Abstract
CD4+ T cells are an essential component of both the primary and secondary immune response against the intracellular protozoan parasite Leishmania major. Our lab has previously shown that CD62Lhigh IL7Rhigh central memory T (TCM) cells mediate protective immunity following secondary challenge. To determine when TCM cells develop, we examined the phenotype of Leishmania-specific CD4+ T cells in the first two weeks following infection. As expected, we identified a population of CD4+ T cells present in the draining lymph node with the characteristics of effector T cells. However, in addition, a second population phenotypically resembling TCM cells emerged coincident with the effector population. These T cells, expressing CD62L, CCR7, and the IL7R, failed to produce IFN-γ but had the capacity to give rise to IFN-γ-producing effector cells. Our studies also demonstrated that the degree of proliferation and the timing of lymph node entry impact TCM cell development. The early generation of TCM cells following L. major infection indicates that TCM cells may not only control secondary infections, but may also contribute to the control of the primary infection.
Keywords: Leishmania major, CD4+ T cells, Memory, CD62L expression
Introduction
A heterogeneous population of memory T cells with distinct phenotypes and functional responses are generated during an immune response. The subset of central memory T (TCM)4 cells maintains the ability to home to the lymph nodes (LNs) due to their expression of CD62L and CCR7 (1-3). TCM cells serve as a reservoir of antigen (ag)-specific T cells that can proliferate and become effector cells upon secondary challenge. A second population of cells, termed effector memory T (TEM) cells, can migrate through the tissues and upon encountering antigen rapidly produce effector cytokines such as IFN-γ or IL-4. Therefore, TEM cells may be poised to immediately respond to eliminate pathogens. How and when these memory T cells are generated remains unclear, and several models have been proposed to account for the heterogeneity in memory T cell populations (4-11). In contrast to models of memory generation following acute infection, it has been proposed that the lack of pathogen clearance may impair long-term memory development through the repeated stimulation of ag-specific T cells (12-15). Recent studies have suggested that the early CD4+ and CD8+ T cell response is heterogeneous, with some cells exhibiting the characteristics of memory T cells (or their precursors) while others have the phenotype of effector T cells (16-18). The majority of these studies have been done with pathogens that are rapidly cleared such as LCMV or Listeria monocytogenes, and whether the early heterogeneity observed in some of these studies will be evident with an infection where the pathogen is never cleared is unknown. Here we have used experimental infection with Leishmania major to ask whether CD4+ T cells with the phenotype of memory T cells develop early after infection.
C57BL/6 mice resolve a primary infection with the intracellular protozoan Leishmania major, although they maintain low levels of persisting parasites. Following resolution of the primary infection, mice are subsequently immune to rechallenge. This immunity is mediated by both CD4+ and CD8+ T cells, although CD4+ T cells alone can transfer resistance (19). We have identified three populations of CD4+ T cells that can participate in both the control of persistent parasites and resistance to reinfection: CD62Llow interleukin-7 receptor (IL7R)low T-bethigh cells, CD62LlowIL7Rhigh T-bethigh cells, and CD62LhighIL7Rhigh T-betlow cells (20). The latter subset has the characteristics of TCM cells since they migrate through LNs, can be maintained without antigen, and, upon restimulation, differentiate into Th1 cells that migrate to the site of infection and mediate parasite control (19). The ability of CD4+ TCM cells generated during L. major infection to differentiate into Th1 cells is dependent upon the presence of IL-12 since TCM cells from immune mice transferred into L. major infected IL-12p40−/− mice develop into Th2 cells (21). Thus, the flexible nature of the TCM cells induced by L. major infection suggests that they are not committed to a particular lineage and may develop prior to the acquisition of effector function. If this is the case, TCM cells could be generated early following infection and provide a pool of ag-specific T cells that upon further activation augment the effector T cell pool.
To determine if the early CD4+ T cell response to infection with L. major involved generation of cells with the characteristics of TCM cells, we examined the responses of both transgenic and polyclonal CD4+ T cells during the early weeks of L. major infection. We found that IL7R, CD62L and CCR7 expression defined two distinct populations of responding CD4+ T cells that could be observed by two weeks post-infection. One population expressed low levels of CD62L, CCR7, and the IL7R, while the other population expressed elevated levels of these molecules, characteristic of TCM cells. The TCM cells did not produce the effector cytokine IFN-γ directly ex vivo but could efficiently give rise to IFN-γ-producing Th1 effector cells following restimulation in vivo. We used a combination of LN-homing blockade, CFSE-labeling, and BrdU administration to show that the CD62Lhigh cells are stimulated in the draining LN within the first few days following infection but many of the TCM cells soon thereafter cease proliferation. These data show that the early CD4+ T cell response to L. major is heterogeneous and suggest that the generation of TCM cells is concurrent with the initiation of the primary immune response to L. major.
Materials & Methods
Mice
C57BL/6, B6.PL-Thy1a/CyJ (Thy1.1), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1), and C57BL/6-Tg(TcraTcrb)425Cbn/J (OTII) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or the National Cancer Institute (Fredericksburg, MD). Thy1.1+ OTII mice were bred and maintained in our facility. Animals were maintained in a pathogen free environment, and experiments were performed in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Parasites and infection
L. major (MHOM/IL/80/Friedlin) and Leish-OVA parasites were grown in Schneider’s insect medium supplemented with 20% heat-inactivated FBS and 2mM glutamine (22). Infectious stage metacyclic parasites were enriched using density gradient centrifugation (23). Mice were infected in the hind footpad or the ear dermis with 1-2 × 106 parasites.
Adoptive transfers and in vivo antibody treatment
CD4+ T cells were enriched from the lymphoid tissue of donor mice prior to transfer using MACS (Miltenyi Biotec). Where indicated, CD4+ cells were sorted from transgenic donor mice prior to transfer on a FACS Aria (Becton Dickinson). All cells were CFSE-labeled (1.25μM) (Molecular Probes), and 5 × 106 cells were transferred to congenic recipients unless otherwise noted in figure legends. In all experiments, the inguinal, axillary, and brachial lymph nodes were harvested and pooled together as representative peripheral LNs (also referred to as non-draining LNs) from individual naïve or infected mice. For detection of low numbers of transferred cells, the harvested tissues were first enriched by negative selection (MACS) for the donor marker prior to analysis by flow cytometry (24). Briefly, samples were incubated with biotinylated antibodies against CD45.1 followed by incubation with anti-biotin microbeads according to the manufacturer’s instructions (Miltenyi). The CD45.2+ donor cells were then negatively selected using LS columns. Blocking antibody against CD62L (MEL-14) was a gift from S. Reiner (University of Pennsylvania, Philadelphia, PA). 250μg of antibody was administered i.p. at the indicated time points.
