Abstract
Previous studies have investigated rabies virus (RABV) epizootiology in Brazilian free-tailed bats (Tadarida brasiliensis) in natural cave roosts. However, little is known about geographic variation in RABV exposure, or if the use of man-made roosts by this species affects enzootic RABV infection dynamics within colonies. We sampled rabies viral neutralizing antibodies in bats at three bridge and three cave roosts at multiple time points during the reproductive season to investigate temporal and roost variation in RABV exposure. We report seropositive bats in all age and sex classes with minimal geographic variation in RABV seroprevalence among Brazilian free-tailed bat colonies in south-central Texas. While roost type was not a significant predictor of RABV seroprevalence, it was significantly associated with seasonal fluctuations, suggesting patterns of exposure that differ between roosts. Temporal patterns suggest increased RABV seroprevalence after parturition in cave colonies, potentially related to an influx of susceptible young, in contrast to more uniform seroprevalence in bridge colonies. This study highlights the importance of life history and roost ecology in understanding patterns of RABV seroprevalence in colonies of the Brazilian free-tailed bat.
Key Words: Brazilian free-tailed bat, Epizootiology, Rabies virus, Roost ecology
Introduction
Rabies virus (RABV) infection was first reported in Brazilian free-tailed bats (Tadarida brasiliensis) in 1954 in California and Texas (Sullivan et al. 1954, Enright et al. 1955), although the virus was likely to be widely circulating in bats long before its detection (Hughes et al. 2005). The initial detection of RABV infection in bats prompted multiple surveillance studies, and RABV-infected Brazilian free-tailed bats were detected across their geographic range in the southern United States (Burns and Farinacci 1955, Burns et al. 1956a, 1956b, Schneider et al. 1957, Maddy et al. 1958, Glass 1959, Dean et al. 1960, Richardson et al. 1966). Die-offs of several thousand Brazilian free-tailed bats in New Mexico (1955, 1956) and Texas (1955) prompted additional surveillance of Brazilian free-tailed bat maternity colonies in the southwestern United States, although RABV infection was confirmed in low proportions of moribund bats collected during periods of massive mortality (Constantine et al. 1968). Systematic surveillance of apparently healthy adult Brazilian free-tailed bats from maternity colonies in New Mexico has documented variable levels of RABV exposure (12–80%), and low levels of central nervous system infection (<1%) (Constantine et al. 1968, Steece and Altenbach 1989), yet few studies have examined the effects of ecological and geographic variation on the dynamics of RABV infection in bats.
Brazilian free-tailed bats aggregate annually in colonies of tens to millions of bats across their geographic range in the southwestern United States (McCracken 2003, Betke et al. 2008) and provide substantial ecosystem and economic services to agricultural regions of south-central Texas (Cleveland et al. 2006, Federico et al. 2008). The largest aggregations of these bats often function as maternity colonies, whose diets are supported by the emergence of insect prey from agricultural croplands in south-central Texas (e.g., corn and cotton) during the summer months (Kunz et al. 1995, Lee and McCracken 2005). This species is known to colonize a variety of roosts, including natural (e.g., caves, trees) and man-made structures (e.g., mines, bridges, bat houses, and buildings). In Texas, large colonies occur in caves (Davis et al. 1962, McCracken 2003, Betke et al. 2008) and, increasingly, in the expansion joints of highway bridges (Keeley and Tuttle 1999, Keeley and Keeley 2004), with smaller colonies found in buildings (Davis et al. 1962, Scales and Wilkins 2007). Bridge colonies are often associated with heavy vehicular or rail traffic, which contributes noise, air, and ground pollution to the local environment. Little is known about how these novel stimuli affect the immune competence and epizootiology of bats living in man-made roosts.
