Abstract
Background
Epidemiologic studies have reported an association between self-reported short sleep duration and high blood pressure. Our objective was to examine both cross-sectional and longitudinal associations between objectively-measured sleep and blood pressure.
Methods
This is an ancillary study to the Coronary Artery Risk Development in Young Adults (CARDIA) cohort study. Blood pressure was measured in 2000-2001 and 2005-2006. Sleep was measured using wrist actigraphy for 3 consecutive days twice between 2003 and 2005. Sleep duration and sleep maintenance (a component of sleep quality) were calculated. Analyses included 578 African-Americans and whites aged 33 to 45 years at baseline. Outcome measures were systolic and diastolic blood pressure, 5-year change in blood pressure and incident hypertension.
Results
After excluding those taking anti-hypertensive medications and adjusting for age, race and sex, shorter sleep duration and lower sleep maintenance significantly predicted higher systolic and diastolic blood pressure cross-sectionally and more adverse changes in systolic and diastolic blood pressure over five years (all p<.05). Short sleep duration also significantly predicted increased odds of incident hypertension (OR 1.37, 95% CI: 1.05, 1.78). Adjustment for 16 additional covariates, including snoring and daytime sleepiness, slightly attenuated the associations between sleep and blood pressure. Sleep duration appeared to mediate the difference between African Americans and Whites in diastolic blood pressure change over time (p=.02).
Conclusions
Reduced sleep duration and consolidation predicted higher blood pressure levels and adverse changes in blood pressure, suggesting the need for studies of whether interventions to optimize sleep may reduce blood pressure.
Nearly one-third of Americans have high blood pressure,1 and worldwide 7 million deaths are attributed to high blood pressure each year.2 Recently, two large epidemiologic studies reported an association between self-reported sleep duration and the prevalence or incidence of hypertension.3, 4 Identifying a novel lifestyle risk factor for high blood pressure could lead to new interventions to prevent or reduce high blood pressure.
Laboratory studies of short-term sleep deprivation have suggested potential mechanisms for a causal link between sleep loss and hypertension. Partial sleep deprivation is associated with increased sympathetic activity estimated from measures of heart rate variability.5, 6 Other studies have observed increased blood pressure after a night of partial 7, 8 or total sleep deprivation.9 Thus, sleep loss may lead to increased sympathetic nervous activity which could cause high blood pressure if sleep loss were chronic.
On a population level, previous epidemiologic studies have observed associations between shorter sleep duration and increased blood pressure.3, 4, 10-13 However, most of these studies were cross-sectional and relied on self-reported usual sleep duration, which is only moderately correlated with objectively-measured sleep duration.14, 15 Furthermore, the possible role of sleep quality independent of sleep duration as a risk factor for high blood pressure has not been explored in adults. The goal of our study was to determine if objectively-measured sleep duration or quality predicted 5-year incidence of hypertension and changes in systolic and diastolic blood pressure in a community-based sample of persons in early middle age.
Methods
This is an ancillary sleep study to a large, on-going cohort study, the Coronary Artery Risk Development in Young Adults (CARDIA) Study. In 1985-1986, CARDIA recruited African-American and white adults aged 18-30 years from four sites in the U.S. Our ancillary study included persons examined at the Chicago site. We invited those who were not pregnant at the CARDIA year 15 examination in 2000-2001 (total eligible was 814) to participate and 670 consented. Participants and non-participants did not differ with respect to self-reported sleep quality or quantity at the year 15 examination.14 The protocol was approved by the institutional review boards of Northwestern University and the University of Chicago and by the CARDIA steering committee, and written informed consent was obtained from each participant.
In 2000-2001, as part of the year 15 CARDIA examination (termed “baseline” in this report), blood pressure was measured along with other clinical, demographic and health variables. The CARDIA clinical examination included self-reported questions about sleep duration and quality. In 2003-2005, sleep measures were collected in two waves approximately one year apart using wrist actigraphy and surveys. After actigraphy data collection was completed, blood pressure, demographic and self-reported sleep covariates were again measured during the year 20 CARDIA examination in 2005-2006 (termed “follow-up” in this report). Since the actigraphy data collection was closer to the follow-up than the baseline; our primary cross-sectional analysis use blood pressure and covariate data from the follow-up examination. Longitudinal analyses use data from both the baseline and follow-up examinations.