Flow cytometry and BrdU treatment
The following antibodies used to detect cell surface markers were purchased from eBioscience: CD4, Thy1.2, Thy1.1, CD45.2, CD45.1, B220, CD11b, NK1.1, CD127 (IL7Rα) (PE or allophycocyanin only), and CD62L. For detection of CCR7, cells were incubated with CCR7 antibody (PE or allophycocyanin only) for 1hr at 37°, washed, and then stained for any remaining surface antigens as above. For intracellular detection of cytokines, cells were stimulated with PMA, ionomycin, and Brefeldin A for 4 hours in vitro and fixed with 2% paraformaldehyde in PBS. Cells were then permeabilized with 0.2% saponin and stained with IL-2-allophycocyanin and IFN-γ-PE-Cy7 (eBioscience). BrdU was purchased from BD Pharmingen, and 1 mg BrdU was administered i.p. every 12 hrs for 3 days (6mg total per mouse). Cells were permeabilized and stained for flow cytometry per the manufacturer’s instructions. Data was acquired on an LSR II or a FACS Canto (Becton Dickinson). Analysis was performed using FlowJo software (Tree Star, Inc.). For all samples, gating was established using a combination of isotype and fluorescence-minus-one (FMO) controls.
Statistics
Statistical analysis was performed using Prism (GraphPad Software, Inc.). For all graphs, data is presented as the mean ± SEM. One-way ANOVA was used to establish significance in combination with the indicated post test, and a p-value of < 0.05 was considered significant.
Results
IL7R expression on CD4+ T cells defines two populations of T cells responding to L. major infection
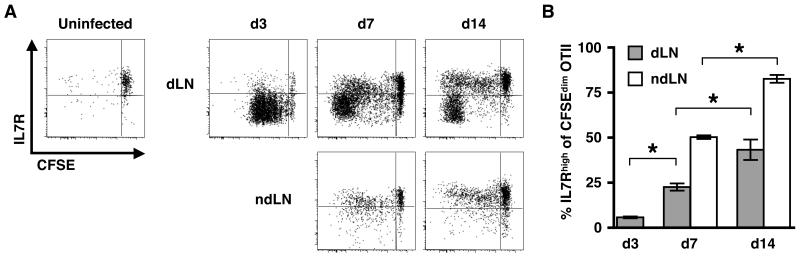
In order to monitor the ag-specific CD4+ T cell response to L. major, we adoptively transferred CFSE-labeled OTII T cells into congenic C57BL/6 mice and challenged the recipient mice with L. major parasites expressing OVA (Leish-OVA) (22). Proliferation and IL7R expression on the ag-specific OTII cells in the LN draining the site of infection (dLN) was assessed on days 3, 7 and 14 following infection (Fig. 1A & 1B). We initially chose to focus on the expression of the IL7R since its expression is required for the survival of memory T cells (25-27). As early as 3 days post infection (pi) there was a robust proliferative response of the OTII cells. Almost all of the proliferating cells downregulated the IL7R, which is consistent with previous studies (25, 28-37). However, we observed two distinct populations of responding cells by 7 and 14 days pi. One group of proliferating CD4+ T cells expressed a low level of the IL7R, a phenotype suggestive of an activated effector T cell that has recently been stimulated via the TCR (38). The other population of responding cells expressed the IL7R at levels equivalent to that of naïve T cells. We also observed that by day 7, proliferated OTII cells could be detected within other peripheral (non-draining; nd) LNs. Most likely these T cells proliferated in the dLN and then migrated to the ndLNs. Interestingly, only the IL7R-expressing T cells were found in ndLNs suggesting that these two T cell populations may exhibit differences in their migratory potential or that migration away from the inflammatory site allows for the upregulation of the IL7R. While parasites can disseminate through the blood to distant sites (39), the lack of an IL7Rlow population in the ndLNs could suggest that priming within the ndLNs is minimal or that the activation of CD4+ cells under such conditions generates cells with a TCM-like phenotype. Thus, based upon expression of the IL7R, within two weeks of L. major infection, two distinct populations of CD4+ T cells are evident.
Figure 1. Dynamic IL7R expression on proliferating ag-specific CD4+ T cells following L. major infection.
(A) OTII cells were CFSE-labeled and transferred to Thy disparate recipients that were infected with Leish-OVA the following day. Draining (dLN) and non-draining (ndLN) lymph nodes were harvested on the indicated days pi, and the donor OTII cell population (CD4+ Thy1.1+) was analyzed for IL7R expression. Plots are representative of 5 mice in 2 separate experiments. Peripheral LNs from uninfected controls were harvested and pooled 15 days following OTII transfer (d14 pi). The percentage of donor cells with an IL7Rhigh phenotype within the CFSEdim population is shown in B for both the dLN (grey bars) and ndLNs (white bars). A Tukey post test was used to compare differences between individual groups. * p < 0.05
