Abstract
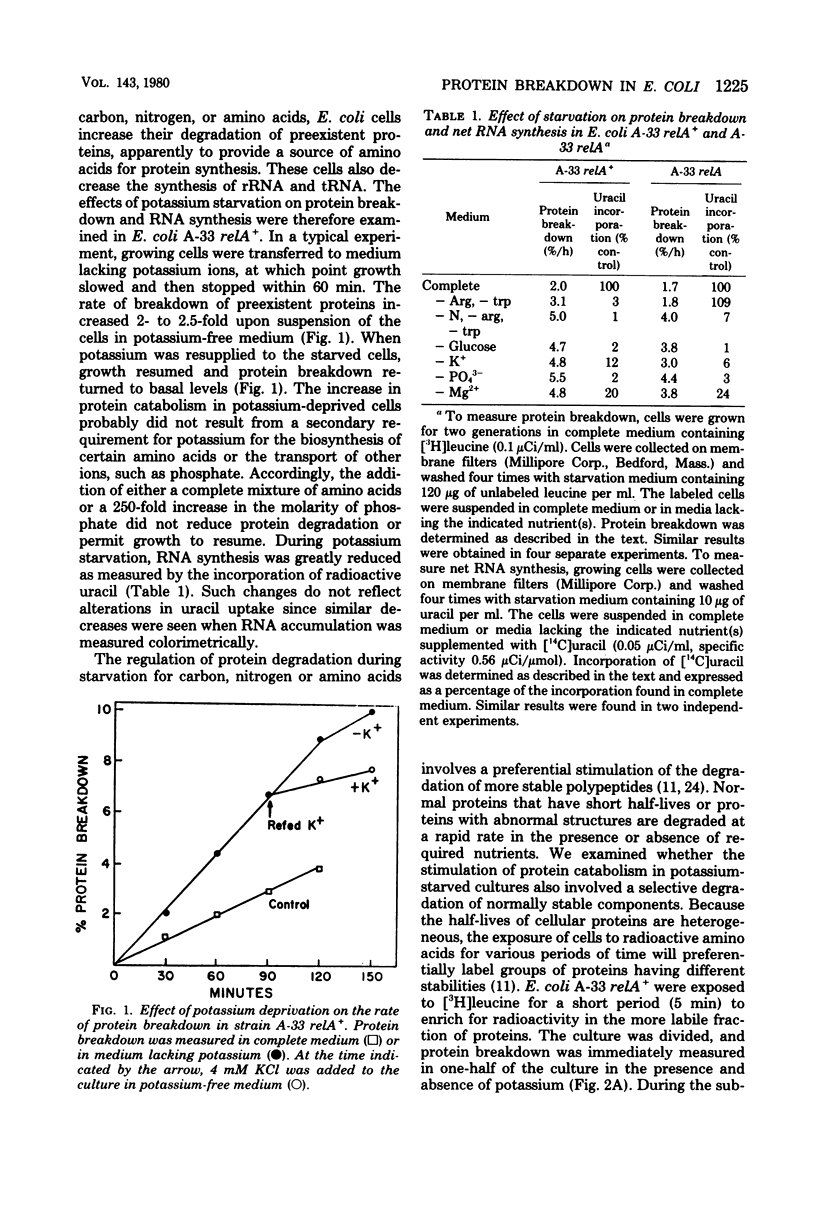
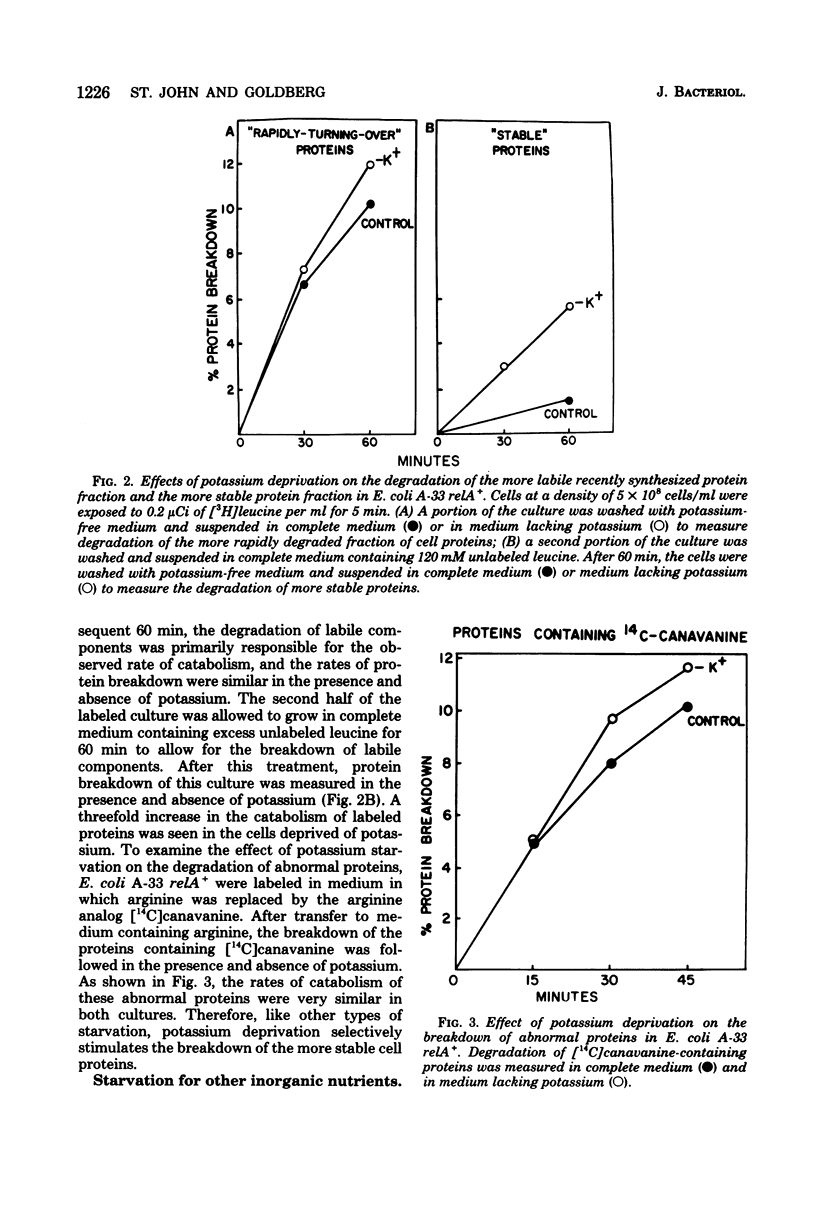
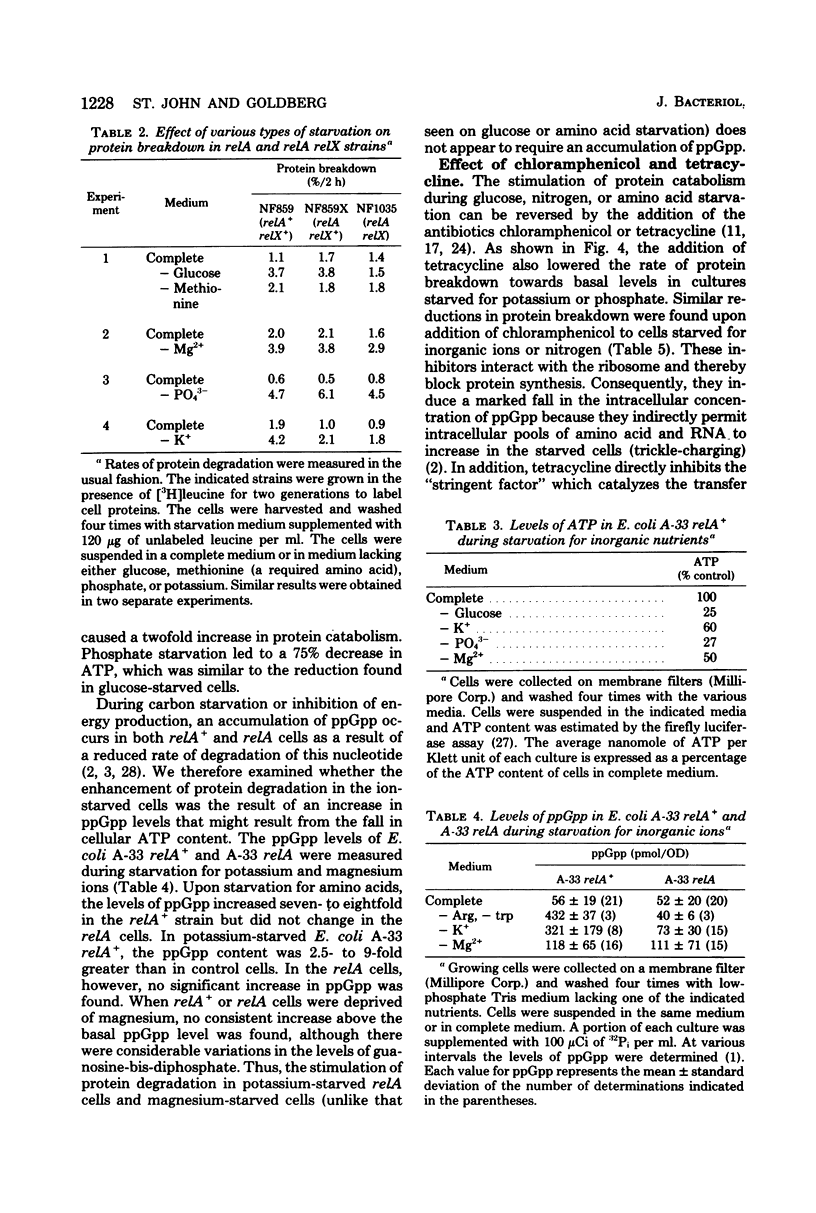
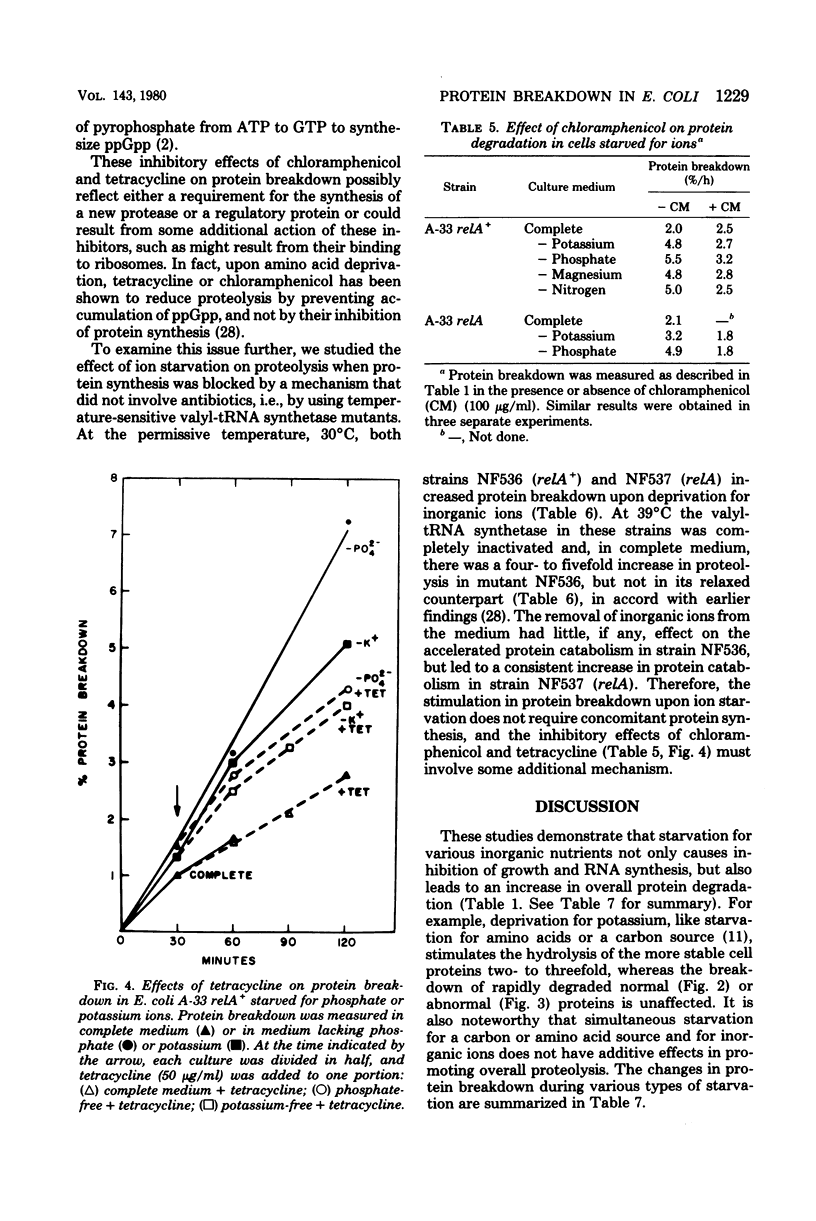
Starvation of Escherichia coli for potassium, phosphate, or magnesium ions leads to a reversible increase in the rate of protein degradation and an inhibition of ribonucleic acid (RNA) synthesis. In cells deprived of potassium, the breakdown of the more stable cell proteins increased two- to threefold, whereas the hydrolysis of short-lived proteins, both normal ones and analog-containing polypeptides, did not change. The mechanisms initiating the enhancement of proteolysis during starvation for these ions were examined. Upon starvation for amino acids or amino acyl-transfer RNA (tRNA), protein breakdown increases in relA+ (but not relA) cells as a result of the rapid synthesis of guanosine-5'-diphosphate-3'-diphosphate (ppGpp). However, a lack of amino acyl-tRNA does not appear to be responsible for the increased protein breakdown in cells starved for inorganic ions, since protein breakdown increased in the absence of these ions in both relA+ and relA cultures, and since a large excess of amino acids did not affect this response. In bacteria in which energy production is restricted, ppGpp levels also rise, and protein breakdown increases. The ion-deprived cultures did show a 40 to 75% reduction in adenosine-5'-triphosphate levels,l similar to that seen upon glucose starvation. However, this decrease in ATP content does not appear to cause