synaptic GABAAR clusters. Similar results were also obtained imaging GABAARs via a SEP-tagged γ2 subunit. The dynamic nature of single SEP-tagged GABAARs was similar to that of endogenous QD-labeled GABAARs. These observations are in agreement with other recent reports showing that GABAARs can be highly dynamic in the plasma membrane (12, 28) and are in line with previous observations of bulk exchange of receptors between synaptic and extrasynaptic locations determined using electrophysiological tagging or fluorescence recovery after photobleaching approaches (10, 11). In contrast to single receptors, GABAAR clusters were stable for prolonged periods under basal conditions. We did, however, observe small asynchronous oscillatory movements of entire receptor clusters, but these movements were ≈50-fold slower than those of single receptors. The small oscillatory movements of α2SEP-GABAAR clusters are similar to those previously reported for the inhibitory scaffold gephyrin (18), suggesting that the entire inhibitory postsynaptic apparatus (receptor and scaffold) may move together. This observation fits well with the recent observation that GABAAR α2 subunits interact directly with gephyrin (15) and suggests a tight coupling of the receptor aggregate and scaffold in live cells.
Live cell imaging of SEP-GABAAR clusters allowed us to directly visualize in real time the role played by neuronal activity in regulating the stability of synaptic GABAAR clusters. Ca2+ entry through NMDARs caused a rapid dispersal of GABAAR clusters without affecting total surface receptor fluorescence, suggesting that dispersal was due to a redistribution of GABAARs out of clusters and into the extrasynaptic membrane. In agreement with this, blocking GABAAR endocytosis, which is dynamin dependent (21), with the dynamin inhibitor dynasore did not block GABAAR cluster dispersal. GluR-dependent dispersal of native synaptic GABAARs without surface down-modulation was also observed. Moreover, GluR-dependent cluster dispersal correlated with an increase in the lateral mobility of endogenous GABAARs and both α2SEP-GABAARs and γ2SEP-GABAARs as determined using QD tracking. Our results are in line with other recent observations demonstrating that increased neuronal activity generated with the potassium channel blocker 4-AP also leads to an increase in GABAAR mobilities in dendrites (28).
Dependent on the levels of postsynaptic Ca2+influx, GluR activation can cause either an increase or a decrease in the number or activity of synaptic GABAARs, via activation of Ca2+-dependent kinases or phosphatases, respectively (2, 27–29). Calcineurin is known to dephosphorylate GABAARs on influx of Ca2+ through NMDARs (27). In support of a key role for calcineurin in regulating synaptic inhibition via GABAAR trafficking, we found that this phosphatase underlies the NMDAR-mediated dispersal of GABAARs from inhibitory synaptic clusters. Interestingly, calcineurin activity was found to have little influence on the stability or intensity of GABAAR clusters under resting conditions, in agreement with reports suggesting calcineurin is recruited to GABAARs only on increased neuronal activity (27).
Whether changes in GABAAR phosphorylation state could influence GABAAR lateral mobility was unknown. Importantly, we found that activity-dependent GABAAR cluster dispersal and increased lateral mobility were dependent on a well-established GABAAR phosphorylation site and known target for calcineurin-mediated dephosphorylation (27). GluR activation reduced the phosphorylation of the native γ2 subunit at S327, and mutation of S327 to alanine (S327A) blocked GluR-dependent GABAAR cluster dispersal. Moreover, by QD tracking GABAARs containing mutant γ2 subunits, we found that S327 directly controlled the GluR-dependent increase in GABAAR lateral mobility and parallel decrease in GABAAR residency time at synaptic clusters. These results suggest the possibility of an active release mechanism of GABAARs from synapses due to S327 dephosphorylation. An interesting topic for future study will be to determine if there is also an activity dependence to GABAAR lateral mobility in receptors containing nonsynaptic subunit combinations that contribute to tonic conductances (e.g., α5- or δ-containing GABAARs).
NMDAR-dependent synaptic GABAAR cluster dispersal has important implications for information processing and is likely to underlie the long-term depression of inhibitory synaptic strength reported to occur during long-term potentiation at excitatory synapses (3, 27). Importantly, imaging SEP-GABAARs for longer periods following the activation of GluRs revealed that NMDAR-mediated GABAAR dispersal is reversible because recovery of cluster fluorescence could be observed on longer (40 min) timescales. Return of receptors on this timescale fits well with the idea that whereas transient GABAAR dispersal leading to reduced inhibition would favor long-term potentiation at excitatory synapses and coupling of increased excitability to increased action potential firing (E-S coupling) (3), an eventual “homeostatic” return of GABAARs back to synapses would prevent a decrease in synaptic inhibition that may lead to runaway excitation and the generation of seizures. We also demonstrate that GABAAR dispersal can act locally when only a few glutamatergic synapses are activated. Thus synaptic GABAAR cluster dispersal could act to locally enhance dendritic branch excitability and facilitate interactions between neighboring excitatory synapses, with important implications for clustered plasticity and information storage in dendrites (30, 31).