Expression of LN homing molecules by CD4+ T cells responding to L. major infection
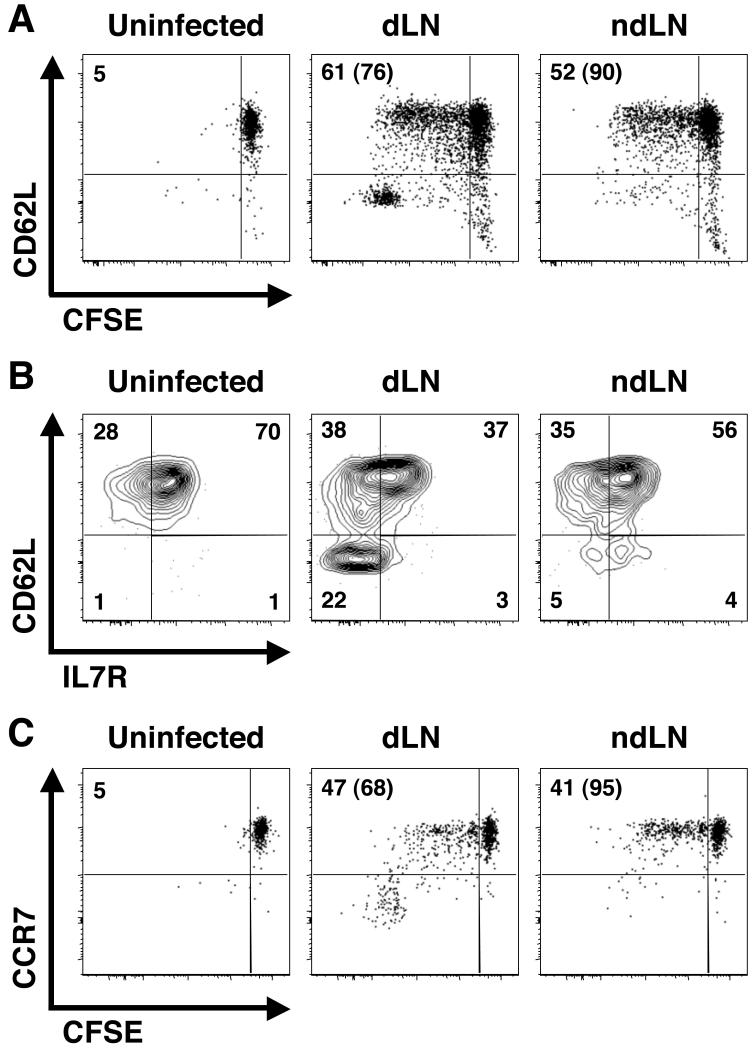
The expression of CD62L and CCR7 is required for efficient entry of T cells into LNs, and their differential expression has also been used to define memory T cell subsets (1). We have previously reported that CD62Lhigh CD4+ TCM cells are present in mice that have resolved a primary infection with L. major (referred to as ‘immune mice’) (19), and we wanted to determine if cells with a similar phenotype were present early after infection. Therefore, we again adoptively transferred CFSE-labeled OTII cells to naïve recipients that were subsequently challenged with Leish-OVA and asked if the T cells responding to infection exhibited differential expression of CD62L and CCR7. As shown in Fig. 2, a pattern similar to that observed with IL7R expression was seen in proliferating (CFSEdim) OTII T cells. Thus, two populations of responding T cells were present early after infection: one population of cells expressed CD62L and CCR7, while the second did not (Fig. 2A and 2C). Furthermore, the CD62Llow cells expressed uniformly low levels of the IL7R while the population of CD62Lhigh cells contained both IL7Rhigh and IL7Rlow cells (Fig. 2B). We confirmed these results with another TCR transgenic mouse (termed ABLE) that recognizes a Leishmania-derived peptide (LACK156-173; Leishmania homolog of receptors for activated C kinase) in the context of MHC II I-Ad and found similar results (data not shown).
Figure 2. CD62L and CCR7 are expressed by a subset of L. major-specific CD4+ T cells early following infection.
(A-C) CFSE-labeled OTII cells were transferred to congenic recipients, which were infected with Leish-OVA the following day. The dLN and several ndLNs were harvested on day 10 pi and stained for surface expression of CD62L or CCR7. Plots have been gated on CD4+ CD45.2+ transgenic donor cells and are representative of 6-7 mice in 2-3 separate experiments. The phenotype of the donor cells in lymphoid tissue of uninfected mice is shown as a control. The numbers in the upper left of each plot (A and C) represent the percentage of donor cells that are CFSEdim with the percentage of CFSEdim cells exhibiting high levels of CD62L or CCR7 surface expression shown in parenthesis. In B, CD62L versus IL7R expression is shown on the proliferated (CFSEdim) cells from infected mice in A. Surface expression of these molecules is shown on naïve donor cells, which are CFSEbri, from uninfected mice as a control. Numbers in B indicate the percentage of cells in each quadrant.
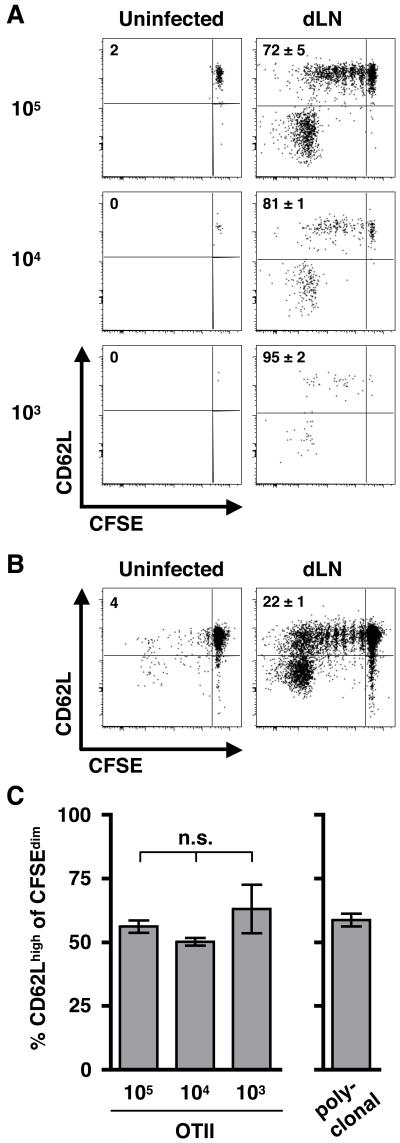
Recent work has demonstrated that adoptive transfer of large numbers of transgenic T cells can impact the emergence of memory T cells (40-43). Thus, to determine if the generation of these two different cell types was due to the transfer of non-physiologic numbers of T cells with the same specificity, we repeated the above experiment but transferred reduced numbers of TCR transgenic cells (105, 104, and 103). Due to the loss of cells following transfer, the actual numbers present in spleen and LN tissues following transfer corresponds roughly to 104, 103, and 102, respectively (44). We then infected the recipient mice with Leish-OVA and isolated the dLN after 10 days. We found that regardless of the initial number of cells transferred, two populations of cells could be defined by CD62L expression, and the relative frequency of each population was the same (Fig. 3A & 3C). However, when lower numbers of cells were transferred, the extent of CFSE dilution was increased as only a small percentage of OTII cells remained CFSEbri after 10 days, and the fold increase in proliferating ag-specific cells over input was significantly increased (Fig. 3A and data not shown). These data are consistent with previously published findings that proliferation is dramatically influenced by the frequency of T cells recognizing a particular epitope (41).
Figure 3. Phenotypic heterogeneity is maintained when varying the initial frequency of ag-specific cells.