The 670 participants did not differ significantly from the 144 eligible non-participants in self-reported sleep duration, sleep quality or blood pressure measurements.
Outcome Measures
For participants not taking anti-hypertensive medication at either clinical examination, we examined the 5-year change in systolic blood pressure (SBP) and diastolic blood pressure (DBP). Blood pressure was measured three times by trained and certified technicians using standardized methods after the participant rested for five minutes. The last two measurements of blood pressure were averaged for analyses. At the baseline examination, Hawksley random zero sphygmomanometers were used and at follow-up blood pressure was measured using OmROn HEM907XL instruments. A calibration study was performed and we used calibrated values for the follow-up measurements to assure comparability. In those without hypertension at baseline, we examined incident hypertension at follow-up, defined as a SBP ≥140 mmHg, or DBP ≥ 90 mmHg or taking anti-hypertensive medications.
Sleep Measures
Sleep measurements were collected between 2003 and 2005. Participants were asked to wear a wrist activity monitor (Actiwatch-16, Mini-Mitter Inc, Bend, OR) for three consecutive days on two occasions approximately one year apart. The wrist activity monitors contain highly sensitive omnidirectional accelerometers that counted wrist movements in 30-second epochs. Wrist actigraphy has been validated against polysomnography, demonstrating a correlation over 0.9 in healthy subjects.16 Unlike polysomnography, actigraphy does not appear to alter sleep behavior, as there is no “first night effect”.17 Validated computer software was used to calculate sleep variables, which include sleep duration and sleep maintenance as described below.
Sleep duration
This is the amount of time between sleep onset and final morning awakening minus the total duration of all awakenings after sleep onset.
Sleep maintenance
This index assesses sleep consolidation, a component of sleep quality and is the percentage of time between initial sleep onset and final waking that is spent sleeping.
For most participants (91%), there were six days of actigraphy recording. The remaining 9% contributed 1-5 days of actigraphy recording. Analyses restricted to those with 6 days of recordings produced similar results as analyses including all subjects for whom at least one day of actigraphy was obtained. For each sleep measure we used the average of all days of actigraphy recording was used.
Covariates
Sociodemographic covariates included in all analyses were race, sex and age at baseline. Baseline income (7-level ordinal variable ranging from <$16,000 to ≥$100,000) and number of years of education (ranging from 4 to 20 years) were also included in the full models.
Other covariates were body mass index (BMI), smoking, alcohol use, and physical activity. For each of these, both baseline status and 5-year change were included in the full models. BMI was calculated from measured height and weight and was analyzed as a continuous variable. Anti-hypertensive medication use, current smoking (yes/no), alcohol use and physical activity were all determined from interview questions at the baseline and follow-up exams. Alcohol use was the average number of drinks per week of wine, liquor and beer. The CARDIA Physical Activity History included questions about 13 categories of sports and exercise over the past 12 months, and was used to determine a total physical activity score in exercise units.18
Risk of sleep apnea was defined based on the Berlin Sleep Apnea Questionnaire.19 The full questionnaire includes three components and a high risk of sleep apnea is defined by the presence of any two of the three components: (1) persistent snoring symptoms, (2) persistent daytime sleepiness and (3) obesity or hypertension. A participant was considered to have persistent snoring symptoms if he/she indicated two of three following conditions on this questionnaire: (1) snored 3 or more times per week, (2) snoring was louder than talking or very loud; (3) experienced breathing pauses 3 or more times per week. A participant was considered to have persistent daytime sleepiness if he/she indicated two of three following condition on this questionnaire: (1) was tired after sleeping 3 or more times per week; (2) was tired during wake time 3 or more times per week; (3) has fallen asleep while driving. Because we were already including measured BMI and change in BMI as covariates and because blood pressure was our outcome, we did not use the summary apnea risk score. Instead the sleepiness and snoring components of apnea risk were each included in the full models.
