Abstract
The precise mechanisms whereby anti-angiogenesis therapy blocks tumour growth or causes vascular toxicity are unknown. We propose that endothelial cells establish a vascular niche that promotes tumour growth and tissue repair not only by delivering nutrients and O2 but also through an ‘angiocrine’ mechanism by producing stem and progenitor cell-active trophogens. Identification of endothelial-derived instructive angiocrine factors will allow direct tumour targeting, while diminishing the unwanted side effects associated with the use of anti-angiogenic agents.
Angiogenesis, the process of new blood vessel formation from pre-existing endothelial cells, has a crucial role in tumour initiation, dormancy, progression and metastasis. It has been suggested that anti-angiogenic factors can disable the capacity of tumour cells to access oxygen or the necessary nutrients for tumour growth and metastasis by inhibiting the functional sprouting and assembly of abnormal tumour vessels1–6. This could result in the regression or growth arrest of certain angiogenesis-dependent tumours. In corroboration of this concept, most of the anti-angiogenic agents have manifested efficacy in blocking tumour invasiveness and progression in mouse tumour models1–6.
Despite the efficacy of some anti-angiogenic agents in improving the survival of tumour-bearing mice, so far the outcome of clinical trials in which anti-angiogenic agents were delivered in conjunction with chemotherapy has been limited to a transient increase in the survival of patients with advanced solid tumours, with most patients ultimately succumbing to tumour progression3. Paradoxically, in certain mouse tumour models the inhibition of specific angiogenic pathways, partly through the induction of hypoxia or the recruitment of alternative angiogenic pathways, has enhanced tumour invasiveness7,8. These data suggest that the mechanism by which endothelial cells, which constitute the main building blocks of tumour vessels, might regulate tumour growth is complex and is not merely driven by establishing normalized passive9 and permissive conduits for delivering O2, nutrients and chemotherapeutic agents to the tumour tissue. It is conceivable that endothelial cells release specific growth factors that might directly regulate tumour growth in a perfusion-independent manner. In support of this concept, it has been shown that during developmental processes the invasion of endothelial cells into incipient organs confers inductive signals to promote organogenesis, even in the absence of blood flow. These data indicate that endothelial cells can produce growth factors that support organogenesis10–12, many of which could also potentially promote the growth of tumours.
Therefore, an alternative mechanism by which endothelial cells directly regulate tumour growth might be through the paracrine release of endothelial-derived growth factors and trophogens, which we refer to as ‘angiocrine factors’. Angiocrine factors comprise growth factors or trophogens (TABLE 1); adhesion molecules such as intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), E-selectin, P-selectin and hyaluronan; and chemokines, such as interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP1; also known as CCL2) and stromal cell-derived factor 1 (SDF1; also known as CXCL12). Angiocrine factors may accelerate tissue repair after treatment with anti-angiogenic and chemotherapeutic agents. Indeed, accumulating evidence from preclinical studies suggests that endothelial cells are not just non-thrombogenic passive conduits of the blood but have the potential for producing physiologically potent tumour- and stem cell-active angiocrine factors13–15. In this scenario, endothelial cells establish a vascular niche (FIG. 1a,b) from which they secrete or express membrane-bound stem cell and progenitor cell active factors and deposit components of the extracellular matrix (ECM) to generate a unique cellular microenvironment that modulates tumour progression, invasiveness, trafficking and metastasis. As angiocrine factors modulate the proliferation of stem and progenitor cells16, it is conceivable that the vascular niche might also directly modulate the homeostasis of tumour-initiating cells17. Although the physiological significance of tumour-initiating cells remains unclear and their proportion may vary in each tumour18, the cellular interaction of tumour-initiating cells with the vascular niche could be crucial for the maintenance and propagation of these cells.
Table 1.
Angiocrine factors produced by the vascular niche
| Angiocrine factors | Function | Organ specificity | Refs |
|---|---|---|---|
| BMP2 and BMP4 | Organogenesis and tumorigenesis | Nonspecific | 66 |
| FGF2 | Organogenesis and tumorigenesis | Nonspecific | 4 |
| BDNF | Neurogenesis | Brain and heart | 68 |
| PEDF | Neurogenesis | Brain and bone marrow | 67 |
| PGF | Angiogenesis and tumorigenesis | Nonspecific | 117 |
| PDGFβ | Angiogenesis and tumorigenesis | Smooth muscle | 60,86,118 |
| VEGFA | Angiogenesis and autocrine loop | Vasculature | 119 |
| ANGPT2 | Angiogenesis remodelling | Vasculature | 26 |
| Jagged 1 and jagged 2 | Haematopoiesis, angiogenesis and tumorigenesis | Bone marrow | 83,110 |
| LAMA4 | Organogenesis and tumorigenesis | Stem cell niches | 11,62 |
| NO | Tumorigenesis and leukaemogenesis | Nonspecific | 76 |
| IL-8, IL-6, CD40, G-CSF, GM-CSF, IGF1, SDF1, EDN1, MCP1 and TGFβ | Tumorigenesis and tissue repair | Nonspecific | 60,106 |
ANGPT2, angiopoietin 2; BDNF, brain-derived nerve growth factor; BMP, bone morphogenetic protein; CSF, colony stimulating factor; EDN1, endothelin 1; FGF, fibroblast growth factor; G-CSF, granulocyte-CSF; GM-CSF, granulocyte-macrophage-CSF; IGF, insulin-like growth factor; IL, interleukin; LAMA4, laminin α4; MCP1, monocyte chemotactic protein 1 (also known as CCL2); NO, nitric oxide; PDGFβ, platelet-derived growth factor-β; PEDF, pigmented epithelial growth factor; PGF, placental growth factor; SDF1, stromal cell-derived factor 1 (also known as CXCL2); TGFβ, transforming growth factor-β; VEGF, vascular endothelial growth factor.