This study investigates ecological and geographic effects on rabies viral neutralizing antibody (VNA) seroprevalence in six colonies of Brazilian free-tailed bats in south-central Texas. Periods of sampling correspond with seasonal changes in reproductive activity of adult female bats (e.g., pregnancy, lactation, and postlactation/nonreproductive periods). We predicted that RABV seroprevalence would increase after synchronized parturition, when the overall population size, contact rates, and the proportion of susceptible bats are expected to increase. We expected lower immune competence from bats in bridge roosts because of the perceived stresses associated with anthropogenic disturbances, potentially resulting in fewer bats mounting immune responses to RABV exposure, and lower VNA seroprevalence (Smith 1981, Smith et al. 1982). We expected periodic fluctuations in RABV seroprevalence to be greater in adult females, compared to adult males, as reproductive females presumably have greater contact with clusters of susceptible young. As sex ratios are more heavily skewed in favoring females at cave roosts, we expected periodic fluctuations in RABV seroprevalence to be greater in cave roosts. For juveniles, we expected higher RABV seroprevalence during early periods of sampling after parturition, as previous data have suggested high levels of RABV infection during early weeks of life (Constantine 1986, Steece and Altenbach 1989).
Materials and Methods
Animal sampling
All capture and handling procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee, and comply with the American Society of Mammalogists guidelines for the use of wild mammals in research (Gannon et al. 2007), under Texas Parks and Wildlife permit #SPR-0305-058. All persons involved with the capture and handling of bats received RABV preexposure prophylaxis (Manning et al. 2008), and appropriate personal protective equipment was worn during sampling.
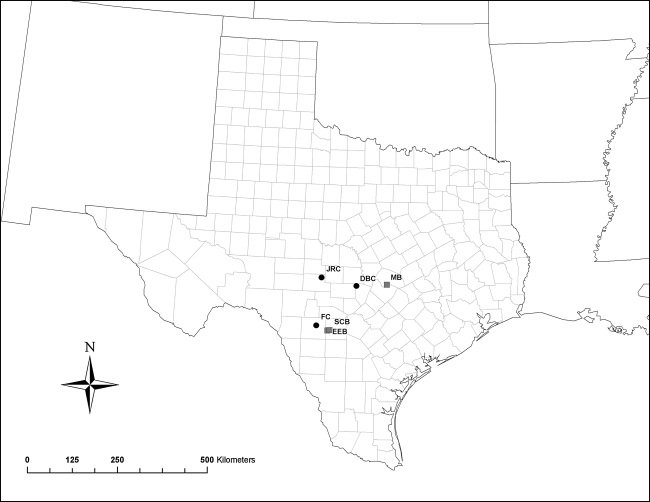
A total of six sites in south-central Texas were sampled between May and October 2005, including three caves and three bridges: Davis Blowout Cave (DBC), Frio Cave (FC), Eckert James River Cave (JRC), McNeil Bridge (MB), Seco Creek Bridge (SCB), and East Elm Creek Bridge (EEB) (Fig. 1). Free-ranging bats were captured at emergence from all sites between 18:00 and 21:00, using a combination of harp trap and hand nets, and all bats were immediately freed from traps or nets and placed into individual cloth bags (Kunz and Kurta 1988). Standard measurements were taken on all bats, including mass, right forearm length, age (Anthony 1988), sex, and reproductive condition (Racey 1988). A sample (80–100 μL) of whole blood was collected in sterile heparinized microcapillary tubes after aseptic preparation and puncture of the interfemoral artery (Kunz and Nagy 1988). Plasma was separated within 2 h of blood draw, and stored at −20°C. Bats were observed for clinical signs of RABV infection during processing (i.e., odd vocalizations, ataxia, paresis, and paralysis). Before release at the site of capture, a nontoxic ink tattoo was applied to all bats to prevent re-sampling of individuals and to assess site fidelity through the sampling season. An index of body condition was calculated by taking the ratio of body mass (g) to length of forearm (mm).
FIG. 1.
The geographic location of all sites sampled in Texas, United States. Cave sites (black circles) include Eckert James River Cave (JRC), Davis Blowout Cave (DBC), and Frio Cave (FC). Bridge sites (gray squares) include Seco Creek Bridge (SCB), East Elm Creek Bridge (EEB), and McNeil Bridge (MB).