(A) Vβ5+ Vα2+ CD4+ T cells were FACS purified from OTII mice and CFSE-labeled, and the indicated numbers of cells were transferred to naïve congenic recipients. The phenotype of the transferred cells (CD45.2+ CD45.1−) in the dLN on d10 is shown, and plots are representative of 3 mice per group in 2 separate experiments. To enhance detection of low frequencies of OTII cells, dLN tissue (infected mice) or pooled peripheral LNs (uninfected controls) were enriched for CD45.2 donor cells prior to flow cytometric analysis as described in the Materials & Methods. Numbers in each plot indicate the percentage of donor cells that are CFSEdim with the percentage of CFSEdim cells that are CD62Lhigh in parenthesis. (B) CD4+ cells were enriched from the spleens and LNs of naïve C57BL/6 mice, and 5 × 106 cells were transferred to naïve CD45.1 recipients following CFSE-labeling. Recipient mice were infected with WT L. major, and the dLN was isolated after 2 weeks. Plots have been gated on CD4+ CD45.2+ cells and are representative of >12 mice in more than 4 separate experiments. (C) Bar graphs indicate the percentage of cells expressing high levels of CD62L for the OTII (A) and polyclonal (B) T cell transfers shown above. Using one-way ANOVA, we found that any differences between the groups were not significant (n.s.).
To further confirm that our results reflected the naturally occurring immune response following infection, we adoptively transferred CFSE-labeled polyclonal CD4+ T cells from naïve conventional C57BL/6 mice to congenic recipients (CD45.1) that were subsequently infected with wild-type L. major parasites. This allows us to monitor naturally occurring Leishmania-specific CD4+ T cells at normal physiologic frequencies within a given pool of naïve T cells based upon their proliferation and dilution of CFSE. We isolated the cells from the dLN after 2 weeks and found that similar to the T cells from the TCR transgenic mice, polyclonal CD4+ T cells from conventional mice exhibited the same degree of heterogeneity, generating both CD62Llow and CD62Lhigh populations of responding CD4+ T cells (Fig. 3B & 3C). These results further support our findings that infection with L. major rapidly generates a heterogeneous population of CD4+ T cells. Thus, we can conclude that the early CD4+ T cell response to L. major infection generates at least two populations of cells that can be distinguished based on their expression of both the IL7R and LN-homing molecules and that one of these populations has the phenotype of TCM cells.
Proliferative arrest generates CD62Lhigh T cells early following infection
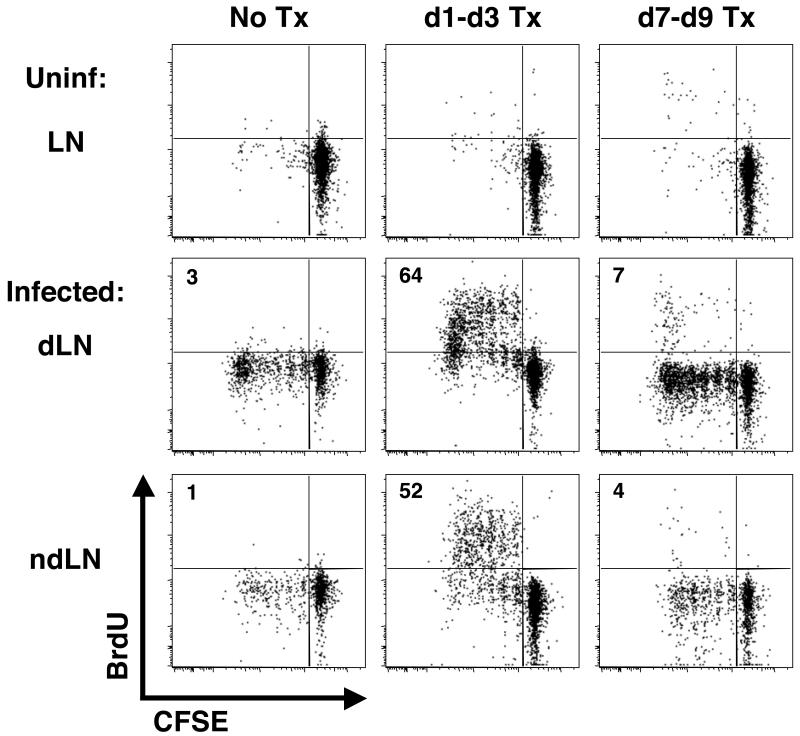
In addition to the differential expression of the IL7R and molecules associated with LN-homing, we noticed that the CD4+ T cells responding to infection could be characterized by how many cell divisions they had undergone with a substantial enrichment in the CD62Lhigh cells in divisions 1 through 5 (Figs. 2 & 3). This enrichment varied, as is evident when looking at Figures 3A and 3B. One cause for such variation could be the specificity of the monoclonal versus polyclonal cells. Alternatively, the dLNs were collected at different times (d10 versus d14 pi), and as the infection proceeds, one might anticipate that the CD62Lhigh cells would have an increased opportunity to be restimulated and further dilute CFSE. By focusing on these early time, points we can examine how cell division might contribute to the generation of the heterogeneous CD4+ T cell response. Since division was assessed by CFSE dilution after 7-14 days of infection, we could not determine when over the course of that time the cells in division 1-5 had actually divided. The cells in divisions 1-5 could represent (1) cells that had only recently begun to divide or (2) T cells that received a signal to proliferate early following infection but had ceased dividing. To distinguish between these two possibilities, we transferred CFSE-labeled OTII cells into congenic recipients that were given BrdU at different times pi to assess when the CFSEdim cells had proliferated. One group of mice was given BrdU from days 1 to 3, and a second group of mice received BrdU from days 7 to 9. Thus, if the cells were proliferating just prior to sacrifice, the CFSEdim OTII cells in divisions 1-5 would also be BrdU+ in mice in mice receiving BrdU from days 7 to 9. However, we found that only when BrdU was administered during the first 3 days following Leish-OVA infection was a large percentage of BrdU+ cells detected in the dLN on day 10 pi (Fig. 4). Interestingly, many of these BrdU+ cells were also detected in the ndLNs, suggesting that these cells proliferated in the first 3 days, likely in the dLN, but then migrated to other lymphoid tissues. When the BrdU was given just prior to the end of the experiment (days 7 to 9), the small percentage of cells that were BrdU+ were present in those cells that had undergone >5 divisions. Thus, through the combination of CFSE dilution and the incorporation of BrdU, we can conclude that many of the cells with a TCM phenotype that are observed early following infection received a signal to proliferate in the first 3 days following infection but then underwent an active cessation in proliferation.
Figure 4. The CD62Lhigh population undergoes early proliferation followed by proliferative arrest.