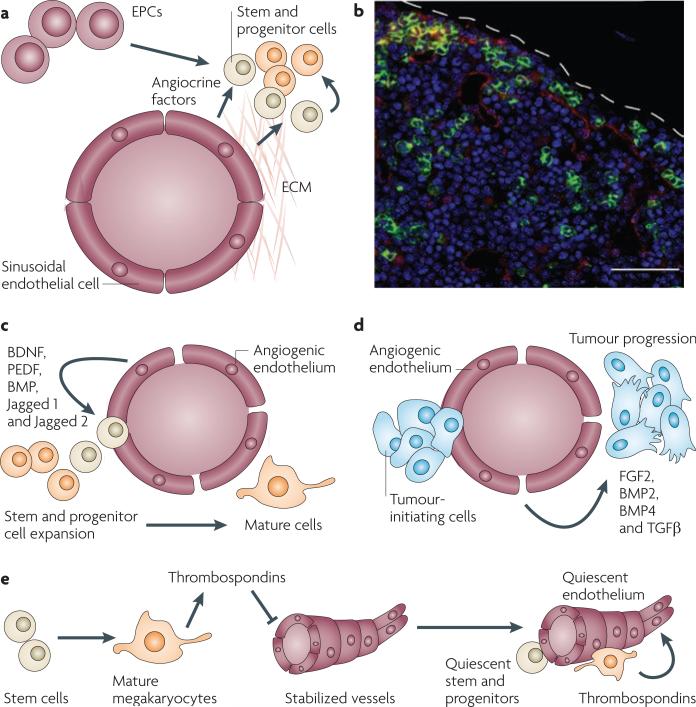
Figure 1. The vascular niche supports the expansion of stem and progenitor cells as well as their malignant counterparts.
a | By expressing angiocrine factors and producing extracellular matrix (ECM), endothelial cells and endothelial progenitor cells (EPCs) establish a microenvironment, referred to as the vascular niche, which supports the expansion of normal and malignant stem and progenitor cells. b | Establishment of the vascular niche by the bone marrow sinusoidal endothelial cells. The confocal image shows a cross-section of the bone marrow taken from transgenic Notch reporter mice 7 days after sublethal irradiation with 6.5 Gy. Regenerating Notch-activated green fluorescent protein (GFP)+ haematopoietic stem and progenitor cells (green fluorescence) could be detected in the proximity of VE-cadherin+ Notch ligand+ sinusoidal endothelial cells (red staining). Therefore, angiocrine factors, such as Notch ligands, reconstitute haematopoiesis. c | Vascular endothelial growth factor A (VEGFA)- or fibroblast growth factor 2 (FGF2)-activated endothelial cells promote the proliferation and lineage-specific differentiation of normal cells by release of angiocrine factors (FGFs, BMPs, jagged 1 and jagged 2) and direct cellular contact. d | Identical pathways to those described in (c) affect malignant stem and progenitor cells. e | The mature progeny of stem cells, such as megakaryocytes, enforce a quiescent state in the endothelial cells by inhibiting their angiogenic activity through the release of anti-angiogenic factors, such as thrombospondins, which in turn promotes stem cell quiescence. BDNF, bone-derived neurotrophic factor; BMP, bone morphogenetic protein; PEDF, pigment epithelium-derived factor; TGFβ, transforming growth factor-β. Bar represents 50 μm.
In this Opinion article, we set forth the idea that the endothelial cells that line the surface of small calibre arteries and veins, in particular the sinusoidal endothelial cells of the haematopoietic compartment (box 1), are not only the building blocks of blood vessels that permit the delivery of essential nutrients and O2, but might also function as a vascular niche that — through the production of angiocrine factors — instructively nurtures tumour growth and initiates tissue regeneration after anticancer therapy. On the one hand, we propose that the selective inhibition of angiocrine factors, without disturbing the integrity of the blood vessels, might still block tumour growth and thereby avoid potential toxic side effects to the normal vasculature. Indeed, the use of currently available anti-angiogenic agents causes certain normal angiogenic blood vessels that promote tissue repair to undergo regression, which might consequently promote hypoxia-induced angiogenesis and enhanced tumour invasiveness7,8,19. On the other hand, maintaining the activity of specific angiocrine factors that support haematopoiesis, but not tumorigenesis, will selectively accelerate bone marrow recovery, thereby diminishing the morbidity and mortality associated with chemotherapy and irradiation. Therefore, balancing the expression of angiocrine factors might not only target tumour cells, but might also ameliorate therapy-induced toxicities. It is important to note that although the endothelial cells lining the wall of the lymphatic vessels could regulate anti-tumour immune responses20 this article focuses on the role of non-lymphatic endothelial cells.
Instructive role of the vascular niche
Before the technological developments that enabled the cultivation of primary endothelial cells derived from the wall of arteries and veins, vascular cells were considered as passive and non-thrombogenic intralumenal vascular cells that primarily provided blood to organs and tumours4,21. Two groups led by Judah Folkman22 and ralph Nachman23 established in vitro cell culture of pure populations of primary endothelial cells from human umbilical vein endothelial cells (HUVECs). These fetal-derived endothelia, which are an extension of the placental blood supply, were proposed to specialize in modulating vascular homeostasis within the fetal–maternal circulation22.
Cultivation of HUVECs and other primary human organ-specific endothelial cells resulted in the identification of angiogenic factors that support endothelial cell survival and proliferation, such as fibroblast growth factors (FGFs) and vascular endothelial growth factors (VEGFs)4,24–27. Furthermore, the propagation of pure populations of endothelial cells enabled the design of in vitro and in vivo quantitative assays28 for assessing vasculogenesis and neo-angiogenesis during adulthood26,27. The isolation of homogenous endothelial monolayers also facilitated surface marker analyses of organ-specific endothelia, demonstrating that there is remarkable phenotypic heterogeneity of the vasculature in each organ29–31. These models allowed screening for angiogenic factors that modulate organ-specific proliferation, survival, tubulogenesis and sprouting of the endothelial cells4,24–27. Propagation of a large number of endothelial cells also accelerated biochemical identification of the factors secreted by the endothelium, and so set forth the idea that the vasculature can directly regulate physiological processes through the production of angiocrine factors (Supplementary information S1 (figure)).
Inflammatory cell recruitment
Primary endothelial cell cultures provided invaluable cells to clone inflammatory cytokines that were secreted by quiescent or immune-stimulated endothelial cells. Endothelial cells were shown to mediate immune responses after stimulation with inflammatory cytokines, including IL1-β and tumour necrosis factor-α (TNFα). Both IL-1β and TNFα are released by inflammatory cells and induce endothelial cells to increase the expression of chemokines, inflammatory adhesion molecules and haematopoietic growth factors (such as interferon, IL-6 and colony stimulating factors (CSFs)) to orchestrate an immune response to infectious agents or tumour tissue4,24–27,32. Upregulation of endothelial-specific adhesion molecules, including E-selectin, and the release of endothelial-derived chemokines, such as IL-8 and MCP1 (ref. 33), were also shown to mediate the recruitment of the inflammatory cells (Supplementary information S1 (figure)).