the increase in protein breakdown or lead to an accumulation of ppGpp. No consistent change in intracellular ppGpp levels was found in relA+ or relA cells starved for these ions. In addition, in relX mutants, removal of these ions led to accelerated protein degradation even though relX cells are unable to increase ppGpp levels or proteolysis when deprived of a carbon source. In the potassium-, phosphate-, and magnesium-deprived cultures, the addition of choramphenicol or tetracycline caused a reduction in protein breakdown toward basal levels. Such findings, however, do not indicate that protein synthesis is essential for the enhancement of protein degradation, since blockage of protein synthesis by inactivation of a temperature-sensitive valyl-tRNA synthetase did not restore protein catabolism to basal levels. These various results and related studies suggest that the mechanism for increased protein catabolism on starvation for inorganic ions differs from that occurring upon amino acid or arbon deprivation and probably involves an enhanced susceptibility of various cell proteins (especially ribosomal proteins) to proteolysis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- De Boer H. A., Bakker A. J., Weyer W. J., Gruber M. The role of energy-generating processes in the degradation of guanosine tetrophosphate, ppGpp, in Escherichia coli. Biochim Biophys Acta. 1976 May 19;432(3):361–368. doi: 10.1016/0005-2787(76)90146-5. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. PRE-RIBOSOMAL PARTICLES FORMED IN POTASSIUM-DEPLETED CELLS. STUDIES ON DEGRADATION AND STABILIZATION. Biochim Biophys Acta. 1965 Apr 19;95:605–623. doi: 10.1016/0005-2787(65)90515-0. [DOI] [PubMed] [Google Scholar]