CFSE-labeled OTII cells were transferred to naïve congenic recipients which were infected with Leish-OVA the following day (d0). Groups of mice were given 6 injections of BrdU over a 3-day period (either d1-d3 or d7-d9). All mice were sacrificed on d10 at which time the incorporation of BrdU by the donor cells in the dLN was analyzed by flow cytometry. All plots have been gated on the CD4+ CD45.2+ CD45.1− donor cells and are representative of 3 mice per group in 2 separate experiments.
The timing of T cell arrival in the draining LN influences their ability to respond
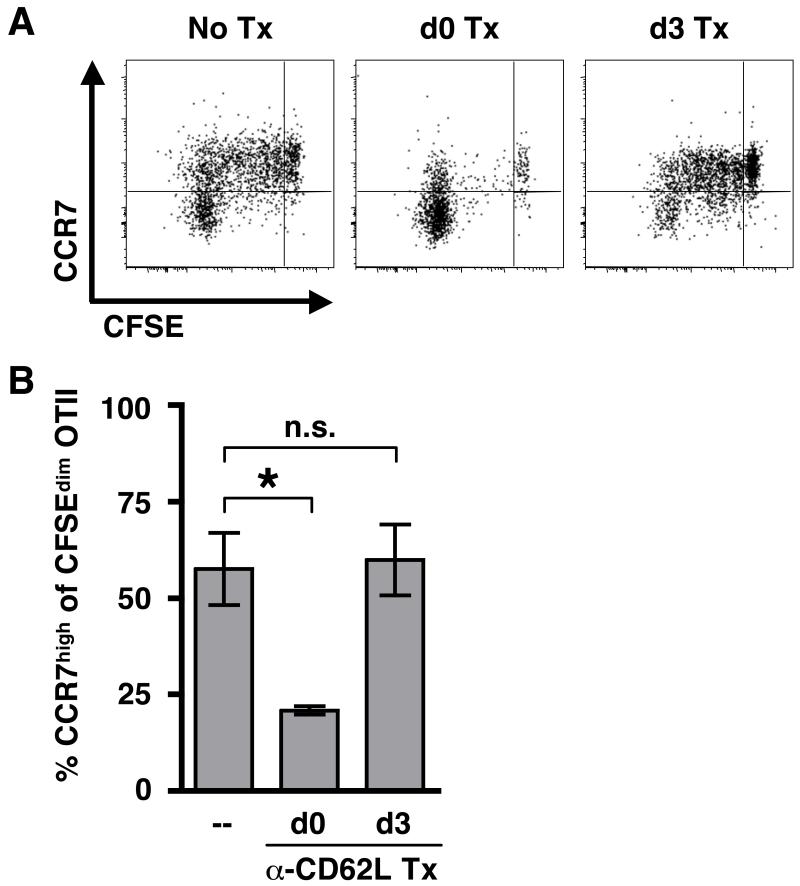
The above results indicate that only some CD4+ T cells continue to divide following infection with L. major, and those with reduced proliferation exhibit the phenotype of TCM cells. In previous studies, we found that naïve T cells proliferated poorly when transferred into already infected mice, suggesting that when T cells are recruited into the immune response could influence how well they respond (45). Similarly, studies in mice immunized with peptide showed that early arriving T cells proliferated more while late arriving T cells proliferated less and were directed towards a TCM fate (46). Thus, we considered the possibility that one difference between the cells that had divided >5 times and those that had ceased proliferating was when they entered the dLN. To determine if this was the case, we assessed the response of T cells that were resident in the dLN at the time of infection by blocking the entry of additional recruited T cells into the LN using monoclonal antibodies against CD62L. In one group of mice, anti-CD62L mAb was administered at the time of Leish-OVA infection (one day after the transfer of OTII cells). A second group of mice did not receive anti-CD62L mAb treatment until 3 days pi, which allowed for transient recruitment of cells into the dLN. Because anti-CD62L mAb treatment prevents surface staining for CD62L, we used expression of CCR7 to differentiate between the two T cell populations. We found that very few cells were in divisions 1-5 when LN recruitment was blocked at the time of infection (Fig. 5A). Most of the cells found in the dLN at day 10 pi had divided >5 times and expressed low levels of CCR7 (Fig 5A & 5B). However, a population of T cells in divisions 1-5 and expressing CCR7 were present in the dLNs of mice when antibody treatment was withheld until day 3. These data suggest that the T cells resident in the dLN at the time of infection are more likely to differentiate into effector T cells, especially in the absence of newly recruited cells, which are more likely to develop a TCM phenotype. In addition, our results suggest that recruited cells can be directed towards a TCM fate within the first 3 days following infection.
Figure 5. LN-homing can impact the early-emerging population of CCR7high cells.
(A) CFSE-labeled OTII cells were transferred to naïve congenic recipients one day prior to infection with Leish-OVA to allow for systemic distribution of naïve transgenic cells. Mice were then treated with MEL-14 (anti-CD62L) (250 μg) on either d0 (at the time of infection) or d3 to block LN-homing. All plots have been gated on the CD4+ CD45.2+ CD45.1− donor cells and are representative of 3 mice per group in 2 separate experiments. (B) The bar graph indicates the percentage of CFSEdim cells expressing high levels of CCR7 for each group of mice. Statistical significance was established using one-way ANOVA followed by a Dunnett post test. * p < 0.05; not significant (n.s.)
