Abstract
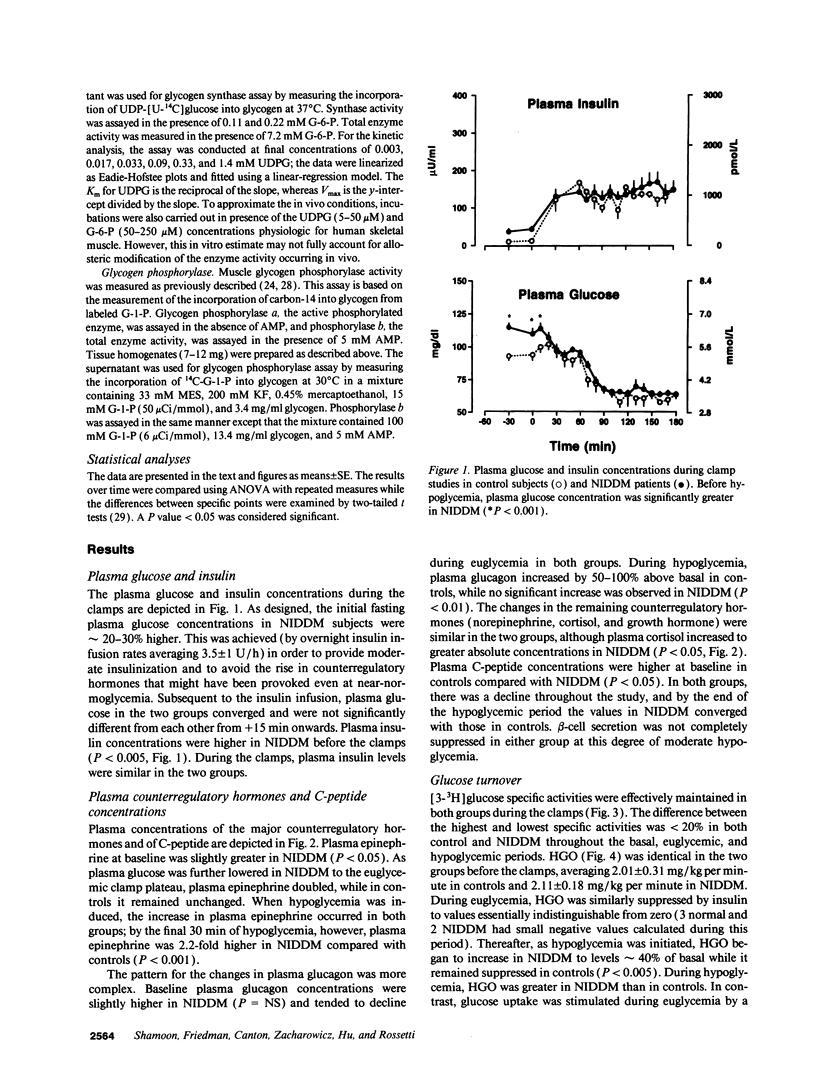
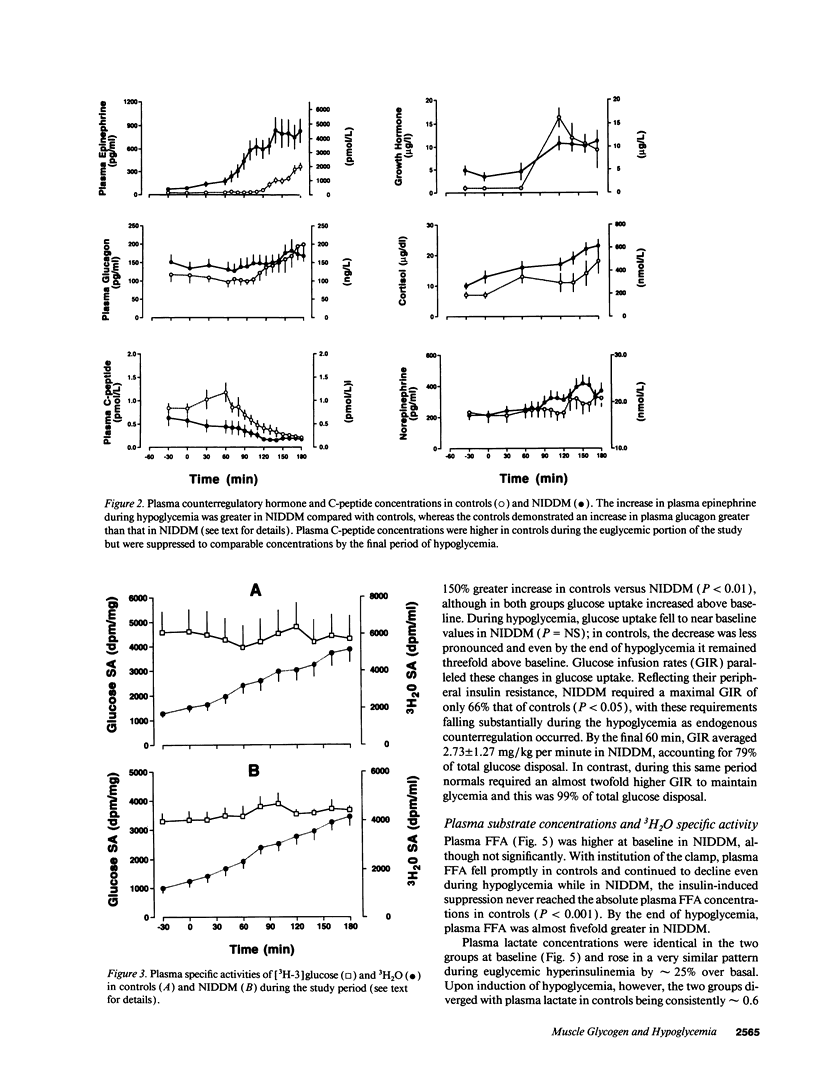
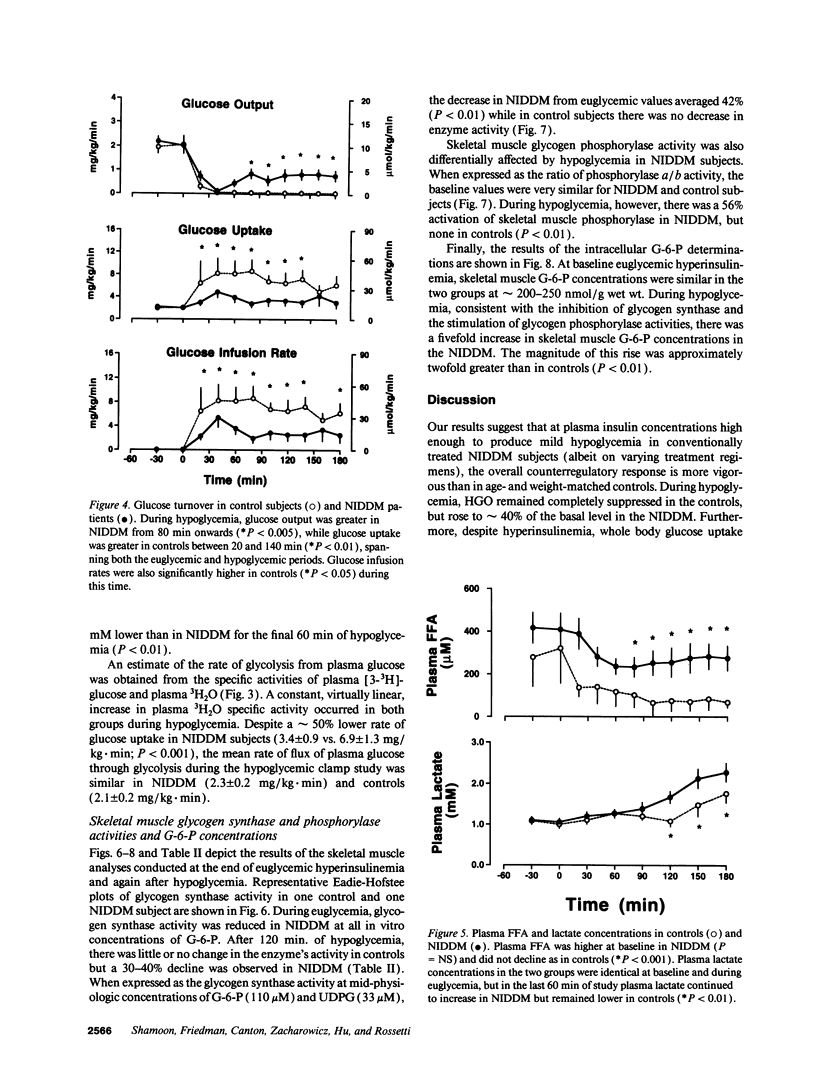
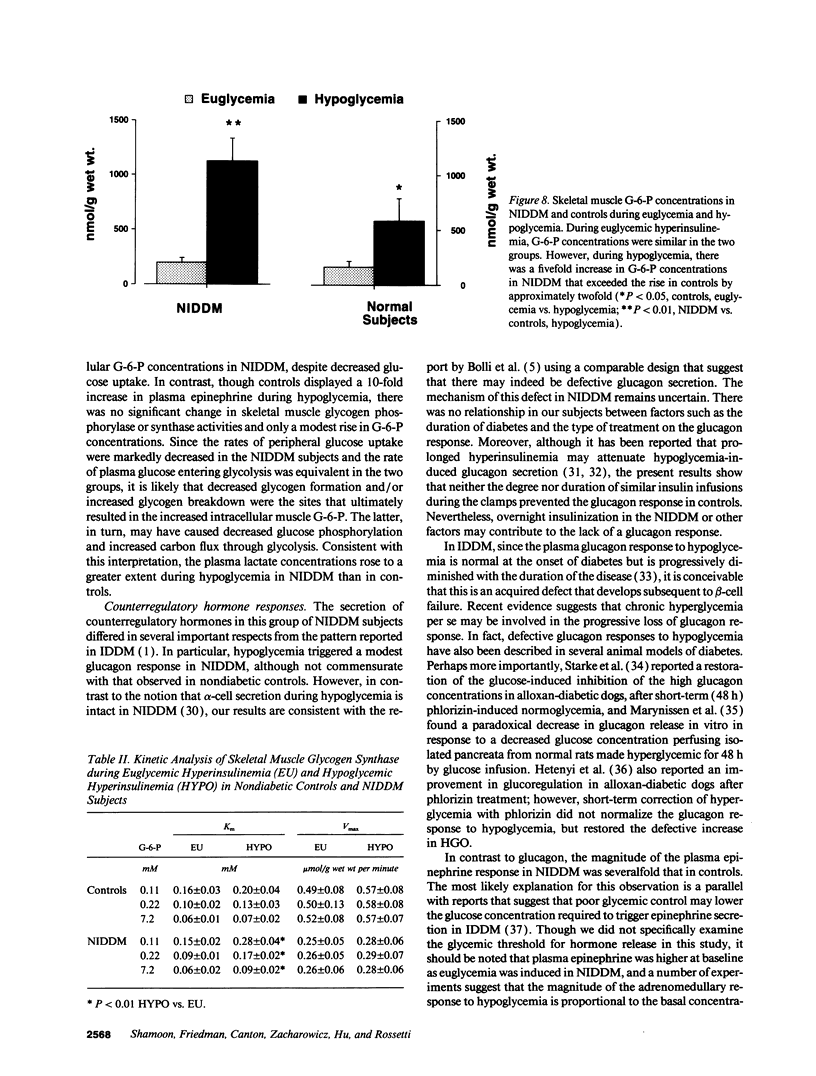
We evaluated skeletal muscle counterregulation during hypoglycemia in nine subjects with non-insulin-dependent diabetes mellitus (NIDDM) (HbA1c 9.4 +/- 0.5%, nl < 6.2%) compared with six normal controls, matched for age (51 +/- 3 and 49 +/- 5 yr, respectively) and body mass index (27.3 +/- 1.2 and 27.0 +/- 2.1 kg/m2). After 60 min of euglycemia (plasma insulin approximately 140 microU/ml), plasma glucose was lowered to 62 +/- 2 mg/dl by 120 min. Hypoglycemia induced a 2.2-fold greater increase in plasma epinephrine in NIDDM (P < 0.001), while the plasma glucagon response was blunted (P < 0.01). Hepatic glucose output ([3H-3]glucose) suppressed similarly during euglycemia, but during hypoglycemia was greater in NIDDM (P < 0.005). Conversely, glucose uptake during euglycemia was 150% greater in controls (P < 0.01) and remained persistently higher than in NIDDM during hypoglycemia. In NIDDM, plasma FFA concentrations were approximately fivefold greater (P < 0.001), and plasma lactate levels were approximately 40% higher than in controls during hypoglycemia (P < 0.01); the rates of glycolysis from plasma glucose were similar in the two groups despite a 49% lower rate of glucose uptake in NIDDM (3.4 +/- 0.9 vs. 6.9 +/- 1.3 mg/kg per minute, P < 0.001). Muscle glycogen synthase activity fell by 42% with hypoglycemia (P < 0.01) in NIDDM but not in controls. In addition, glycogen phosphorylase was activated by 56% during hypoglycemia in NIDDM only (P < 0.01). Muscle glucose-6-phosphate concentrations rose during hypoglycemia by a twofold greater increment in NIDDM (P < 0.01). Thus, skeletal muscle participates in hypoglycemia counterregulation in NIDDM, directly by decreased removal of plasma glucose and, indirectly, by providing lactate