Acute and chronic inflammation through the activation of endothelial cells and recruitment of protumorigenic haematopoietic cells promotes tumour angiogenesis, growth and metastasis. In particular, tumour cells can not only activate endothelial cells but also induce mobilization of the inflammatory haematopoietic cells from bone marrow to the tumour microenvironment by secreting numerous proinflammatory cytokines, including IL-1β, TNFα, SDF1 and osteopontin34,35. Of the mouse bone marrow-derived pro-angiogenic haematopoietic cells34, Gr1+CD11b+ (also known as integrin αM)36–38 cells, colony stimulating factor receptor 1+ (CSFR1+; also known as M-CSFR or CD115) monocytic cells39, CXCR4+VEGFR1+ progenitor cells40,41, endothelial-specific receptor tyrosine kinase+ (TEK+; also known as TIE2+) myeloid cells42–44 and subsets of lymphoid cells45 lodge in the tumour microenvironment through adhesion to activated endothelial cells and support tumour angiogenesis and growth. Recruitment of Gr1+CD11b+ cells could also confer chemotherapy resistance to solid tumours46.
In addition, SDF1 and VEGFA support the recruitment of endothelial progenitor cells (EPCs) to the tumour microenvironment, fostering tumour blood vessel assembly34,40,47–57. Recruited EPCs are incorporated either intralumenally or perivascularly into the vessel wall and could potentially produce angiocrine factors that support tumour angiogenesis58. EPCs could also convert micrometastatic lesions into macrometastatic invasive tumours through the production of as yet unrecognized angiocrine signals58,59 (FIG. 1a). In this setting, the activated tumour endothelial cells function as vascular conduits to facilitate the recruitment of the pro-angiogenic inflammatory haematopoietic cells and EPCs to the tumour microenvironment thereby maintaining tumour neo-angiogenesis and growth.
Role of angiocrine factors in mediating tumorigenesis
In addition to orchestrating the immune response and tumour angiogenesis, another mechanism by which endothelial cells could, in a perfusion-independent manner, directly promote tumour progression is by the release of pro-tumorigenic angiocrine factors60. Malignant transformation is often associated with the reactivation of molecular pathways that regulate developmental processes during organogenesis21. Evidence from developmental mouse models suggests that blood vessel development precedes organ specification, and sprouting capillaries are found throughout the developing embryo61,62 raising the possibility that endothelial cells might confer inductive signals that enable proper organogenesis. Embryonic endothelial cells expressing the tyrosine kinase VEGFR2 establish a vascular niche10, which by producing undefined angiocrine factors and ECM components such as laminin61,62, induces pancreatic and liver organogenesis. These data indicate that during development, direct interaction between endothelial cells and tissue-specific cells supports organogenesis.
Similarly, the proximity of the endothelial cells to stem and progenitor cells, as well as their malignant counterparts, in adults indicates that angiocrine factors could directly stimulate tumour growth. The first in vitro model supporting this hypothesis was the demonstration that the co-culture of primary bone marrow-derived sinusoidal endothelial cells (BMECs) supplemented with serum and endothelial growth factors, including VEGFA and FGF2, was capable of transiently maintaining human CD34+ haematopoietic stem and progenitor cells for several days63,64 (boxes 1,2; FIG. 1c). Various sources of endothelial cells, including brain-derived endothelial cells65–67 and HUVECs68,69, cultivated in the presence of serum also stimulated the expansion of the neuronal precursor cells by the secretion of neuronal active cytokines, including brain-derived neurotrophic factor (BDNF), bone morphogenetic proteins (BMPs) and pigment epithelium-derived factor (PEDF; also known as SERPINF1) (TABLE 1).
Endothelial cells could also support the survival and proliferation of leukaemic cells through direct cellular contacts. Potential interactions between leukaemic cells and endothelial cells have been shown to be mediated through VCAM1–fibronectin–integrin α4β1 (also known as VLA4)70 binding and CD44–hyaluronan71 binding. These in vitro data were supported by in vivo observations, whereby leukaemic cells were shown to reside in apposition of endothelial cells both in the bone marrow72,73 (FIG. 2a) and in distant organs, such as the liver74 (FIG. 2b). In a series of in vitro and in vivo studies, leukaemias were shown to secrete angiogenic factors, including VEGFA, which activated endothelial cells to release leukaemic trophogens, supportingthe expansion of leukaemic cells72,73,75 (FIG. 2c). Endothelial cells, by secretion of cytokines, including IL-6, IL-3, granulocyte-CSF (G-CSF; also known as CSF3), granulocyte-macrophage-CSF (GM-CSF), IL-1 and nitric oxide (NO)76,77, were shown to promote leukaemic proliferation, suggesting that activated angiogenic endothelial cells could provoke leukaemic cell growth through paracrine signalling. Inhibition of endothelial cells by the injection of anti-angiogenic agents into mice with human leukaemias was sufficient to block the proliferation of the leukaemic cells72,73,75. It is plausible that interfering with the angiogenic activity of endothelial cells could target specific types of leukaemic cells, thereby abrogating leukaemic progression and tissue infiltration.
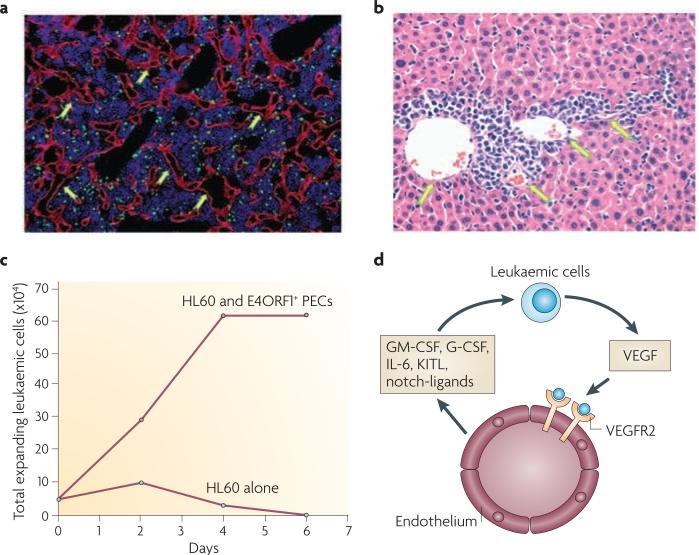
Figure 2. The vascular niche supports the progression of leukaemic cells.