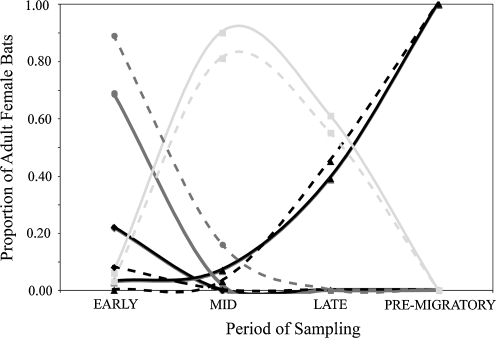
Free-flying bats at all six colonies were sampled periodically on a minimum of three occasions (Early, Mid, and Late) during four life history cycles: Early—May through mid-June (pregnancy); Mid—mid-June through July (lactation); Late—August (postlactation/nonreproductive); and Premigratory—September through October (nonreproductive) (Fig. 2). Mating in this species is thought to occur among colonies in Mexico before the migration of bats into Texas, but has been documented in colonies during March and April (Davis et al. 1962, Keeley and Keeley 2004). However, none of the male bats that we sampled showed evidence of reproductive activity (i.e., descended testes) during any of the periods sampled. In the Early period, colonies are still assembling as individuals arrive from Mexico at maternity sites that are primarily comprised of adult females in the early to middle stages of gestation. The Mid period immediately follows synchronized parturition, which occurs in early June (Davis et al. 1962, Constantine 1967a, McCracken and Gustin 1991). The majority of adult female bats are nursing pups during the Mid period, and milk is easily expressed from nursing females that have visibly swollen nipples that are devoid of hair. Colony sizes peak and are most stable during the Mid period (Davis et al. 1962, Constantine 1967a, Betke et al. 2008). In the Late period, most adult females are in the late stages of lactation and transitioning into a postlactation (nonreproductive) state, as juveniles achieve adult size and begin to forage independently. Postlactational adult females are characterized by the growth of new hair around the nipple and no milk expression after palpation. During the Premigratory phase, all adult bats and young-of-the-year are considered nonreproductive, and are preparing for autumn migration to Mexico (Fig. 2).
FIG. 2.
The reproductive activity of adult female bats measured during four periods: Early, Mid, Late, and Premigratory. Solid lines represent cave roosts, and dashed lines represent bridge roosts. Reproductive status was as follows: not determined (diamond), pregnant (circle), lactating (square), or nonreproductive (triangle).
The timing and pattern of reproductive activity in adult female bats observed during this study are in agreement with previously published accounts (Davis et al. 1962, Constantine 1967a, McCracken and Gustin 1991). The data suggest that, despite greater proportions of male bats in bridge colonies, reproductive schedules and dynamics for adult female bats are similar in cave and bridge roosts (Fig. 2).
Detection of RABV neutralizing antibodies
A modified rapid fluorescent focus inhibition test (Smith et al. 1996, Jackson et al. 2008), using rabies challenge virus standard (CVS-11, V399; Briggs et al. 1998), was used to assay for RABV-specific VNA in the blood plasma of individual bats. The lowest bat plasma dilution tested in the rapid fluorescent focus inhibition test assay was 1:4, and sequential twofold dilutions were tested up to 1:2048. Rabies VNA endpoint titers of individual bats were calculated by the Reed-Muench method (Reed and Muench 1938), and were converted to international units (IU/mL) by comparison to standard rabies immune globulin control containing 2 IU/mL. Final titers less than 0.06 IU/mL were considered negative for rabies VNA. Positive VNA titers (≥0.06 IU/mL) were demonstrated by complete neutralization of the challenge virus dose (50 focus forming doses) at a 1:4 serum dilution. The choice of this cutoff value follows previous studies for Lyssavirus surveillance using bat and nonbat sera (Shankar et al. 2004, Lumlertdacha et al. 2005, Rupprecht et al. 2005, Blanton et al. 2007, Jackson et al. 2008). A previous study has demonstrated that the immunoglobulin G fraction of the bat serum is responsible for neutralization activity against RABV (Shankar et al. 2004).