Statistical Analyses
Linear regression models were used to examine the cross-sectional association between objective sleep and blood pressure at the follow-up examination after adjusting for age, race and sex and excluding those on anti-hypertensive medication. Linear regression models were also used to predict the 5-year change in SBP and in DBP while controlling for age, race, sex and baseline blood pressure, excluding participants taking anti-hypertensive medications at either baseline or follow-up. Modeling the follow-up blood pressure and including baseline blood pressure as a covariate is exactly the same as modeling the 5-year change in blood pressure and adjusting for baseline blood pressure. Then, among those without hypertension at baseline, logistic regression analyses were used to predict incident hypertension as a function of sleep adjusting for age, race and sex. Finally, all of the covariates were added to the linear and logistic regression models. We also tested whether the associations between sleep and blood pressure varied by race or sex by adding interaction terms between race or sex and the sleep measure to the models. We further examined whether the association between snoring and blood pressure varied by sex by introducing an interaction term between sex and snoring to the sleep duration models. Variables were centered at their means. Separate models were estimated for sleep duration and sleep maintenance. The robust variance estimator was used in linear regression models to calculate confidence intervals and p values. In regression models, sleep duration and sleep maintenance were entered as continuous variables. For data presentation, sleep duration was divided into five categories: <4 hours, 4-<5 hours, 5-<6 hours, 6-<7 hours and ≥7 hours, and sleep maintenance was divided into five categories: <80%, 80-<85%, 85-<90%, 90-<95%, ≥95%. In regression models, sleep duration and sleep maintenance were entered as continuous variables.
We also explored whether the race-sex differences in 5-year change in SBP and DBP were mediated by race-sex differences in sleep duration. To do this, we estimated the percentage of the mean 5-year change in SDP and DBP for African-American men, African-American women and white men relative to white women that was eliminated by adding sleep duration to the models.20 In order to determine if the effect of adding sleep duration to the models had a significant effect on coefficients of the race-sex groups, we modified the approach suggested by Lin, Fleming and DeGruttola21 for application to linear regression. This approach involves doubling the data by making a copy of each observation and running a joint model that estimates the race effect both with and without adjusting for sleep. The joint model permits testing whether the race effect differs after adjusting for sleep by using robust variance-covariance estimation clustering on the individual identification numbers. We used a 3 degree of freedom chi-square test for any mediation across the 4 race-sex groups.
All statistical analyses were preformed using Stata 9.2 (StataCorp, College Station, TX).
Results
In the cross-sectional analysis, we excluded those who were missing valid actigraphy data (n=3), those who were on anti-hypertensive medication at baseline (n=45), those who were missing a baseline measure of diastolic blood pressure (n=43), and those who were missing a follow-up measure of diastolic blood pressure (n=1). This resulted in a final sample size of 578. In the longitudinal analysis predicting change in blood pressure, we further excluded those who were on anti-hypertensive medication at follow-up (n=73), which left 505 participants for these analyses. Of the 667 participants with actigraphy data, 53 participants were missing data for hypertension status at years 15 and/or 20, and an additional 69 participants had hypertension in year 15, which resulted in a sample size of 535 participants for the incident hypertension analyses.