- Fallon A. M., Jinks C. S., Yamamoto M., Nomura M. Expression of ribosomal protein genes cloned in a hybrid plasmid in Escherichia coli: gene dosage effects on synthesis of ribosomal proteins and ribosomal protein messenger ribonucleic acid. J Bacteriol. 1979 May;138(2):383–396. doi: 10.1128/jb.138.2.383-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiil N. P., von Meyenburg K., Friesen J. D. Accumulation and turnover of guanosine tetraphosphate in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):769–783. doi: 10.1016/s0022-2836(72)80037-8. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. A role of aminoacyl-tRNA in the regulation of protein breakdown in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Feb;68(2):362–366. doi: 10.1073/pnas.68.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. Magnesium binding by Escherichia coli ribosomes. J Mol Biol. 1966 Feb;15(2):663–673. doi: 10.1016/s0022-2836(66)80134-1. [DOI] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Synthesis and turnover of ribosomal proteins in the absence of 60S subunit assembly in Saccharomyces cerevisiae. Mol Gen Genet. 1977 Dec 9;157(3):327–332. doi: 10.1007/BF00268670. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irr J. D. Control of nucleotide metabolism and ribosomal ribonucleic acid synthesis during nitrogen starvation of Escherichia coli. J Bacteriol. 1972 May;110(2):554–561. doi: 10.1128/jb.110.2.554-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Mizuno D. Ribosome degradation and the degradation products in starved Escherichia coli. I. Comparison of the degradation rate and of the nucleotide pool between Escherichia coli B and Q-13 strains in phosphate deficiency. Biochim Biophys Acta. 1970 Jan 21;199(1):159–165. [PubMed] [Google Scholar]
- Natori S., Nozawa R., Mizunod The turnover of ribosomal RNA of Escherichia coli in a magnesium-deficient stage. Biochim Biophys Acta. 1966 Feb 21;114(2):245–253. doi: 10.1016/0005-2787(66)90306-6. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. III. Effects of rpsD mutations on expression of some ribosomal protein genes. Mol Gen Genet. 1979 Feb 1;169(3):271–278. doi: 10.1007/BF00382273. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A gene involved in the metabolic control of ppGpp synthesis. Mol Gen Genet. 1978 Jan 17;158(3):271–277. doi: 10.1007/BF00267198. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Gallant J. A new nucleotide involved in the stringent response in Escherichia coli. Guanosine 5'-diphosphate-3'-monophosphate. J Biol Chem. 1979 Feb 10;254(3):688–692. [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D., Epstein D., Hershko A. Roles of protein synthesis and tRNA aminoacylation in the regulation of intracellular protein breakdown in E. coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):899–905. doi: 10.1016/0006-291x(74)90240-x. [DOI] [PubMed] [Google Scholar]