Statistical analyses
Rabies VNA seroprevalence was treated as a binomial response variable for all analyses. For statistical analyses, the data were partitioned into adult females (n = 305), adult males (n = 148), and juveniles (n = 56). For statistical analyses on the adult cohorts, the Premigratory period was excluded owing to uniformly low sample sizes and uneven sampling across sites. For the analysis of adult males, low sample sizes and uneven sampling across periods also led to the exclusion of two cave sites (DBC and JRC). For the analysis of juveniles, we had no a priori expectation for differences in RABV exposure based on sex, and thus data for both sexes were combined for analyses. Lastly, adult male and female data were combined (n = 463) to test for sex differences in VNA seroprevalence.
A series of hierarchical logistic models were tested using SAS v.9.1 (SAS Institute, Cary, NC) to investigate significant ecological predictors of rabies VNA seroprevalence. The central question focused on testing for effects of roost type on rabies VNA seroprevalence, particularly in maternity colonies of reproductively active adult female bats. A nested mixed logistic model (PROC GLIMMIX) was used to control for variation among sites that represent the two roost types (cave vs. bridge) sampled in this study, with site treated as a random effect nested within roost type, and fixed effects of roost type and period (α = 0.1). In the absence of significant effects of roost type, models were simplified to nonnested logistic models, with site, period, and sex treated as fixed effects (α = 0.05). In the nested and nonnested models, individual body condition was tested as a covariate. All two- and three-way fixed effect and covariate interactions were tested (α = 0.05). Nonsignificant covariate-fixed effect interactions were removed from the model before testing fixed effects (Engqvist 2005). Marginally significant (α < 0.10) fixed effect interactions were retained in the models. The likelihood ratio chi-square (LRχ2), and Hosmer-Lemeshow goodness of fit (Hosmer and Lemeshow 2000) test statistics were used to compare the fit of various models. For models with significant fixed effects, pair-wise contrasts among all levels of the significant fixed effect were tested (α = 0.05). Figures show proportions of rabies VNA seroprevalence, with the upper bound of the 95% confidence interval on each proportion.
Although tested as a covariate in the seroprevalence models, body condition during the Early, Mid, and Late periods was also analyzed separately for male and female adult cohorts, and during the Late and Premigratory periods for the juvenile cohort, to investigate roost type and seasonal effects. A nested mixed analysis of variance (ANOVA) model was used to test for roost-type differences in body condition among adult female bats at cave and bridge colonies (n = 351, α = 0.05). All nested, fixed, and random effects were treated as described above. As the adult male cohort (n = 158) included adequate sampling for one cave (FC) and three bridge colonies (EEB, MB, SCB), a nonnested ANOVA model with fixed effects of site, period, and an interaction term was tested. A nested mixed ANOVA model was used to test for roost-type effects on body condition of the juvenile cohort (n = 45) across evenly sampled colonies (DBC, FC, MB, and SCB) for the Late and Premigratory periods. Nonnested ANOVA models were also used to identify significant predictors of juvenile body condition. Tukey's post hoc means separation test was used for pair-wise comparisons between all levels for significant fixed effects (α = 0.05). Figures show untransformed mean (± standard error) body condition indices.
Results
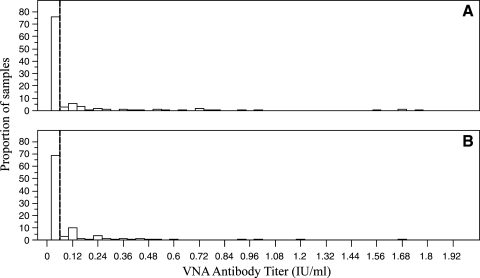
A total of 615 Brazilian free-tailed bats were sampled for rabies VNA between May and October 2005, and VNA titers were determined from 534 samples (Fig. 3; Tables 1 and 2). None of the bats presented clinical signs of RABV infection during handling and sampling procedures. None of the bats were re-captured during the sampling season.
FIG. 3.
A histogram of the subset of viral neutralizing antibody (VNA) titers that were less than 2 IU/mL (97%; 519 of 534), from (A) female (n = 329) and (B) male (n = 190) bats sampled in Texas during 2005 across adult and juvenile cohorts. The cutoff for positive VNA titers is shown with a dashed vertical line.
Table 1.