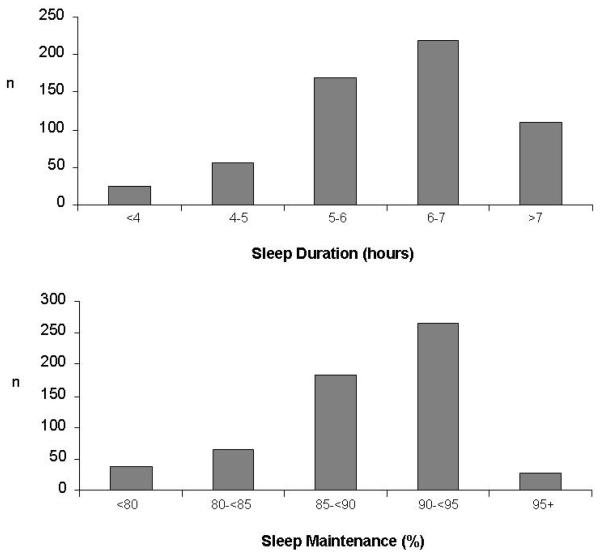
The cross-sectional sample (n=578) included 93 (16%) African-American men, 146 (25%) African-American women, 159 (28%) white men and 180 (31%) white women. Mean ages at baseline was 40.1 ±3.6 years. Table 1 presents summary statistics for key variables. On average, participants slept 6 hours and were awake approximately 11% of the time after falling asleep. The distributions of sleep duration and sleep maintenance are illustrated in Figure 1. Almost half of the sample (43%) slept less than 6 hours per night. Only 7 participants (1%) averaged 8 or more hours of sleep. Over five years, on average, systolic blood pressure increased and diastolic blood pressure decreased. Of those without hypertension at baseline, 14% (75/535) of the full sample developed hypertension. Seventy-five participants (14%) in the full sample reported persistent snoring symptoms and 183 (32%) reported daytime sleepiness. Snoring was more common in men than women (17% of men vs. 11% of women), in African-Americans than whites (17% of African-Americans vs. 11% of whites), and in the obese (BMI≥30kg/m2) than in the non obese (20% in the obese vs. 10% in the non-obese).
Table 1.
Means (standard deviation) or proportions (%) of key variables excluding those taking anti-hypertensive medications at baseline (n=578).
| Variable | CARDIA EXAM Baseline 2000-2001 |
Sleep Assessment 2003-2005 |
CARDIA EXAM Follow-up 2005-2006 |
5-year change |
|---|---|---|---|---|
| Systolic Blood Pressure (mm/Hg) |
110.1 (13.1) | 114.4 (14.4) | +4.3 (11.7) | |
| Diastolic Blood Pressure (mm/Hg) |
73.7 (10.1) | 71.1 (11.4) | −2.6 (9.8) | |
| Body Mass Index (kg/m2) |
27.9 (6.3) | 28.7 (6.7) | 0.7 (2.4) | |
| Sleep Duration (h) | 6.1 (1.1) | |||
| Sleep Maintenance (%) |
88.8 (5.5) |
Figure 1.
Distribution of participants in sleep duration and sleep maintenance categories (n=578).
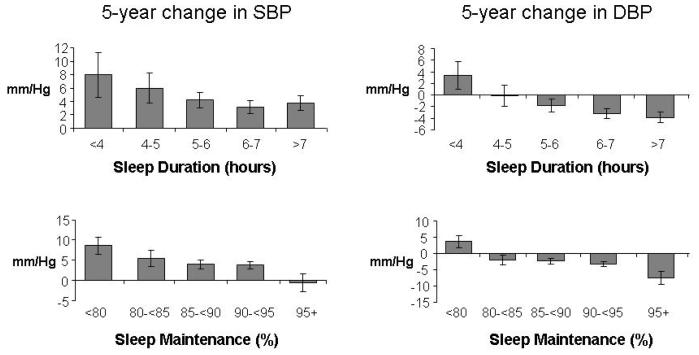
Table 2 presents the results from linear regression models used to examine both cross-sectional and longitudinal associations between sleep and blood pressure after adjusting for age, race and sex. Figure 2 illustrates the longitudinal associations between the adjusted 5-year change in SBP and DBP by sleep duration and sleep maintenance categories. Shorter sleep duration and lower sleep maintenance were both significantly associated with higher systolic and diastolic blood pressure at year 20 as well as smaller decreases or even increases in systolic and diastolic blood pressure over the five-year period.
Table 2.