for hepatic gluconeogenesis. Consequently, in addition to inherent insulin resistance in NIDDM, the enhanced plasma epinephrine response during hypoglycemia may partially offset impaired glucagon secretion and counteract the effects of hyperinsulinemia on liver, fat, and skeletal muscle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader M., Bergman R. N. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am J Physiol. 1990 Jun;258(6 Pt 1):E1020–E1032. doi: 10.1152/ajpendo.1990.258.6.E1020. [DOI] [PubMed] [Google Scholar]
- Bedinger P., Moriarty A., von Borstel R. C., 2nd, Donovan N. J., Steimer K. S., Littman D. R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988 Jul 14;334(6178):162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- Bergström J., Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19(3):218–228. doi: 10.3109/00365516709090629. [DOI] [PubMed] [Google Scholar]
- Bolli G. B., Tsalikian E., Haymond M. W., Cryer P. E., Gerich J. E. Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. J Clin Invest. 1984 Jun;73(6):1532–1541. doi: 10.1172/JCI111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Schwartz N. S., Shah S. D., Clutter W. E., Cryer P. E. Plasma glucose concentrations at the onset of hypoglycemic symptoms in patients with poorly controlled diabetes and in nondiabetics. N Engl J Med. 1988 Jun 9;318(23):1487–1492. doi: 10.1056/NEJM198806093182302. [DOI] [PubMed] [Google Scholar]
- Bradley D. C., Poulin R. A., Bergman R. N. Dynamics of hepatic and peripheral insulin effects suggest common rate-limiting step in vivo. Diabetes. 1993 Feb;42(2):296–306. doi: 10.2337/diab.42.2.296. [DOI] [PubMed] [Google Scholar]
- Caprio S., Amiel S., Tamborlane W. V., Gelfand R. A., Sherwin R. S. Defective free-fatty acid and oxidative glucose metabolism in IDDM during hypoglycemia. Influence of glycemic control. Diabetes. 1990 Feb;39(2):134–141. doi: 10.2337/diab.39.2.134. [DOI] [PubMed] [Google Scholar]