Proportion of Adult Brazilian Free-Tailed Bats with Rabies Viral Neutralizing Antibodies Across Six Sites in South-Central Texas
| Roost | Site | Period | Sex | n | Seroprevalence | Roost | Site | Period | Sex | n | Seroprevalence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cave | FC | Early | F | 22 | 0.32 | Bridge | MB | Early | F | 17 | 0.53 |
| M | 8 | 0.25 | M | 11 | 0.36 | ||||||
| Mid | F | 24 | 0.33 | Mid | F | 21 | 0.38 | ||||
| M | 7 | 0.71 | M | 11 | 0.55 | ||||||
| Late | F | 18 | 0.50 | Late | F | 15 | 0.47 | ||||
| M | 2 | 0.50 | M | 5 | 0.40 | ||||||
| Premigratory | F | 2 | 0.00 | Premigratory | F | 1 | 0.00 | ||||
| M | 2 | 0.50 | M | — | — | ||||||
| DBC | Early | F | 22 | 0.32 | SCB | Early | F | 3 | 1.00 | ||
| M | 7 | 0.43 | M | 25 | 0.64 | ||||||
| Mid | F | 23 | 0.74 | Mid | F | 8 | 0.50 | ||||
| M | — | — | M | 23 | 0.52 | ||||||
| Late | F | 18 | 0.33 | Late | F | 12 | 0.75 | ||||
| M | — | — | M | 8 | 0.25 | ||||||
| Premigratory | F | 2 | 0.50 | Premigratory | F | 1 | 0.00 | ||||
| M | — | — | M | 7 | 0.14 | ||||||
| JRC | Early | F | 30 | 0.17 | EEB | Early | F | 11 | 0.18 | ||
| M | 3 | 0.00 | M | 16 | 0.06 | ||||||
| Mid | F | 28 | 0.36 | Mid | F | 7 | 0.57 | ||||
| M | — | — | M | 24 | 0.58 | ||||||
| Late | F | 20 | 0.00 | Late | F | 6 | 0.33 | ||||
| M | — | — | M | 8 | 0.50 | ||||||
| Premigratory | F | — | — | Premigratory | F | — | — | ||||
| M | — | — | M | — | — |
Data in bold are included in the statistical analyses.
FC, Frio Cave; DBC, Davis Blowout Cave; JRC, Eckert James River Cave; MB, McNeil Bridge; SCB, Seco Creek Bridge; EEB, East Elm Creek Bridge.
Table 2.
Proportion of Juvenile Brazilian Free-Tailed Bats with Rabies Viral Neutralizing Antibodies Across Six Sites in South-Central Texas
| Roost | Site | Period | n | Seroprevalence | Roost | Site | Period | n | Seroprevalence |
|---|---|---|---|---|---|---|---|---|---|
| Cave | FC | Early | — | — | Bridge | MB | Early | — | — |
| Mid | — | — | Mid | — | — | ||||
| Late | 3 | 0.00 | Late | 4 | 0.50 | ||||
| Premigratory | 6 | 0.17 | Premigratory | 3 | 0.00 | ||||
| DBC | Early | — | — | SCB | Early | — | — | ||
| Mid | 6 | 0.80 | Mid | — | — | ||||
| Late | 1 | 0.00 | Late | — | — | ||||
| Premigratory | 9 | 0.56 | Premigratory | 6 | 0.50 | ||||
| JRC | Early | — | — | EEB | Early | — | — | ||
| Mid | — | — | Mid | — | — | ||||
| Late | 3 | 0.00 | Late | 15 | 0.47 | ||||
| Premigratory | — | — | Premigratory | — | — |
Data in bold are included in the analyses.