CD62Lhigh T cells can become IFN-γ producing T cells upon restimulation
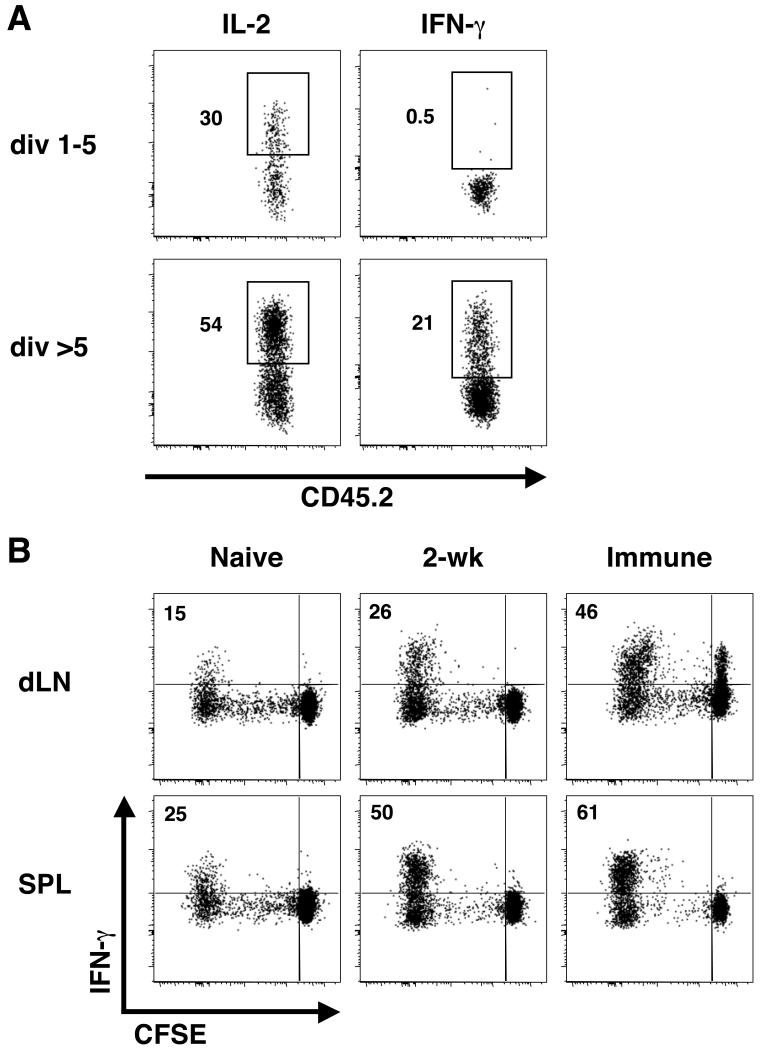
The lack of IFN-γ production by TCM cells is one of the critical characteristics that was shown to distinguish TCM cells from effector cells following L. major infection (19). Therefore, we next assessed the ability of the different types of early responding CD4+ T cells to produce IFN-γ directly ex vivo. Naïve polyclonal CD4+ T cells were enriched from the spleens and LNs of C57BL/6 mice, labeled with CFSE, and transferred to naïve congenic recipients. The recipient mice were infected with L. major the following day, and at 2 weeks pi, the dLN was isolated to determine cytokine production by responding (CFSEdim) CD4+ T cells in the dLN. Because in vitro stimulation with PMA and ionomycin can influence surface expression of CD62L, we used the extent of CFSE dilution to compare TCM cells in divisions 1-5 to the heterogeneous population of CD62Lhigh TCM and CD62Llow effector T cells in >5 divisions. While both populations had the capacity to make IL-2, IFN-γ was only produced by cells that had divided more than 5 times (Fig. 6A). Thus, similar to the TCM cells that we observed >12 weeks pi, the early responding T cells that have ceased dividing and adopted a TCM phenotype fail to make IFN-γ. To determine if the cells with a TCM phenotype that emerge in the early weeks following infection could differentiate into IFN-γ-producing cells upon restimulation, we purified CD4+ CD62Lhigh cells from mice that had been infected with L. major for only 2 weeks. Based on the results shown in Fig. 3B, this population of donor cells would include a mixture of Leishmania-specific cells that have undergone varying rounds of division. We then CFSE-labeled the cells and adoptively transferred them into congenic recipients that were subsequently challenged with L. major. As controls, we also purified cells with this phenotype from naïve and immune mice. We isolated the dLNs and spleens after 2 weeks and compared the frequency of IFN-γ-producing cells within the total population of CFSEdim cells. This comparison allowed us to normalize for the expected differences in ag-specific cells that would be present in these three different populations of donor T cells. We found that while a small percentage of cells derived from naïve mice could produce IFN-γ after 2 weeks, this percentage was increased ~2-fold when the donor cells were derived from mice that had been infected for 2 weeks prior to isolation, transfer, and rechallenge (Fig. 6B). Thus, we found that at least some of the CD62Lhigh cells generated early after infection had the capacity to differentiate into Th1 cells, similar to the TCM cells we characterized in immune mice. Using current protocols, it is unknown whether the Th1-generating cells had undergone more or less rounds of division prior to restimulation. The increase in the percentage of IFN-γ-producing T cells by donor-derived cells from 2-week infected mice compared with naïve donor cells, in combination with the lack of IFN-γ production by cells in divisions 1-5, suggests that while the CD62Lhigh cells may not produce IFN-γ directly ex vivo, some of the cells may already be predisposed along a pathway towards Th1 cytokine production. This would be consistent with our recent findings that some CD62Lhigh cells, while not making IFN-γ, express T-bet, the transcription factor required for IFN-γ production (20).
Figure 6. Cytokine production by TCM cells.
(A) Polyclonal CD4+ T cells were enriched from naïve C57BL/6 mice and CFSE-labeled prior to transfer to naïve CD45.1 recipients. The recipient mice were infected with WT L. major the following day. After 2 weeks, the extent of CFSE dilution within the CD4+ CD45.2+ CD45.1− donor cells in the dLN was used to gate the plots shown in A which are representative of >12 mice in more than 4 separate experiments. Intracellular cytokine production was detected ex vivo following a 4hr in vitro stimulation. Numbers indicate the percent cytokine positive. (B) CD4+ CD62Lhigh cells from the spleens and LNs of naïve mice, mice that had been infected with 2 × 106 WT L. major 2 weeks prior, and immune mice (>12 weeks post L. major infection) were MACS purified, CFSE-labeled, and transferred to congenic recipients that were challenged with WT L. major the following day. After 2 weeks, the dLN and spleen (SPL) were harvested, and cytokine production by the CD4+ CD45.2+ CD45.1− donor cells was determined as above. Plots are representative of 3-4 mice per group in 2 separate experiments. Numbers indicate the percentage of CFSEdim cells that are producing IFN-γ.
Discussion
The hallmark of adaptive immunity is the ability to develop immunologic memory, such that following a primary infection we are better able to respond when re-exposed to the same pathogen. While immunologic memory is a critical component of the immune response, how and when memory develops is still not completely understood. We previously demonstrated that following infection with L. major, C57BL/6 mice develop CD4+ TCM cells that can mediate resistance to reinfection (19). Here, we find that CD4+ T cells with a TCM phenotype develop within the first few weeks following L. major infection, coincident with the development of CD4+ Th1 effector cells. These TCM cells expressed high levels of CD62L and CCR7, which promote migration through lymphoid tissues, and high levels of the IL7R, which is required for the long-term survival of memory T cells. Cytokine production by these early emerging TCM cells was limited to IL-2 and required antigen-induced proliferation in order to give rise to IFN-γ-producing Th1 cells. Lastly, we provided evidence that the phenotype of the CD4+ T cells is influenced by the timing of their arrival in the dLN and involves the proliferative arrest of ag-specific T cells, despite the continued presence of parasites. Taken together, these results indicate that CD4+ T cells with a TCM phenotype develop during the early stages of an immune response.