Cross-sectional and longitudinal associations from linear regression models predicting SBP and DBP (n=505).
| Cross-sectional | Longitudinal a | |||||||
|---|---|---|---|---|---|---|---|---|
| Year 20 SBP | Year 20 DBP | 5-year Change in SBP | 5-year Change in DBP | |||||
| Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
|
| Sleep Duration (effect per hour) |
−1.80 mmHg | −3.07, −0.52 (p=.006) |
−1.70 mmHg | −2.72, −0.68 (p=.001) |
−1.26 mmHg | −2.34, −0.17 (p=.023) |
−1.56 mmHg | −2.41, −0.71 (p<.001) |
| Sleep Maintenance (effect per 10%) |
−4.30 mmHg | −6.70, −1.89 (p<.001) |
−4.56 mmHg | −6.26, −2.87 (p<.001) |
−3.09 mmHg | −5.10, −1.07 (p=.003) |
−3.88 mmHg | −5.31, −2.44 (p<.001) |
Covariates included age and race-sex groups, and excluded those taking antihypertensive medications at follow-up for the cross-sectional analysis and at both baseline and follow-up for the change models
Model also adjusts for baseline blood pressure
Figure 2.
Predicted 5-year change in SBP and DBP by sleep duration and sleep maintenance from regression analyses adjusting for age and race-sex and excluding those taking anti-hypertensive medication at either baseline or follow-up (n=505). Error bars represent the standard error of the predicted change (the beta coefficient).
In logistic regression models predicting incident hypertension, short sleep duration was significantly associated with increased odds of hypertension (OR 1.37, 95% CI: 1.05, 1.78) adjusting for age and race-sex groups. Each hour reduction in sleep duration was associated with a 37% increase in odds of incident hypertension. Sleep maintenance was not significantly associated with incident hypertension (OR 0.77 per 10% of maintenance, 95% CI: 0.50, 1.20).
In the fully adjusted models (Table 3), the associations between sleep duration and blood pressure were reduced, however, sleep duration was still significantly negatively associated with 5-year change in DBP (p=.03). The associations between sleep maintenance and blood pressure were also somewhat reduced but remained significant for all models. The association between short sleep duration and odds of incident hypertension in the fully adjusted logistic regression model was also attenuated (OR=1.30, 95% CI: 0.96, 1.75). Finally, the interaction terms between race or sex and sleep duration or maintenance were not significant, indicating that the associations between sleep and blood pressure did not vary by race or by sex. The interaction term between sex and snoring was not significant in the cross-sectional or longitudinal models predicting SBP or DBP, however, it was significant in the logistic model predicting hypertension (p=.015). When stratifying by sex, snoring did not significantly predict incident hypertension in men (OR 0.75, 95% CI: 0.22, 2.57), but it did in women (OR 4.59, 95% CI: 1.95, 10.76).
Table 3.
Fully adjusted linear regression models predicting SBP and DBP.
| Cross-sectional | Longitudinal a | |||||||
|---|---|---|---|---|---|---|---|---|
| Year 20 SBP | Year 20 DBP | 5-year Change in SBP | 5-year Change in DBP | |||||
| n | 492 | 491 | 491 | 491 | ||||
| Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
Regression coefficient |
95% CI (p value) |
|
| Sleep Duration (effect per hour) |
−1.18 mmHg |
2.44, 0.08 (p=.066) |
−0.86 mmHg |
1.87, 0.15 (p=.094) |
−0.91 mmHg |
1.96, 0.14 (p=.088) |
−0.90 mmHg |
−1.74, −0.07 (p=.034) |
| Sleep Maintenance (effect per 10%) |
−2.66 mmHg |
−5.19, −0.13 (p=.039) |
−2.70 mmHg |
4.45, −0.94 (p=.003) |
−2.56 mmHg |
4.57, −0.55 (p=.013) |
−2.67 mmHg |
4.12, −1.22 (p<.001) |
Covariates included age, race, sex, snoring, daytime sleepiness, income, education, smoking, BMI, physical activity, alcohol, and 5-year change in smoking, alcohol, BMI and physical activity, and excluded those taking antihypertensive medications at follow-up for the cross-sectional analysis and at both baseline and follow-up for the change models
Model also adjusts for baseline blood pressure