Adult females
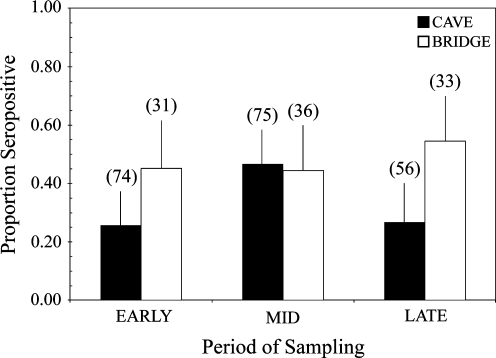
The nested logistic model including roost and period fixed effects provided the best fit to the VNA seroprevalence data for adult female bats. The nested logistic model with only roost type as a fixed effect was not significant (p = 0.33). However, when period was included as a fixed effect in the nested model, there was a significant interaction of roost and period effects (F = 2.52, p < 0.1) (Fig. 4). Data were subdivided by roost type for additional testing of period and site effects.
FIG. 4.
Mean rabies VNA seroprevalence among colonies of adult female bats, from three cave (black; FC, DBC, and JRC) and three bridge (white; MB, SCB, and EEB) roosts, across time periods (n = 305). Sample sizes are listed parenthetically above histogram bars.
The model including site and period fixed effects was significant in explaining variation in VNA seroprevalence of adult females roosting in caves (LRχ2 = 22.9, p = 0.0001; Lack of Fit χ2 = 13.1, p = 0.07). Log-transformed body condition was not a significant covariate (p = 0.77), and was not included in the final model. The site by period interaction was also nonsignificant (p = 0.12), and was removed from the final model. Period was a significant predictor in the model (p = 0.01), and pair-wise contrasts indicate that VNA seroprevalence during the Mid period is significantly higher compared to the Early (p = 0.008) and Late (p = 0.02) periods. However, VNA seroprevalence between the Early and Late periods did not differ (p = 0.99). Site was also a significant predictor in the model (p = 0.002). Rabies VNA seroprevalence at JRC was significantly lower than at DBC (p = 0.004) and at FC (p = 0.02); however, VNA seroprevalence did not vary between FC and DBC (p = 0.23).
The model including site effects, and body condition as a covariate, was significant (LRχ2 = 9.84, p = 0.02; Lack of Fit χ2 = 14.4, p = 0.07) in explaining VNA seroprevalence of adult female bats roosting in bridges, but period was not a significant predictor (p = 0.06). Pair-wise contrasts indicate that VNA seroprevalence at SCB was significantly higher than at EEB (p = 0.02), but all other contrasts between sites were nonsignificant (p > 0.1). Body condition was a marginally predictive covariate in the model (p = 0.08), and bridge females with lower body condition were more likely to be seropositive.
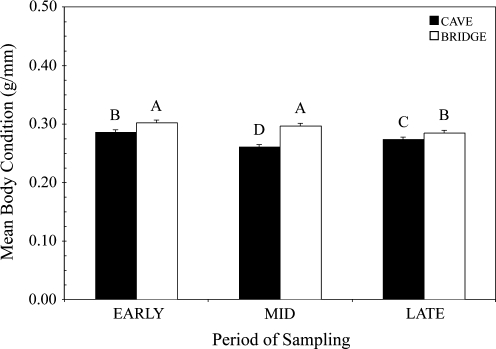
The nested mixed ANOVA model including site as a random effect nested within roost type, and with fixed effects of roost type, period, and the interaction term explained a quarter of the variation in body condition of adult female bats (R2 = 0.25). There was a highly significant roost type by period interaction (F = 7.81, p = 0.0005). Tukey post hoc comparisons indicate that the body condition of adult female bats living in bridge colonies is significantly higher than the body condition of females in cave colonies, at each time period (i.e., Early, Mid, and Late) (Fig. 6).
FIG. 6.
Mean (± standard error) body condition indices among adult female bats, from three cave (black; FC, DBC, and JRC) and three bridge (white; MB, SCB, and EEB) roosts, across time periods (n = 351). Different letters above the histogram bars represent significantly different mean body condition indices.