The prototypical angiogenic factor vascular endothelial growth factor A (VEGFA) released by leukaemic cells (green fluorescent protein+ cells in (a) and infiltrating cells in (b)) activates the tyrosine kinase VEGF receptor 2 (VEGFR2) expressed on the endothelial cells and this promotes the proliferation of VE-cadherin+ vessels in the bone marrow (a, arrows) and liver (b, arrows). In vitro E4ORF1+ primary endothelial cells (PECs) support the long term expansion of HL60 leukaemic cells in serum- and cytokine-free conditions (c), and in the absence of the E4ORF1+ primary endothelial cells HL60 leukaemic cells undergo cell death (c). The mechanism by which endothelial cells support the proliferation of the leukaemic cells is through the VEGFA–VEGFR2-mediated upregulation of the pro-leukaemic factors (d) leading to the uncontrolled proliferation of the leukaemic cells. G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte-macrophage-colony stimulating factor; IL-6, interleukin-6; KITL, KIT ligand. Part (c) of this figure is reproduced from ref. 13.
Endothelial cells might also directly support the homeostasis of glioma-initiating cells15. Using in vitro andorthotopic in vivo models, gliomas were detected in association with blood vessels. Increasing the number of endothelial cells through the secretion of paracrine factors stimulated the propagation of nestin+CD133+ (also known as prominin 1) gliomas, and anti-angiogenic factors abrogated the expansion of the gliomas. It was postulated that endothelial-derived BDNF and PEDF, both of which stimulate the self-renewal of neuronal precursor cells, could have stimulated the growth of the tumour cells67,78. In addition, medulloblastomas localized to the perivascular niche were shown to be resistant to irradiation. Inhibition of AKT signalling sensitized medullobalstomas in the perivascular region to radiation-induced apoptosis79. These data also suggested that as yet unidentified angiocrine factors expressed by the endothelial cells confer tumours with radiation resistance.
The vascular niche and tumour-initiating cells
Several studies60,80–83 have fostered the idea that through a perfusion-independent mechanism angiocrine factors could also modulate the homeostasis of the multipotent cells that have been proposed to initiate tumours. In support of this idea, putative organ-specific stem and progenitor cells were detected in the vicinity of the vascular niche. The vascular niche localized in the subventricular zone (SVZ) houses neural stem and progenitor cells that could initiate gliomas84–86. Likewise, mesenchymal87 and adipose88 progenitor cells, which have been implicated in initiating sarcoma and adenocarcinomas, localize in apposition to the vascular niche. Even undifferentiated spermatogonial cells, which are thought to give rise to a subset of adult germ cell tumours, reside in the vicinity of the testicular vasculature89. These data indicate that the vascular niche could directly interact with putative precursors of tumour-initiating cells.
Box 1 | organ specificity of specialized endothelial cells.
Sinusoidal endothelial cells: bone marrow, spleen and liver
These are fenestrated and malleable vessels that lack investment of conventional smooth muscle cells. They support the expansion of normal and malignant haematopoietic stem and progenitor cells.
Tumour endothelium
This term is used to describe atypical, dilated, leaky and often haemorrhagic tumour vessels. Such vessels could potentially support tumour growth through the release of angiocrine factors.
Nascent neo-angiogenic vessels
These are proliferating, sprouting vessels detected during embryonic development, ischemic revascularization and organ regeneration. They promote organ-specific stem and progenitor cell expansion.
Although the direct link between putative organ-specific stem and progenitors and their contribution to solid tumours is not formally validated, it is now well established that haematopoietic stem and progenitor cells can initiate leukaemias at high frequency90,91. Remarkably, Notch-activated haematopoietic stem and progenitor cells have also been detected in close proximity to the bone marrow vasculature16,92 (FIG. 1a,b). Bone marrow endothelial cells interact with haematopoietic stem and progenitor cells, including their leukaemic derivatives, through the engagement of the adhesion molecules VCAM1–integrin α4β1 and CD44–hyaluronan to sustain their proliferation and expansion93–95 (FIG. 1c,d).
Notably, the release of anti-angiogenic factors, such as thrombospondins, by mature megakaryocytes inhibited further expansion of the haematopoietic compartment by decelerating angiogenesis, and this was shown to increase apoptosis and force the differentiation of leukaemic cells, thereby preventing leukaemogenesis (FIG. 1e)96. Inhibition of CD44 and integrin α4β1 binding also interferes with the interaction of leukaemic cells with vascular cells74, rendering the leukaemic cells sensitive to chemotherapeutic agents.
Taken together, the remarkable proximity of the vasculature to the stem and progenitor cells (FIG. 1b,c,d) lends credence to the concept that endothelial cells might be pathophysiologically reprogrammed to support tumorigenesis once organ-specific stem and progenitor cells undergo malignant transformation. Alternatively, the vascular niche might be endowed with the ability to sustain both the putative tumour-initiating cells and the tumour cell populations that do not have the capacity to initiate tumours but maintain tumour mass (as has been shown with melanomas18).
Remodelling the ECM
Deposition of a complex ECM by endothelial cells in vascular niches establishes a highly specialized vascular basement membrane that might regulate tumour progression and organogenesis. The endothelial cell basement membrane, which variably comprises laminin α4 (LAMA4), fibronectin, hyaluronan and collagen-α type IV, provides a large ‘sink’ that could modulate the exposure of various angiocrine factors to organ-specific stem and progenitor cells. For example, expression of LAMA4 by VEGFR2+ endothelial cells stimulates insulin production by the pancreatic tissue, a unique feature of endocrine pancreatic cells11,97. Secretion of various proteases, including disintegrin and metalloproteinase domain-containing protein 17 (ADAM17)98,99, matrix metalloproteinase 2 (MMP2) and MMP10 by the endothelial cells, through remodelling of the basement membrane might alter the bioavailability of diffusible cytokines deposited in the ECM, thereby fostering tumour growth.
Remodelling of the ECM by components of the activated coagulation cascade might also have a role in remodelling the ECM in the tumour vascular microenvironment. Tissue factor, which could be produced by either the tumour cells or vascular cells, promotes tumour growth100–104. Collectively, these data indicate that the vascular niche can support organogenesis and the expansion of tumour cells through production of ECM.