There were significant race-sex differences in 5-year changes in SBP and DBP (Table 4). We explored whether these differences were partly explained by differences in sleep duration. White women were chosen as the reference group because they had the smallest average increase in SBP and the longest average sleep duration relative to the other three race-sex groups. On average their SBP increased by 3.61 mm/Hg and their DBP decreased by 3.14 mm/Hg over 5 years. Compared to white women, white men and African-American men experienced significantly greater increases in SBP and African-American men and women experienced significantly smaller decreases in DBP over 5 years (Table 4). To determine whether sleep duration mediated the association between race-sex and changes in blood pressure, we compared two linear regression models: one model predicted change in blood pressure from race-sex group only adjusting for age and the second model added sleep duration as a predictor (Table 4). For African-American men, the change in SBP relative to that in white women was reduced by 36% and the change in DBP again relative to that in white women was reduced by 84% when sleep duration was included in the model. For white men, the change in SBP relative to white women was reduced by 21% and for African-American women the change in DBP relative to white women was reduced by 37% when sleep duration was included. The overall mediation effect of including sleep duration on the coefficients of the race-sex groups was statistically significant for the change in DBP (p=.02) but not SBP (p=.38). When we compared models that included all covariates (age, income, education, smoking, 5-year change in smoking, BMI, 5-year change in BMI, physical activity, 5-year change in physical activity, alcohol use, 5-year change in alcohol use, persistent snoring symptoms, and persistent daytime sleepiness), the effect of adding sleep duration was similar. These results suggest that sleep duration partially mediates the larger increases in blood pressure that is associated with African-American race or male sex, particularly for diastolic blood pressure.
Table 4.
Proportion (%) of race-sex effect on 5-year change in SBP (n=506) and DBP (n=505) associated with differences in sleep duration.
| Sleep Duration (hours) |
5- year Change in SBP (95% CI) | 5- year Change in DBP (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Not adjusted for sleep duration |
Adjusted for sleep duration |
Effect of adjusting for sleep (%) * |
Not adjusted for sleep duration |
Adjusted for sleep duration |
Effect of adjusting for sleep (%) * |
|
| African- American Men |
5.2 (1.1) |
+4.45 mm/Hg (1.59, 7.32) |
+2.87 mm/Hg (−0.41, 6.16) |
−36% |
+2.70 mm/Hg (0.32, 5.08) |
+0.43 mm/Hg (−2.28, 3.14) |
−84% |
| African- American Women |
5.9 (0.8) | +1.25 mm/Hg (−1.54, 4.04) |
+0.36 mm/Hg (−2.49, 3.22) |
+3.48 mm/Hg (1.15, 5.80) |
+2.20 mm/Hg (−0.19, 4.59) |
−37% | |
| White Men | 6.1 (0.9) |
+2.85 mm/Hg (0.56, 5.15) |
+2.26 mm/Hg (−0.04, 4.55) |
−21% | −0.36 mm/Hg (−2.43, 1.71) |
−1.22 mm/Hg (−3.29, 0.86) |
|
| White Women | 6.7 (0.8) | reference | reference | reference | reference | ||
|
3 df Chi- square test for mediation |
χ2=1.02 | p=.38 | χ2=3.16 | p=.02 | |||
Percent of excess change in blood pressure relative to white women accounted for by adding sleep duration. This is only calculated when the 5-year change in blood pressure is significantly different (p<.05) from the reference group (white women).
Bold indicates statistical significance (p<.05).
Participants taking anti-hypertensive medication at baseline or follow-up are excluded.
Comment
Among this sample of early middle-aged adults, shorter objectively-measured sleep duration or lower sleep maintenance (one component of sleep quality) predicted higher levels of systolic and diastolic blood pressure as well as greater increases in systolic blood pressure and smaller decreases in diastolic blood pressure over five years. Sleep duration predicted increased odds of incident hypertension after adjustment for age, race and sex. Inclusion of numerous socioeconomic, health and sleep-related covariates weakened some of the associations between sleep and blood pressure, however most remained statistically significant. When examined separately, no single covariate had a strong effect, but all of them considered together did attenuate the associations. Because the mechanisms underlying the relationship between sleep and blood pressure regulation are not fully understood, some of these covariates may not be confounders but rather mediating variables on the causal pathway between sleep and increased blood pressure.