Adult males
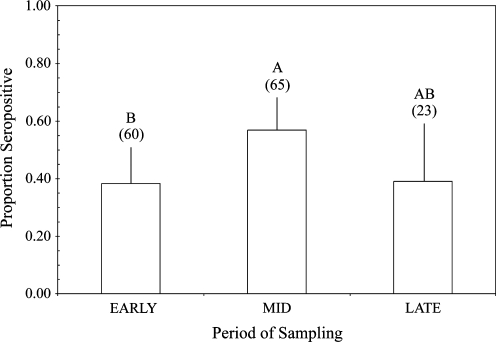
The best-fit logistic model to the VNA seroprevalence data from adult male bats included site and period effects (LRχ2 = 23.4, p = 0.02; Lack of Fit χ2 = 0.0, p = 1.0). Log-transformed body condition was not a significant covariate (p = 0.67), and was not included in the final model. In the nonnested model including site, period, and the interaction term, the interaction of site and period was marginally significant (p = 0.06) and retained in the final model. Period was a significant predictor of RABV exposure (p = 0.02), but site was not (p = 0.66). Rabies VNA seroprevalence increased significantly from the Early to Mid period (p = 0.006), but all other pair-wise contrasts between periods were nonsignificant (p > 0.1) (Fig. 5).
FIG. 5.
Mean rabies VNA seroprevalence among colonies of adult male bats, from three bridges and one cave colony (MB, SCB, EEB, and FC), across time periods (n = 148). Sample sizes are listed parenthetically above the histogram bars, and different letters represent significantly different seroprevalence proportions.
There was significant variation in the body condition of adult males at one cave (FC) and three bridge (EEB, MB, and SCB) colonies in the ANOVA model including site, period, and the interaction term (F = 3.94, p < 0.0001). The interaction of site and period on body condition was highly significant (F = 5.04, p < 0.0001), and each site was subsequently analyzed separately to examine period effects. At the two smaller bridge colonies (EEB and SCB), body condition of adult male bats did not vary across the Early, Mid, and Late periods (p > 0.05). However, at the larger cave and bridge colonies (FC and MB), male body condition was seasonally variable, and highest during the Mid period (FC F = 9.28, p = 0.003; MB F = 6.20, p = 0.007).
All adults
The logistic model with sex as a fixed effect was not significant in explaining variation in seroprevalence among all adult bats (LRχ2 = 2.23, p = 0.14). The logistic model with sex and period effects was significant in explaining variation in the adult seroprevalence data (LRχ2 = 12.5, p = 0.006; Lack of Fit χ2 = 0.42, p = 0.98), but only period was a significant predictor (p = 0.006).
Juveniles
Limited sampling prevented the testing of nested models to explain variation in the VNA seroprevalence of juvenile bats. Sex was not a significant predictor of variation in VNA seroprevalence of juvenile bats (p = 0.47). A logistic model with period as a fixed effect and body condition as a covariate marginally fit the data (LRχ2 = 10.4, p = 0.02; Lack of Fit χ2 = 16.3, p = 0.02), but period was not a significant predictor (p = 0.10). The logistic model including body condition as a predictor was a better fit to the data (LRχ2 = 4.78, p = 0.03; Lack of Fit χ2 = 5.96, p = 0.54), and juvenile bats with lower body condition were more likely to be seropositive (p = 0.04).
There was no significant effect of roost type (p = 0.59), or interaction of period and roost type (p = 0.82), on juvenile body condition across four sites (DBC, FC, MB, and SCB) in the Late and Premigratory periods. There also was no effect of sex (p = 0.80), nor a significant interaction of sex and period (p = 0.89), on the body condition of juvenile bats. Body condition did not differ across sites (p = 0.28), nor was a significant interaction of site and period (p = 0.28) detected. Period was the only significant predictor explaining the body condition of juvenile bats (F = 8.35, p = 0.006), with significantly higher values in the Premigratory period compared to the Late period.
Discussion
The exposure of Brazilian free-tailed bats to RABV, as evidenced by VNA seroprevalence, is highly variable, but the patterns of seroprevalence in this study are consistent with previous research for this species. Rabies VNA seroprevalence varied from low levels of exposure (0–5%) to extremely high levels (>50%) among colonies. Seropositive bats were detected in all colonies, further supporting wide geographic prevalence of infection in colonies of Brazilian free-tailed bats, and consistent with previous surveys (Burns et al. 1956b, Constantine et al. 1968, Steece and Altenbach 1989). Our results indicate that roosting ecology and reproductive activity are important factors affecting rabies VNA seroprevalence among colonies of these bats.