There are several models that attempt to explain how memory T cells develop, and while some postulate that memory T cells slowly develop after the pathogen has been cleared, others propose that memory T cells–or their precursors–develop very soon after infection (4-11). Our data indicate that CD4+ T cells with a TCM phenotype (CD62Lhigh CCR7high IL7Rhigh) develop quite rapidly following infection with L. major. These cells do not immediately produce IFN-γ upon restimulation, suggesting that they develop prior to becoming effector cells. These results are consistent with our previous studies indicating that TCM cells in immune mice do not make IFN-γ and that, in the absence of IL-12, they can differentiate into Th2 cells (19, 21). Catron et al. described a similar population of CD62Lhigh CD4+ T cells that were generated following peptide immunization (46), suggesting that these early emerging TCM cells are not unique to leishmaniasis and may represent a normal component of the CD4+ T cell response. Thus, while cytokine-producing cells can contribute to the pool of long-lived memory T cells (47), our data suggests that progression through an effector phase is not a requirement for TCM generation. It is also possible that depending on the nature of the infection, TCM-like cells might develop both prior to and after becoming effector cells (48).
The mechanism(s) driving the heterogeneity of the early T cell response is not well understood. The initial interaction between a T cell and the APC can lead to asymmetric cell division, such that one daughter cell becomes an effector cell while the other a less differentiated cell that exhibits a memory phenotype (18). Alternatively, Lanzavecchia and Sallusto proposed the signal strength hypothesis as a mechanism to explain the heterogeneity of the CD4+ T cell response (49). Their hypothesis suggests that those cells receiving weaker signals will become TCM cells while those cells receiving the strongest signals will become fully differentiated effector cells that exert their function and then die. Support for this hypothesis was provided by Wu et al. who showed that acquisition of IFN-γ production by multiple rounds of stimulation with antigen led to the inability of a CD4+ T cell to survive long-term (50). However, more recent studies have shown that IFN-γ-producing cells can contribute to the pool of long-lived memory T cells (47). Despite their expression of the IL7R, it is not thought that signals through the IL7R drive TCM cell development (32, 51, 52). Also, whether or not a CD4+ T cell receives successive stimulation can have a direct impact on the degree of proliferation and the generation of memory (15, 53, 54) It is probable that a range of signal strengths exist with differential outcomes on individual CD4+ T cells. Therefore, the overall extent of stimulation can impact the type of memory T cell generated and depends on the cumulative signals imparted on a given cell through successive interactions with DCs, costimulation, and the inflammatory milieu (6, 40, 55).
By using OTII cells to track the ag-specific CD4+ T cell response, we found that most of the TCM cells evident two weeks after L. major infection have ceased proliferating with many of them having divided only a few times. Why they fail to continue to proliferate is unknown. It is possible that T cells receive an inhibitory signal that actively inhibits proliferation (56). However, since continued TCR stimulation is required for CD4+ T cells to continue to divide (57), the simplest explanation for the proliferative arrest may be a loss of TCR signaling. Thus, the proliferative arrest might result from the lack of a positive signal rather than the presence of a negative signal. For example, it has been proposed that weaker signals are the result of a stochastic event caused by migration away from the site of antigen presentation (49, 58). Consistent with this idea are our findings that some of the TCM cells are present in ndLNs soon after infection. These cells have the potential to migrate back through the dLN where they can be stimulated once again and give rise to Th1 cells as needed to control the parasite burden. Competition among responding T cells can also have a negative impact on the ag-specific T cell response as indicated in several studies using TCR transgenic cells (40, 41). Similar to these studies, we found that altering the number of ag-specific T cells influenced the extent of proliferation. However, it did not change the percentage of T cells that exhibited a TCM phenotype. Moreover, polyclonal responses—where a diverse repertoire of ag-specific T cells is maintained at physiologic frequencies—exhibited the same early development of TCM cells. Thus, while competition with non-physiologic numbers of T cells can influence the magnitude of the proliferative response, it does not appear to be responsible for the generation of TCM cells following L. major infection. On the other hand, we found that when T cells arrived in the dLN did influence the nature of the T cells that responded such that only the cells recruited into the dLN developed a TCM phenotype. Using a peptide immunization model with similar results, it was suggested that this was due to decreasing antigen over time (46). However, following L. major infection, the parasite burden continues to increase during the first several weeks of infection, although it has also been proposed that there are two waves of antigen presentation with the second wave commencing after two weeks (59, 60). Thus, a more likely explanation for our findings might be the ability of Leishmania to impair antigen presentation by DCs resulting in an overall decrease in the extent of T cell activation (61-64). By two weeks pi, those cells that had fully diluted CFSE (>5 div) were a heterogeneous population containing both CD62Lhigh and CD62Llow cells.
Infection of C57BL/6 mice with L. major is associated with the development of a cutaneous lesion that resolves after several weeks due to the development of CD4+ Th1 response. However, a small number of parasites persist in these mice. The control of these remaining parasites is dependent upon the maintenance of an effective Th1 response, such that treatment of mice that have resolved a primary L. major infection with anti-IFN-γ leads to the reactivation of the disease. How this Th1 immunity is maintained is not well understood. One possibility involves the continued recruitment of naïve T cells into the response, yet we have found that naïve Leishmania-specific T cells respond poorly in the presence of previously activated T cells (45). Alternatively, the TCM cell population that we have described here may serve as an additional source of ag-experienced T cells that can recirculate through the dLN and receive additional signals to further differentiate into Th1 effector cells as needed to help control the infection. Certainly, if most highly activated Th1 cells eventually die, the maintenance of a population of TCM cells might be essential to control the parasites that persist for the lifetime of the animal.
In summary, we have demonstrated that the early CD4+ T cell response to L. major infection involves the generation of both effector T cells and T cells with the characteristics of TCM cells. The heterogeneity of the response is characterized by differential expression of CD62L, CCR7, and the IL7R, as well as the ability to produce the effector cytokine IFN-γ. These TCM cells are available as an expanded pool of ag-specific T cells able to be restimulated and differentiate into effector cells, a function that may be critical in controlling not only secondary infections but also the parasites that persist in L. major infections.