Consistent with other studies,22, 23 we observed higher blood pressure in men, particularly African-American men. Also, as described in a previous report from this study, African-American men slept much less than white women.14 These two observations suggested the intriguing possibility that the well-documented higher blood pressure in African-Americans and men might be partly related to sleep duration. Indeed, our results suggest that sleep duration did partially mediate the excess increase in blood pressure associated with being male and being African-American relative to white women. As previously reported, the race-sex differences in sleep duration persist after controlling for numerous sociodemographic confounders, including income, education, employment status, marital status and presence of children.14 Thus, the present study revealed shorter sleep as a previously unrecognized potential mechanism that may underlie blood pressure disparities associated with African-American race.
In secondary analyses, we found that self-reported frequent snoring was associated with increased odds of incident hypertension in women but not men. These sex differences could reflect true differential risk of hypertension associated with snoring between men and women. Alternatively, a greater proportion of men who report frequent snoring may not have obstructive sleep apnea compared to women who report frequent snoring, and/or that women who report frequent snoring have more severe sleep apnea than do men who report snoring.
These observed associations between objectively-measured sleep duration and blood pressure change are consistent with some recent epidemiologic studies that have found associations between subjective measures of sleep duration and hypertension. The Sleep Heart Health Study observed a U-shaped cross-sectional association between self-reported sleep duration and prevalent hypertension: the odds ratio for prevalent hypertension was 1.66 for those reporting sleeping <6 hours per night and 1.30 for 9 or more hours relative to 7-<8 hours.4 A French cross-sectional study observed a significant negative association between self-reported sleep time and DBP but not SBP after adjustment for covariates.10 A recent study of Japanese women also reported a cross-sectional association between self-reported poor sleep and increased DBP but not SBP.11 Our study also found that sleep duration was more weakly associated with change in SBP than change in DBP when adjusting for all covariates. Two studies also examined blood pressure prospectively. The NHANES I Epidemiologic follow-up observed that adults aged 32-59 years who reported sleeping 5 hours or less per night had an adjusted hazard ratio of 1.60 for the development of hypertension over 8-10 years relative to those sleeping 7-8 hours.3 Data from the Whitehall II Study found associations between short sleep duration and increased incident hypertension among women only.12 However, our analyses did not find a gender difference in the association between sleep and blood pressure. Finally, two studies among elderly adults, one in the Netherlands and one in Brazil, found no association between sleep duration and blood pressure, 24, 25 which suggests that effect of sleep on blood pressure may be age dependent. Taken together, these studies along with the results of our study support the hypothesis that short sleep duration is associated with higher blood pressure in young to middle-aged adults. One other study used wrist actigraphy in a sample of adolescents and found that low sleep efficiency was associated with increased prevalence of prehypertension (defined as SBP or DBP ≥90th percentile for age, sex and height).26 However, the present study is the first to obtain objective measures of sleep duration and quality in a large sample of adults and to reveal associations between poor sleep quality and adverse effect on blood pressure regulation and the risk of hypertension in early middle-aged adults.
Strengths of our study include the longitudinal measures of blood pressure and the objective measures of sleep duration and quality from six days of wrist activity recordings. Average sleep duration and quality over the 5-year follow up period were derived from two 3-day recording sessions separated by approximately one year. We do not have sleep measurements that span the five-year follow-up period. Previous analysis of these actigraphy data found that the variability between the two years was minimal in this cohort,27 which suggests that our sleep measures may be valid representations of sleep over the five years between clinical examinations.
An important limitation of the present study is that we used wrist actigraphy, which only measures movement, rather than the gold-standard of sleep measurement, polysomnography. As such, we are not able to assess diagnostic sleep characteristics such as the presence and severity of sleep disordered breathing (SDB), a well-documented risk factor for hypertension.28, 29 Instead, risk of SDB was estimated using habitual snoring from the Berlin questionnaire, a validated instrument to evaluate the risk of sleep apnea.19 The proportion of snorers in men, African-Americans and the obese in our sample were consistent with current estimations of the prevalence of SDB by sex, race and weight status.30, 31 When snorers are excluded from our analyses, the impact of sleep duration and maintenance on 5-year changes in blood pressure was similar. Nevertheless, although habitual snorers are more likely to have SDB,30 not all cases of SDB will be identified and our models do not fully adjust for SDB. Future studies need to include an objective measure of sleep disordered breathing in order to better understand the association between hypertension and sleep duration or sleep maintenance that is independent of SDB.