- Caprio S., Gelfand R. A., Tamborlane W. V., Sherwin R. S. Oxidative fuel metabolism during mild hypoglycemia: critical role of free fatty acids. Am J Physiol. 1989 Mar;256(3 Pt 1):E413–E419. doi: 10.1152/ajpendo.1989.256.3.E413. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. R., Shamoon H. Counterregulatory adaptation to recurrent hypoglycemia in normal humans. J Clin Endocrinol Metab. 1991 Nov;73(5):995–1001. doi: 10.1210/jcem-73-5-995. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Fanelli C., Calderone S., Epifano L., De Vincenzo A., Modarelli F., Pampanelli S., Perriello G., De Feo P., Brunetti P., Gerich J. E. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest. 1993 Oct;92(4):1617–1622. doi: 10.1172/JCI116746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrace S., Rossetti L. Hyperglycemia markedly enhances skeletal muscle glycogen synthase activity in diabetic, but not in normal conscious rats. Diabetes. 1992 Nov;41(11):1453–1463. doi: 10.2337/diab.41.11.1453. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Frizzell R. T., Hendrick G. K., Biggers D. W., Lacy D. B., Donahue D. P., Green D. R., Carr R. K., Williams P. E., Stevenson R. W., Cherrington A. D. Role of gluconeogenesis in sustaining glucose production during hypoglycemia caused by continuous insulin infusion in conscious dogs. Diabetes. 1988 Jun;37(6):749–759. doi: 10.2337/diab.37.6.749. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Bier D. M., Cryer P. E., Pagliara A. S. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974 Dec;23(12):982–986. doi: 10.2337/diab.23.12.982. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Giacca A., Fisher S. J., Shi Z. Q., Gupta R., Lickley H. L., Vranic M. Importance of peripheral insulin levels for insulin-induced suppression of glucose production in depancreatized dogs. J Clin Invest. 