Vascular niche and tissue repair
The potential of endothelial cells to produce stem and progenitor cell-active angiocrine factors might represent a double-edged sword. Angiocrine factors might not only promote tumour growth, but might also facilitate the repair of normal tissues targeted by chemotherapeutic agents or radiotherapy. The repair of normal tissues might provide a therapeutic advantage as one of the major obstacles in the treatment of cancer patients is the limit to the dose of chemotherapy and irradiation that can be delivered to the tumour owing to normal tissue toxicity. This is an important issue as cyclic doses of chemotherapy given on a monthly basis impairs the capacity of the haematopoietic cells in the bone marrow to recover, resulting in low white blood cell, red blood cell and platelet counts, which predisposes patients to life-threatening infection, bleeding and anaemia.
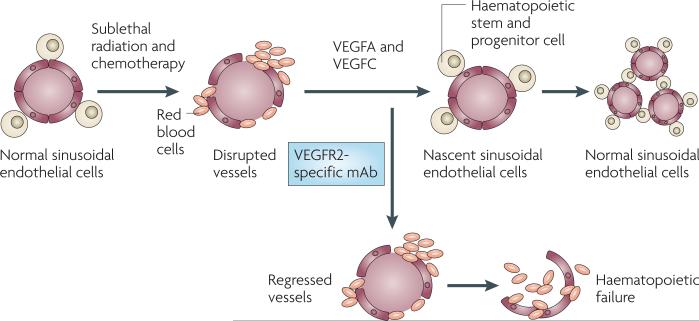
Currently, clinical approaches to diminish haematopoietic suppression after chemotherapy are not effective. One mechanism by which chemotherapy might induce bone marrow suppression is through impairing the function of the bone marrow vasculature. It was recently demonstrated that regeneration of the functional sinusoidal endothelial cells in the bone marrow was essential for haematopoietic recovery after severe myelosuppression16 (FIG. 3). Notably, interfering with the regeneration of endothelial cells through the inhibition of VEGFR2 after radiation injury resulted in a complete failure of haematopoietic stem and progenitor cells to reconstitute the haematopoietic system, which led to haematopoietic failure. The degree of damage to the sinusoidal endothelial cells directly correlated with the capacity of haematopoietic cells to regenerate, establishing the new concept that sinusoidal endothelial cells have a crucial role in the reconstitution of haemato poiesis through modulating the activity of stem and progenitor cells.
Figure 3. Activation of VEGFR2 tyrosine kinase signalling pathways is essential for the regeneration and remodelling of the sinusoidal endothelial cells in the bone marrow.
Timely regeneration of the sinusoidal endothelial cells supports regeneration of the haematopoietic stem and progenitor cells, guaranteeing prompt reconstitution of haematopoiesis after treatment with myeloablative chemotherapy and irradiation. As such, bone marrow sinusoidal endothelial cells establish a vascular niche that, through the release of angiocrine factors, promotes the reconstitution of the haematopoietic stem and progenitor cells. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2.
Box 2 | Models to interrogate the instructive role of the vascular niche.
In vitro models
Primary endothelial co-culture with organ-specific stem cells or tumour cells. Potential problems include the requirement for supplementation with serum and angiogenic factors to maintain endothelial survival. Both serum and angiogenic factors could promote tumour growth independently of the endothelial cells.
Simian virus 40 (SV40) or polyoma middle T and TERT-immortalized endothelial cells. Potential problems include the requirement for supplementation with serum and angiogenic factors to maintain endothelial survival. Diminished angiogenic function and high metabolic rate interfere with optimal expansion of stem and progenitor cells and their malignant counterparts.
E4ORF1- transduced endothelial cells. Advantages include that these cells maintain an angiogenic repertoire of endothelial monolayers in the absence of serum or exogenous angiogenic factors. They mimic primary endothelial cell metabolic demand and growth kinetics and are highly effective in promoting long-term expansion of stem and progenitor as well as tumour cells.
In vivo models
Bone marrow suppression. This evaluates the role of sinusoidal endothelial cells in stem cell recovery and expansion, and assesses the localization and growth kinetic of leukaemias and solid tumours. Advantages include the chronological assessment of the contribution of the vascular niche to stem and progenitor cell reconstitution.
Selective conditional knock down of angiocrine factors in endothelial cells. Potential problems include the possible impaired permissive function of the endothelial cells.
Mouse orthotopic and xenograft models. Potential problems include the possible failure of certain growth factors, such as the stem cell factor kit ligand (KITL) and interleukin-6 (IL-6) to activate human stem or tumour cells.
These data allow us to propose that, similar to the instructive role of endothelial cells promoting the growth of tumours, sinusoidal endothelial cells are not just passive conduits delivering nutrients to bone marrow, but are crucial organ-specific vascular cells that modulate haematopoietic stem cell homeostasis through an instructive mechanism. It is conceivable that specific angiocrine factors that promote tumour growth are distinct from those that support haematopoietic recovery. As such, the selective inhibition of endothelial factors that promote tumour growth, such as VEGFs, may provide an effective means to block tumour growth, and activation of other factors, such as specific Notch ligands105, might diminish haematopoietic toxicity. Therefore, the identification of angiocrine factors that differentially support tumour growth and tissue repair will have a major effect on treating patients with combination chemotherapy and anti-angiogenic therapy, while ameliorating haematopoietic failure.
Vascular activation and tumour growth
One dilemma is how the vascular niche both maintains tissue homeostasis and promotes tumour growth. One plausible explanation is that the activation of endothelial cells during inflammation or neo-angiogenesis might switch on specific programmes to enable the endothelial cells in the vascular niche to secrete cytokines that accelerate tissue repair and promote tumour growth. Indeed, at steady state conditions in adult mammals, most endothelial cells are quiescent and secrete factors that maintain organ homeostasis and favour tumour dormancy4. However, in response to certain physiological stresses, such as hypoxia, inflammation and oncogene-induced malignant transformation, the upregulation and release of angiogenic factors, including VEGFA, FGFs and angiopoietins, stimulates endothelial cells to proliferate and undergo sprouting angiogenesis. Angiogenic factors could also provoke endothelial cells to secrete specific cytokines that reciprocally support the regeneration of normal and malignant stem cells. In this regard, activation of the vascular niche through the induction of angiogenic pathways will stimulate tumour growth and tissue regeneration.