Acknowledgements
We thank Paul Kaye and Deborah Smith for Leish-OVA parasites, Steve Reiner for MEL-14 monoclonal antibody, and the Penn Flow Cytometry & Cell Sorting Facility for their technical assistance.
Footnotes
This work was supported by the National Institutes of Health grant AI35914 (to P.S.).
- TCM
- central memory T
- TEM
- effector memory T
- Th1
- T helper type 1
- Th2
- T helper type 2
- pi
- post-infection
- dLN
- draining lymph node
- ndLN
- non-draining lymph node
- WT
- wild-type
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 5.Lefrancois L, Marzo AL. The descent of memory T-cell subsets. Nat Rev Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]
- 6.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. Trends Immunol. 2006;27:261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Intlekofer AM, Wherry EJ, Reiner SL. Not-so-great expectations: re-assessing the essence of T-cell memory. Immunol Rev. 2006;211:203–213. doi: 10.1111/j.0105-2896.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell EB, Westermann J. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends Immunol. 2008;29:405–411. doi: 10.1016/j.it.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Jelley-Gibbs DM, Strutt TM, McKinstry KK, Swain SL. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunol Cell Biol. 2008;86:343–352. doi: 10.1038/icb.2008.13. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 12.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 13.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jelley-Gibbs DM, Dibble JP, Filipson S, Haynes L, Kemp RA, Swain SL. Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med. 2005;201:1101–1112. doi: 10.1084/jem.20041852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 17.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 19.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 20.Colpitts SL, Dalton NM, Scott P. IL-7 receptor expression provides the potential for long-term survival of both CD62Lhigh central memory T cells and Th1 effector cells during Leishmania major infection. J Immunol. 2009;182:5702–5711. doi: 10.4049/jimmunol.0803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakpour N, Zaph C, Scott P. The central memory CD4(+) T cell population generated during Leishmania major infection requires IL-12 to produce IFN-gamma. J Immunol. 2008;180:8299–8305. doi: 10.4049/jimmunol.180.12.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prickett S, Gray PM, Colpitts SL, Scott P, Kaye PM, Smith DF. In vivo recognition of ovalbumin expressed by transgenic Leishmania is determined by its subcellular localization. J Immunol. 2006;176:4826–4833. doi: 10.4049/jimmunol.176.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 24.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 26.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 29.Bixby LM, Tarleton RL. Stable CD8+ T cell memory during persistent Trypanosoma cruzi infection. J Immunol. 2008;181:2644–2650. doi: 10.4049/jimmunol.181.4.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbin GA, Harty JT. Duration of infection and antigen display have minimal influence on the kinetics of the CD4+ T cell response to Listeria monocytogenes infection. J Immunol. 2004;173:5679–5687. doi: 10.4049/jimmunol.173.9.5679. [DOI] [PubMed] [Google Scholar]
- 31.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 32.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 33.Koesters SA, Alimonti JB, Wachihi C, Matu L, Anzala O, Kimani J, Embree JE, Plummer FA, Fowke KR. IL-7Ralpha expression on CD4(+) T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36:336–344. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 34.Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T, Probst HC, Hengartner H, Zinkernagel RM. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 35.Tripathi P, Mitchell TC, Finkelman F, Hildeman DA. Cutting Edge: Limiting amounts of IL-7 do not control contraction of CD4+ T cell responses. J Immunol. 2007;178:4027–4031. doi: 10.4049/jimmunol.178.7.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Leeuwen EM, de Bree GJ, Remmerswaal EB, Yong SL, Tesselaar K, ten Berge IJ, van Lier RA. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106:2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 37.Wilson DC, Matthews S, Yap GS. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol. 2008;180:5935–5945. doi: 10.4049/jimmunol.180.9.5935. [DOI] [PubMed] [Google Scholar]
- 38.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolas L, Sidjanski S, Colle JH, Milon G. Leishmania major reaches distant cutaneous sites where it persists transiently while persisting durably in the primary dermal site and its draining lymph node: a study with laboratory mice. Infect Immun. 2000;68:6561–6566. doi: 10.1128/iai.68.12.6561-6566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitmire JK, Benning N, Eam B, Whitton JL. Increasing the CD4+ T cell precursor frequency leads to competition for IFN-gamma thereby degrading memory cell quantity and quality. J Immunol. 2008;180:6777–6785. doi: 10.4049/jimmunol.180.10.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray PM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major. J Immunol. 2006;177:925–933. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catron DM, Rusch LK, Hataye J, Itano AA, Jenkins MK. CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 48.Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J Immunol. 2006;177:511–518. doi: 10.4049/jimmunol.177.1.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 50.Wu CY, Kirman JR, Rotte MJ, Davey DF, Perfetto SP, Rhee EG, Freidag BL, Hill BJ, Douek DC, Seder RA. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 51.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doyle AM, Mullen AC, Villarino AV, Hutchins AS, High FA, Lee HW, Thompson CB, Reiner SL. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med. 2001;194:893–902. doi: 10.1084/jem.194.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yarke CA, Dalheimer SL, Zhang N, Catron DM, Jenkins MK, Mueller DL. Proliferating CD4+ T cells undergo immediate growth arrest upon cessation of TCR signaling in vivo. J Immunol. 2008;180:156–162. doi: 10.4049/jimmunol.180.1.156. [DOI] [PubMed] [Google Scholar]
- 58.Harbertson J, Biederman E, Bennett KE, Kondrack RM, Bradley LM. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J Immunol. 2002;168:1095–1102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- 59.Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, Aebischer T. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol. 2004;34:715–725. doi: 10.1002/eji.200324391. [DOI] [PubMed] [Google Scholar]
- 60.Iezzi G, Frohlich A, Ernst B, Ampenberger F, Saeland S, Glaichenhaus N, Kopf M. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J Immunol. 2006;177:1250–1256. doi: 10.4049/jimmunol.177.2.1250. [DOI] [PubMed] [Google Scholar]
- 61.Fruth U, Solioz N, Louis JA. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–1864. [PubMed] [Google Scholar]
- 62.Antoine JC, Prina E, Courret N, Lang T. Leishmania spp.: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv Parasitol. 2004;58:1–68. doi: 10.1016/S0065-308X(04)58001-6. [DOI] [PubMed] [Google Scholar]
- 63.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carvalho LP, Pearce EJ, Scott P. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-alpha, whereas bystander dendritic cells are activated to promote T cell responses. J Immunol. 2008;181:6473–6480. doi: 10.4049/jimmunol.181.9.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]