1992 Nov;90(5):1769–1777. doi: 10.1172/JCI116051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A., Swislocki A. L., Chen Y. D., Reaven G. M. Relationships between plasma-free fatty acid concentration, endogenous glucose production, and fasting hyperglycemia in normal and non-insulin-dependent diabetic individuals. Metabolism. 1987 Jul;36(7):692–696. doi: 10.1016/0026-0495(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Herrera M. G., Kamm D., Ruderman N., Cahill Non-hormonal factors in the control of gluconeogenesis. Adv Enzyme Regul. 1966;4:225–235. doi: 10.1016/0065-2571(66)90017-3. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Gauthier C., Byers M., Vranic M. Phlorizin-induced normoglycemia partially restores glucoregulation in diabetic dogs. Am J Physiol. 1989 Feb;256(2 Pt 1):E277–E283. doi: 10.1152/ajpendo.1989.256.2.E277. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Klein S., Wolfe R. Mechanism of regulation of glucose production by lipolysis in humans. Am J Physiol. 1992 Mar;262(3 Pt 1):E353–E358. doi: 10.1152/ajpendo.1992.262.3.E353. [DOI] [PubMed] [Google Scholar]
- Jenssen T., Nurjhan N., Consoli A., Gerich J. E. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest. 1990 Aug;86(2):489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlander S., Roovete A., Vranić M., Efendić S. Glucose and fructose 6-phosphate cycle in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E530–E536. doi: 10.1152/ajpendo.1986.251.5.E530. [DOI] [PubMed] [Google Scholar]
- Kleinbaum J., Shamoon H. Impaired counterregulation of hypoglycemia in insulin-dependent diabetes mellitus. Diabetes. 1983 Jun;32(6):493–498. doi: 10.2337/diab.32.6.493. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- Lecavalier L., Bolli G., Cryer P., Gerich J. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol. 1989 Jun;256(6 Pt 1):E844–E851. doi: 10.1152/ajpendo.1989.256.6.E844. [DOI] [PubMed] [Google Scholar]