Our findings are also likely to be relevant to neurological disease where prolonged pathological glutamate release, as occurs in epilepsy or stroke, could cause a more sustained shift of GABAARs out of synapses and sustained dispersal. This would lead to disinhibition and facilitation of runaway excitation, contributing to neuronal excitotoxicity and disrupted information processing (32, 33). Indeed, NMDAR-mediated dispersal of GABAARs out of synapses and into extrasynaptic locations may be the first step that occurs before the activity-dependent down-modulation of surface GABAARs that leads to the generation of self-sustaining seizures in status epilepticus (5, 34). Thus, targeting calcineurin and S327-mediated synaptic GABAAR dispersal could also provide a unique therapeutic target for pathological disinhibition in diseases such as epilepsy.
Materials and Methods
Constructs.
The N-terminally tagged α2-SEP DNA was a kind gift from S. Moss (Tufts University, Cambridge, MA) and has been described previously (15). For details of construction of mutants, see SI Materials and Methods.
Biochemistry.
Cell Culture and Transfections.
Rat hippocampal neurons were prepared and cultured from embryonic day 18 rat brains. Cells were transfected by Amaxa nucleofection as previously described (35). See SI Materials and Methods.
Live Cell Imaging.
Imaging media used for all experiments contained 125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, and 10 mM Hepes and was adjusted to pH 7.4 with NaOH before use. Cells were imaged under perfusion (4 mL/min) and heating (35–37 °C). See SI Materials and Methods.
Fixed Cell Imaging.
Image Analysis.
Images were analyzed in ImageJ. QD tracking was performed using detection and tracking algorithms written in Mathematica (Wolfram Research). See SI Materials and Methods.
Local Stimulation.
Local electrical stimulation of axons was made using a stimulating electrode via a patch pipette (resistance ∼1 MΩ) filled with imaging media (36). See SI Materials and Methods.
Statistical Analysis.
All experiments were performed on neurons from at least three individual preparations. Unless otherwise stated, P values given are from two-tailed t tests (type 2) and values are given as mean ± SEM. Error bars represent SEM.
Supplementary Material
Acknowledgments
This work was supported by the United Kingdom Medical Research Council (J.T.K.). J.M. is in the Centre for Mathematics and Physics in the Life Sciences and Experimental Biology Ph.D. Program at University College London.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000589107/-/DCSupplemental.
References
- 1.Arancibia-Cárcamo IL, Kittler JT. Regulation of GABA(A) receptor membrane trafficking and synaptic localization. Pharmacol Ther. 2009;123:17–31. doi: 10.1016/j.pharmthera.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Stelzer A, Slater NT, ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- 3.Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- 4.Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: Mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 5.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: Implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 7.Vithlani M, Moss SJ. The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition. Biochem Soc Trans. 2009;37:1355–1358. doi: 10.1042/BST0371355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tretter V, et al. Deficits in spatial memory correlate with modified γ-aminobutyric acid type A receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci USA. 2009;106:20039–20044. doi: 10.1073/pnas.0908840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, et al. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 10.Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- 11.Jacob TC, et al. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lévi S, et al. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron. 2008;59:261–273. doi: 10.1016/j.neuron.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the beta 1, gamma 2S, and gamma 2L subunits of the gamma-aminobutyric acid type A receptor. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- 15.Tretter V, et al. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannenberg K, Sieghart W, Reuter H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. Eur J Neurosci. 1999;11:1256–1264. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 17.Arancibia-Cárcamo IL, et al. Ubiquitin-dependent lysosomal targeting of GABA(A) receptors regulates neuronal inhibition. Proc Natl Acad Sci USA. 2009;106:17552–17557. doi: 10.1073/pnas.0905502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanus C, Ehrensperger MV, Triller A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci. 2006;26:4586–4595. doi: 10.1523/JNEUROSCI.5123-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahan M, et al. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 20.Groc L, et al. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 21.Kittler JT, et al. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kittler JT, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kittler JT, et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci USA. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogdanov Y, et al. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, et al. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannai H, et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 32.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 33.Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci. 2009;12:483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twelvetrees AE, et al. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macaskill AF, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.