Supporting this idea, angiogenic factors have been shown to activate AKT in endothelial cells, which through the inactivation of the forkhead box O (FOXO) signalling pathway induces the secretion of cytokines, matrix metalloproteinases (such as MMP10), chemokines (such as IL-8) and ECM components (such as laminin)106. Furthermore, chronic AKT activation in the tumour vasculature could also promote tumour growth through the recruitment of the mTOR pathway. Rapamycin, an inhibitor of mTOR, blocked tumour growth and tumour vascular permeability by changing the activation status of AKT in endothelial cells107. The anti-tumour effects of rapamycin might directly affect tumour cells but might also silence endothelial cells by suppressing angiogenesis or the permeability of the tumour vasculature107. These data also raise the possibility that activation of the AKT–mTOR pathway in endothelial cells results in the production of angiocrine factors that promote tumour growth and metastasis or maintain quiescence of tumour-initiating cells107, and the release of anti-angiogenic factors, including thrombospondins, could potentially force endothelial cells into quiescence, thereby suppressing tumorigenesis (FIG. 1e). As the angiogenic state of the endothelial cells might be regulated by VEGFA and FGF2 signalling pathways, it is conceivable that the activation of AKT, ERK or JNK might result in differential upregulation of the angiocrine factors, modulating the growth of normal and malignant stem cells.
Moreover, evidence from microarray analyses indicates that angiogenic factors stimulate endothelial cells to secrete stem cell active angiocrine factors, such as FGFs, EGFs65,108,109 and neuronal growth factors, including BDNF68,69, BMPs66 and PEDF67,78. These endothelial-derived factors have been shown to promote the expansion of neuronal precursor cells for a few days. Similarly, inflammatory cytokines, such as TNFα, induce the expression of the notch ligands jagged 1 and jagged 2 by bone marrow endothelial cells110. Notch activation has a major role in the progression of both T cell leukaemias and solid tumours, suggesting that the expression of Notch ligands on the endothelial cells stimulates tumour growth83.
Collectively, these data suggest that the activation state of the vascular niche balances the performance of the endothelial cells in either suppressing or stimulating the growth of tumours or accelerating tissue repair. Upregulation of angiogenic factors and inflammatory cytokines switch the vascular niche to secrete stem and progenitor cell active angiocrine factors, thereby promoting tumour growth and tissue repair. By contrast, a quiescent vascular niche might sustain tumour dormancy and tissue homeostasis.
Models to study the vascular niche
Most of the prototypical endothelial cell–tumour co-culture models designed to interrogate the role of endothelial cells in the proliferation of solid tumours are carried out in culture conditions that are supplemented with serum and angiogenic factors, including VEGFA, FGF2, EGF and crude brain extracts, all of which could exert a pro-tumorigenic effect independently of the angiocrine factors produced by the endothelial cells. Therefore, it has been unclear whether the expansion of the tumour cells or stem and progenitor cells was the direct result of the interaction with the endothelial cells or partly due to the supplementation of the numerous exogenous angiogenic factors that are essential to maintain the integrity of the endothelial cells during co-culture experiments15,65. As such, current co-culture conditions introduce considerable complexity in defining the role of the endothelial cells in supporting the expansion of tumour-initiating cells, as the exogenous inclusion of recombinant FGF2, EGF and VEGFA could promote stem cell proliferation and tumour growth4,24–27 independently of the angiocrine factors produced by endothelial cells (box 2). The obligate necessity for the use of serum also diminishes the effectiveness of these cultures, as serum (the product of clotted blood) contains stem cell active cytokines such as TGFβ that could introduce culture artefacts by negatively or positively influencing the homeostasis of stem cells111. Furthermore, chronic supplementation with angiogenic factors causes endothelial cells to lose their vasculogenic repertoire and fail to respond to angiogenic factors, thereby hampering the study of their instructive role in the regulation of tumorigenesis13. Therefore, developing strategies to maintain endothelial cells in serum and cytokine free co-culture with tumour cells without altering their angiogenic phenotype is necessary.
To address this issue, long-lasting endothelial cells have been generated by using oncogenic factors, including simian virus 40 (SV40) large-T antigen112, polyoma middle-T113 and telomerase reverse transcriptase (TERT)114,115. However, immortalization of endothelial cells by these approaches results in the chronic activation of the MAPK signalling pathway, which leads to the generation of highly proliferative and hypermetabolic endothelial cells that have lost many of their essential angiogenic and/or adhesive features, including impaired responsiveness to angiogenic factors, and the expression of cytokines that are not physiologically expressed by endothelial cells. Accordingly, establishing long-term durable endothelial cells that could maintain their angiogenic profiles in the absence of exogenous cytokines and angiogenic factors would facilitate the identification of vascular-specific factors that directly support tumour growth or organ-specific stem cell regeneration.
To this end, a new strategy was devised to generate durable endothelial cells that could propagate, while maintaining their long-term angiogenic profile without oncogenic transformation13. To achieve this goal, the E4ORF1 gene of the adenovirus serotype 5 or serotype 9 was introduced into primary endothelial cells. This resulted in endothelial cell survival in serum- and growth factor-free conditions without altering the angiogenic repertoire of the endothelial cells through the chronic activation of AKT, but not MAPK13. E4ORF1+ endothelial cells could support the expansion of the myelocytic HL60 leukaemic cell line in serum-free and cytokine-free conditions for more than 7 days (FIG. 2d). Furthermore, E4ORF1+ endothelial cells could support the long-term proliferation of certain solid tumour cell lines, such as human teratocarcinoma cell lines13 in serum- and cytokine-free conditions, allowing the establishment of screening assays to identify stem cell and tumour-specific growth factors. These studies not only establish the potential of the endothelial cells to directly support the expansion of tumour cells but also lay the foundation for identifying angiocrine factors that directly support tumour growth.
Presumably, most of the stem and progenitor cell-active cytokines are expressed in a redundant manner by a wide variety of stromal cells and endothelial cells. It is possible that in certain tumours the selective expression of angiocrine factors by the endothelial cells will be the key to provoking tumour growth. As it is technically challenging and ethically difficult to assess the role of endothelial cells in tumour growth in clinical studies, mouse models are ideal for determining how selective knock down of a particular growth factor in the vascular cells, but not other stromal cells, influences tumour growth and metastasis. Furthermore, the mechanism by which expression of these angiocrine factors are selectively regulated in endothelial cells but not stromal cells is unknown. Therefore, the generation of transgenic mice genetically engineered to be deficient in the expression of defined angiocrine factors in endothelial cells only will provide important information regarding the mechanism by which vascular niche cells modulate tumorigenesis through the induction of specific subsets of angiocrine factors or ECM components. The knowledge gained from such mouse models could then be corroborated in cancer patients by analysing the expression of the angiocrine factors in tumour samples obtained from patients treated with anti-angiogenic chemotherapy.