- Liu D., Moberg E., Kollind M., Lins P. E., Adamson U. A high concentration of circulating insulin suppresses the glucagon response to hypoglycemia in normal man. J Clin Endocrinol Metab. 1991 Nov;73(5):1123–1128. doi: 10.1210/jcem-73-5-1123. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynissen G., Leclercq-Meyer V., Sener A., Malaisse W. J. Perturbation of pancreatic islet function in glucose-infused rats. Metabolism. 1990 Jan;39(1):87–95. doi: 10.1016/0026-0495(90)90153-4. [DOI] [PubMed] [Google Scholar]
- Mellman M. J., Davis M. R., Shamoon H. Effect of physiological hyperinsulinemia on counterregulatory hormone responses during hypoglycemia in humans. J Clin Endocrinol Metab. 1992 Nov;75(5):1293–1297. doi: 10.1210/jcem.75.5.1430091. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Roach P. J., Larner J. Rabbit skeletal muscle glycogen synthase. II. Enzyme phosphorylation state and effector concentrations as interacting control parameters. J Biol Chem. 1976 Apr 10;251(7):1920–1925. [PubMed] [Google Scholar]
- Rossetti L., Farrace S., Choi S. B., Giaccari A., Sloan L., Frontoni S., Katz M. S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol. 1993 Jan;264(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Hu M. Skeletal muscle glycogenolysis is more sensitive to insulin than is glucose transport/phosphorylation. Relation to the insulin-mediated inhibition of hepatic glucose production. J Clin Invest. 1993 Dec;92(6):2963–2974. doi: 10.1172/JCI116919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lee Y. T., Ruiz J., Aldridge S. C., Shamoon H., Boden G. Quantitation of glycolysis and skeletal muscle glycogen synthesis in humans. Am J Physiol. 1993 Nov;265(5 Pt 1):E761–E769. doi: 10.1152/ajpendo.1993.265.5.E761. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Saloranta C., Franssila-Kallunki A., Ekstrand A., Taskinen M. R., Groop L. Modulation of hepatic glucose production by non-esterified fatty acids in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Jun;34(6):409–415. doi: 10.1007/BF00403179. [DOI] [PubMed] [Google Scholar]