In summary, the present study provides evidence for a link between the duration and quality of sleep and high blood pressure using objectively measured sleep characteristics. The findings are supported by previous studies of an association between self-reported sleep characteristics and blood pressure,3, 4, 10, 11 and by laboratory evidence for increased sympathetic nervous activity as a likely mechanism underlying the increase in blood pressure following sleep loss.5 Because of the major adverse health consequences of high blood pressure, the identification of a new and potentially modifiable risk factor has clinical implications. Intervention studies are needed to determine if optimizing sleep duration and quality can reduce the risk of increased blood pressure.
Acknowledgement
Research for this study was supported by grant AG 11412 from the National Institute on Aging. CARDIA is supported by US Public Health Service contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050, and NO1-HC-95095 from the National Heart, Lung, and Blood Institute. The National Heart, Lung, and Blood Institute reviewed and approved this manuscript. The authors have no conflicts of interest to report. Knutson has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
These data were presented as an oral presentation at the 47th Annual Conference on Cardiovascular Disease Epidemiology and Prevention, Orlando, Florida, March 2, 2007.
Authors have no financial disclosures to report.
Final version available at: http://archinte.ama-assn.org/cgi/content/full/169/11/1055
References
- 1.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Reducing risks, promoting healthy life. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 3.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev P, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 7.Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9(5):503–5. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 8.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27(6):1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35(5):1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 10.Houyez F, Degoulet P, Cittee J, et al. Sommeil et hypertension artérielle. Arch Mal Coeur Vaiss. 1990;83(8):1085–8. [PubMed] [Google Scholar]
- 11.Kotani K, Saiga K, Sakane N, Mu H, Kurozawa Y. Sleep status and blood pressure in a healthy normotensive female population. Int J Cardiol. 2007;125(3):425–7. doi: 10.1016/j.ijcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells JC, Hallal PC, Reichert FF, Menezes AM, Araujo CL, Victora CG. Sleep patterns and television viewing in relation to obesity and blood pressure: evidence from an adolescent Brazilian birth cohort. Int J Obes (Lond) 2008;32(7):1042–9. doi: 10.1038/ijo.2008.37. [DOI] [PubMed] [Google Scholar]
- 14.Lauderdale D, Knutson K, Yan L, et al. Objectively measured sleep characteristics among early middle-aged adults: The CARDIA Study. American Journal of Epidemiology. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 15.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Perceptual & Motor Skills. 1997;85(1):207–16. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Jacobs DR, Jr., Sidney S, Haskell WL, Anderssen N, Oberman A. Physical activity in young black and white women. The CARDIA Study. Ann Epidemiol. 1993;3(6):636–44. doi: 10.1016/1047-2797(93)90087-k. [DOI] [PubMed] [Google Scholar]
- 19.Netzer N, Stoohs R, Netzer C, Clark K, Strohl K. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Annals of Internal Medicine. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd ed. Jones and Bartlett Publishers; Boston: 2007. [Google Scholar]
- 21.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16(13):1515–27. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr., Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13(1):13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114(25):2780–7. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg JF, Tulen JH, Neven AK, et al. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50(3):585–9. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- 25.Lima-Costa MF, Peixoto SV, Rocha FL. Usual sleep duration is not associated with hypertension in Brazilian elderly: The Bambui Health Aging Study (BHAS) Sleep Med. 2008;9(7):806–7. doi: 10.1016/j.sleep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knutson KL, Rathouz P, Yan L, Liu K, Lauderdale D. Intra-Individual Daily and Yearly Variability in Actigraphically Recorded Sleep Measures: the CARDIA Study. Sleep. 2007;30(6):793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Jama. 2000;283(14):1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 29.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 30.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New England Journal of Medicine. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 31.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]