Alternatively, human tumour xenograft models could be used to determine the angiocrine role of endothelial cells in tumour growth. In human xenograft models, mouse endothelial cells will presumably modulate human tumour growth. Therefore, the isolation of mouse endothelial cells supporting human tumour cells will allow the identification of angiocrine factors that support tumour growth. However, isolation and cultivation of primary mouse endothelial cells is cumbersome. Mouse endothelial cells have been cultivated from transgenic mice expressing SV40 large-T antigen116. These immortalized mouse endothelial cells may have lost their angiogenic repertoire and so do not represent physiologically relevant tumour vascular cells with which the role of angiocrine factors could be studied. Therefore, the development of technology to propagate and maintain primary mouse endothelial cells is necessary to interrogate the role of endothelial cell-specific paracrine factors in various transgenic mouse models in mediating tumorigenesis and organogenesis.
Conclusions and future directions
Emerging evidence demonstrates that endothelial cells are not simply passive conduits for delivering O2 and nutrients or for waste disposal. By contrast, endothelial cells could be conceived as specialized organ-specific vascular niches that, on activation by secretion of specific angiocrine factors and deposition of ECM, could instructively support the maintenance and reconstitution of normal and malignant stem and progenitor cells. By releasing as yet unrecognized diffusible, membrane- and ECM-bound factors endothelial cells balance the extent of self-renewal and differentiation of the stem and progenitor cells in tumours and injured organs, such as myeloablated bone marrow exposed to high doses of chemotherapy and irradiation. Although endothelial cells are known to produce stem cell active factors, including BMP2, BMP4, TGFβ, BDNF, jagged 1 and jagged 2, and angiogenic factors, such as placental growth factor (PGF), angiopoietin 2 (ANGPT2), VEGFA, FGF2 and platelet-derived growth factor (PDGF), the precise identity of functional organ-specific angiocrine factors released by the endothelial cells that support organogenesis, tissue repair and tumorigenesis remains to be determined.
Whether endothelial cells have a direct role in maintaining tumour-initiating cells is also unknown. Targeting endothelial-specific angiocrine factors that might nurture tumour-initiating cells will lay the foundation for clinical studies to treat tumours that are dependent on tumour-initiating cells for their long-term maintenance and abrogate resistance to chemotherapeutic agents. The availability of durable endothelial cells to interrogate the interaction with tumour cells, such as E4ORF1+ human endothelial cells, will pave the way for the identification of endothelial cell-derived and ECM-bound growth factors that support tumorigenesis and organogenesis.
To formally interrogate the paracrine potential of the endothelial cells in supporting tumour growth and tissue repair requires the development of mouse models whereby tumour and stem cell active angiocrine factors could be selectively knocked down in adult mice. This approach will allow the determination of the instructive role of endothelial cells in tumorigenesis and organ regeneration. However, as certain stem cell active cytokines, such as Notch ligands, might also have an essential role in maintaining the permissive function of the vasculature, it might be difficult to define the instructive function of the endothelial cells in tumorigenesis83 and tissue repair. To circumvent this problem, the development of technology to selectively knock down angiocrine factors in the endothelial cells of a particular organ is necessary to define the role of endothelial cells in organ-specific tissue repair and organogenesis.
Overall, the therapeutic potential of exploiting vascular derived-tumour specific angiocrine factors for targeting tumours and diminishing therapy-induced vascular toxicity is enormous. Delivery of the angiocrine stem cell active factors might enhance organ regeneration, such as by promoting haematopoietic recovery after treatment with chemotherapy and irradiation, and targeting specific pro-tumorigenic factors released by the endothelial cells will inhibit tumour growth. In this regard, identifying the mechanism by which endothelial cells convey instructive and inductive signals that promote tumour growth or regenerate therapy-damaged organs may provide insight for the design of new therapeutic strategies to eradicate angiogenesis-dependent tumours, while minimizing end-organ toxicity associated with life-threatening myeloablative and anti-angiogenic treatments.
Supplementary Material
Acknowledgements
J.M.B. is supported by Starr Stem Cell Scholars Fellowship and Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant. S.R. is supported by Howard Hughes Medical Institute, Ansary Stem Cell Institute, Anbinder and Newmans Own Foundation, National Heart Lung and Blood Institute grants HL075234 and HL097797, Qatar National Priorities Research Program, Empire State Stem Cell Board and the New York State Department of Health grant NYS C024180.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
E4ORF1
National cancer institute Drug Dictionary:
http://www.cancer.gov/drugdictionary/Rapamycin
UniProtKB: http://www.uniprot.org
α4 | β1 | BDNF | CD44 | FGF2 | fibronectin | ICAM1 | IL1-β | IL-8 | MCP1 | PEDF | SDF1 | TNFα | VCAM1 | VEGFA
SUPPLEMENTARY INFORMATION
see online article: S1 (figure)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev. Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 5.Duda DG, Jain RK, Willett CG. Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J. Clin. Oncol. 2007;25:4033–4042. doi: 10.1200/JCO.2007.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nature Rev. Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 7.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 10.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 11.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech. Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 13.Seandel M, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc. Natl Acad. Sci. USA. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naumov GN, Folkman J, Straume O, Akslen LA. Tumor-vascular interactions and tumor dormancy. APMIS. 2008;116:569–585. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg JI, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA, Jr, Cotran RS, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folkman J, Klagsburn M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Yancopoulos GD, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 28.Nachman RL, Jaffe EA. Endothelial cell culture: beginnings of modern vascular biology. J. Clin. Invest. 2004;114:1037–1040. doi: 10.1172/JCI23284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aird WC. Molecular heterogeneity of tumor endothelium. Cell Tissue Res. 2009;335:271–281. doi: 10.1007/s00441-008-0672-y. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 31.Yano K, et al. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood. 2007;109:613–615. doi: 10.1182/blood-2006-05-026401. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Barros M, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seandel M, Butler J, Lyden D, Rafii S. A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell. 2008;13:181–183. doi: 10.1016/j.ccr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Palma M, et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 43.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 44.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nature Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 45.DeNardo DG, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 47.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 49.Peters BA, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nature Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 50.Madlambayan GJ, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114:4310–4319. doi: 10.1182/blood-2009-03-211342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 52.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 54.Shaked Y, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbich C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J. Mol. Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 57.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 58.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 59.Rafii S, Lyden D. Cancer. A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pirtskhalaishvili G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:77–87. doi: 10.1002/1097-0045(20000615)44:1<77::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 61.Nikolova G, et al. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev. Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 62.Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Rafii S, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–19. [PubMed] [Google Scholar]