- Shilo S., Sotsky M., Shamoon H. Islet hormonal regulation of glucose turnover during exercise in type 1 diabetes. J Clin Endocrinol Metab. 1990 Jan;70(1):162–172. doi: 10.1210/jcem-70-1-162. [DOI] [PubMed] [Google Scholar]
- Sotsky M. J., Shilo S., Shamoon H. Regulation of counterregulatory hormone secretion in man during exercise and hypoglycemia. J Clin Endocrinol Metab. 1989 Jan;68(1):9–16. doi: 10.1210/jcem-68-1-9. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Starke A., Grundy S., McGarry J. D., Unger R. H. Correction of hyperglycemia with phloridzin restores the glucagon response to glucose in insulin-deficient dogs: implications for human diabetes. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1544–1546. doi: 10.1073/pnas.82.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The Berson memorial lecture. Insulin-glucagon relationships in the defense against hypoglycemia. Diabetes. 1983 Jun;32(6):575–583. doi: 10.2337/diab.32.6.575. [DOI] [PubMed] [Google Scholar]
- Virkamäki A., Puhakainen I., Nurjhan N., Gerich J. E., Yki-Järvinen H. Measurement of lactate formation from glucose using [6-3H]- and [6-14C]glucose in humans. Am J Physiol. 1990 Sep;259(3 Pt 1):E397–E404. doi: 10.1152/ajpendo.1990.259.3.E397. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Kauppila M., Kujansuu E., Lahti J., Marjanen T., Niskanen L., Rajala S., Ryysy L., Salo S., Seppälä P. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1992 Nov 12;327(20):1426–1433. doi: 10.1056/NEJM199211123272005. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Wolfe-Lopez D., Mott D. M. Muscle glycogen synthesis and disposition of infused glucose in humans with reduced rates of insulin-mediated carbohydrate storage. Diabetes. 1988 Mar;37(3):303–308. doi: 10.2337/diab.37.3.303. [DOI] [PubMed] [Google Scholar]