- Rafaeli-Eshkol D., Hershko A. Regulation of intracellular protein breakdown in stringent and relaxed strains of E. coli,. Cell. 1974 May;2(1):31–35. doi: 10.1016/0092-8674(74)90005-1. [DOI] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of reduced energy production on protein degradation, guanosine tetraphosphate, and RNA synthesis in Escherichia coli. J Biol Chem. 1978 Apr 25;253(8):2705–2711. [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Protein turnover in amino acid-starved strains of Escherichia coli K-12 differing in their ribonucleic acid control. J Biol Chem. 1969 Nov 25;244(22):6304–6306. [PubMed] [Google Scholar]
- Voellmy R., Goldberg A. L. Guanosine-5'-diphosphate-3'-diphosphate (ppGpp) and the regulation of protein breakdown in Escherichia coli. J Biol Chem. 1980 Feb 10;255(3):1008–1014. [PubMed] [Google Scholar]
- Wallace B. J., Davis B. D. Cyclic blockade of initiation sites by streptomycin-damaged ribosomes in Escherichia coli: an explanation for dominance of sensitivity. J Mol Biol. 1973 Apr 5;75(2):377–390. doi: 10.1016/0022-2836(73)90028-4. [DOI] [PubMed] [Google Scholar]
- Warner J. R. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa cells are synthesized normally and degraded rapidly. J Mol Biol. 1977 Sep 25;115(3):315–333. doi: 10.1016/0022-2836(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in non-growing cells of Escherichia coli. Biochem J. 1967 May;103(2):453–461. doi: 10.1042/bj1030453. [DOI] [PMC free article] [PubMed] [Google Scholar]