- 64.Rafii S, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- 65.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 66.Mathieu C, et al. Endothelial cell-derived bone morphogenetic proteins control proliferation of neural stem/progenitor cells. Mol. Cell Neurosci. 2008;38:569–577. doi: 10.1016/j.mcn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez-Castillejo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nature Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 68.Leventhal C, Rafii S, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from adult mammalian subependyma. Mol. Cell. Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 69.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 70.Matsunaga T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nature Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 71.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nature Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 72.Dias S, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J. Clin. Invest. 2000;106:511–521. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dias S, et al. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc. Natl Acad. Sci. USA. 2001;98:10857–10862. doi: 10.1073/pnas.191117498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petit I, et al. The microtubule-targeting agent CA4P regresses leukemic xenografts by disrupting interaction with vascular cells and mitochondrial-dependent cell death. Blood. 2008;111:1951–1961. doi: 10.1182/blood-2007-05-089219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dias S, Shmelkov SV, Lam G, Rafii S. VEGF165 promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532–2540. doi: 10.1182/blood.v99.7.2532. [DOI] [PubMed] [Google Scholar]
- 76.Koistinen P, et al. Regulation of the acute myeloid leukemia cell line OCI/AML-2 by endothelial nitric oxide synthase under the control of a vascular endothelial growth factor signaling system. Leukemia. 2001;15:1433–1441. doi: 10.1038/sj.leu.2402217. [DOI] [PubMed] [Google Scholar]
- 77.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nature Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 78.Pumiglia K, Temple S. PEDF: bridging neurovascular interactions in the stem cell niche. Nature Neurosci. 2006;9:299–300. doi: 10.1038/nn0306-299. [DOI] [PubMed] [Google Scholar]
- 79.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Folkins C, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 81.Nicosia RF, Tchao R, Leighton J. Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Res. 1983;43:2159–2166. [PubMed] [Google Scholar]
- 82.Rak JW, Hegmann EJ, Lu C, Kerbel RS. Progressive loss of sensitivity to endothelium-derived growth inhibitors expressed by human melanoma cells during disease progression. J. Cell Physiol. 1994;159:245–255. doi: 10.1002/jcp.1041590208. [DOI] [PubMed] [Google Scholar]
- 83.Zeng Q, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 87.Christov C, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol. Biol. Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 90.Barabe F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- 91.Jin L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 92.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 93.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 94.Kopp HG, et al. Tie-2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 96.Kopp HG, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J. Clin. Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- 98.Swendeman S, et al. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ. Res. 2008;103:916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urbich C, et al. FOXO-dependent expression of the proapoptotic protein Bim: pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005;19:974–976. doi: 10.1096/fj.04-2727fje. [DOI] [PubMed] [Google Scholar]
- 100.Rak J, Milsom C, Yu J. Tissue factor in cancer. Curr. Opin. Hematol. 2008;15:522–528. doi: 10.1097/MOH.0b013e3283063a3e. [DOI] [PubMed] [Google Scholar]
- 101.Rak J, Milsom C, Magnus N, Yu J. Tissue factor in tumour progression. Best Pract Res. Clin. Haematol. 2009;22:71–83. doi: 10.1016/j.beha.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Milsom C, et al. The role of tumor-and host-related tissue factor pools in oncogene-driven tumor progression. Thromb. Res. 2007;120:S82–S91. doi: 10.1016/S0049-3848(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 103.Milsom C, Yu J, May L, Magnus N, Rak J. Diverse roles of tissue factor-expressing cell subsets in tumor progression. Semin. Thromb. Hemost. 2008;34:170–181. doi: 10.1055/s-2008-1079257. [DOI] [PubMed] [Google Scholar]
- 104.Palumbo JS, Degen JL. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb. Res. 2007;120:S22–S28. doi: 10.1016/S0049-3848(07)70127-5. [DOI] [PubMed] [Google Scholar]
- 105.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 106.Potente M, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phung TL, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerritsen ME, et al. Branching out: a molecular fingerprint of endothelial differentiation into tube-like structures generated by Affymetrix oligonucleotide arrays. Microcirculation. 2003;10:63–81. doi: 10.1038/sj.mn.7800170. [DOI] [PubMed] [Google Scholar]
- 109.Kahn J, et al. Gene expression profiling in an in vitro model of angiogenesis. Am. J. Pathol. 2000;156:1887–1900. doi: 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez L, et al. Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp. Hematol. 2008;36:545–558. doi: 10.1016/j.exphem.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr. Opin. Hematol. 2008;15:307–311. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Candal FJ, et al. BMEC-1: a human bone marrow microvascular endothelial cell line with primary cell characteristics. Microvasc. Res. 1996;52:221–234. doi: 10.1006/mvre.1996.0060. [DOI] [PubMed] [Google Scholar]
- 113.Oostingh GJ, Schlickum S, Friedl P, Schon MP. Impaired induction of adhesion molecule expression in immortalized endothelial cells leads to functional defects in dynamic interactions with lymphocytes. J. Invest. Dermatol. 2007;127:2253–2258. doi: 10.1038/sj.jid.5700828. [DOI] [PubMed] [Google Scholar]
- 114.Buser R, Montesano R, Garcia I, Dupraz P, Pepper MS. Bovine microvascular endothelial cells immortalized with human telomerase. J. Cell Biochem. 2006;98:267–286. doi: 10.1002/jcb.20715. [DOI] [PubMed] [Google Scholar]
- 115.Nisato RE, et al. Generation and characterization of telomerase-transfected human lymphatic endothelial cells with an extended life span. Am. J. Pathol. 2004;165:11–24. doi: 10.1016/S0002-9440(10)63271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamaguchi T, et al. Development of a new method for isolation and long-term culture of organ-specific blood vascular and lymphatic endothelial cells of the mouse. FEBS J. 2008;275:1988–1998. doi: 10.1111/j.1742-4658.2008.06353.x. [DOI] [PubMed] [Google Scholar]
- 117.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nature Rev. Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 118.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Lee S, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.