Adult females
All of the sites sampled in this study contain seasonal colonies of bats that migrate from Mexico (Davis et al. 1962, Villa and Cockrum 1962, Cockrum 1969), and are predominantly inhabited by adult female bats and their offspring. Contrary to our expectations, roost type alone was not a significant predictor of RABV seroprevalence, but seasonal fluctuations in seroprevalence were affected by roost type. In cave colonies, RABV seroprevalence significantly increased after parturition, whereas RABV seroprevalence at bridge colonies was more uniform across the time periods sampled (Fig. 4). The increase in RABV seroprevalence after parturition among adult females at cave colonies may be associated with heightened RABV infection in susceptible young (Constantine 1986, Steece and Altenbach 1989), and an increase in contact rates associated with adult females nursing among clusters of young (McCracken and Gustin 1991). Although we did not obtain comparative measurements of the densities of bats on roost surfaces in caves and bridges, census data suggest lower colony sizes in bridges when compared to caves (Constantine 1967a, Keeley and Keeley 2004, Betke et al. 2008). Sex ratios of adult bats are significantly skewed at cave colonies when compared to bridge or building colonies, with caves having almost exclusively adult female bats before parturition (Davis et al. 1962, Constantine 1967a, McCracken and Gustin 1991, Keeley and Keeley 2004). The proportional number of adult female bats is particularly important in regard to the increase in colony size after parturition; that is, caves support larger colonies and greater proportions of adult females before parturition and therefore are subject to greater increases in colony size. Thus, we expect greater increase in contact rates in caves after parturition, when compared to bridge colonies. However, the largest bridge colony sampled (MB) had a population size comparable to cave colonies sampled (MB =0.75 million; DBC, FC, and JRC = 0.43–1.3 million [Keeley and Keeley 2004, Betke et al. 2008]), but did not vary in RABV seroprevalence across the sample periods. We conclude that parturition, sex ratio, and colony size contribute to the periodicity in RABV seroprevalence detected among cave colonies of adult female bats.
When similar statistical analyses are performed on previously published data from colonies of adult female bats at Carlsbad Cavern, NM (Table 4 in Constantine et al. 1968), we detect a significant increase in rabies VNA seroprevalence between May 1956, and the period after parturition (July 1956), that decreases by August 1956 (LRχ2 = 7.38, p = 0.03). While the estimates of rabies VNA seroprevalence are not directly comparable to the estimates obtained in this study, the seasonal patterns of seroprevalence in cave colonies of adult female bats are consistent. However, a later study at Lava Cave, NM, did not report significant fluctuation in RABV infection or VNA seroprevalence in colonies of adult female bats during the reproductive season, despite a peak of RABV infection in juveniles in the early weeks after parturition (Steece and Altenbach 1989). The discrepancy in patterns among colonies of adult females may result from ecological variation across years and locations, but may also reflect differences in diagnostics and survey techniques (e.g., capture in roost vs. during emergence) (Constantine et al. 1968, Steece and Altenbach 1989).
Contrary to our expectations, bridge colonies of adult female bats had higher VNA seroprevalence when compared to cave colonies of females during Early and Late reproductive periods (Fig. 4). Despite the perceived stresses associated with anthropogenic disturbance at man-made roosts, bridge females had higher body condition overall than females roosting in caves (p = 0.002), with the greatest differences observed in the Mid period (Fig. 6). The body condition data suggest that bridge colonies of females may have greater energy to allocate to mounting immune defenses when compared with cave colonies of females, although a companion study found equivocal evidence for greater overall immune status of bats in cave or bridge roosts (Allen et al. 2009). Alternatively, there may be differences in RABV infection prevalence between cave and bridge roosts. Constantine (1968) estimated RABV infection prevalence at a bridge colony of Brazilian free-tailed bats in New Mexico near Carlsbad Cavern. Although the infection estimates were higher in normal-appearing bats at the bridge colony, it is unclear whether bats at the two different roosts were collected in the same manner (i.e., collected by hand vs. in flight). In particular, most (four of five) of the infected individuals at the bridge roost were immature young, and perhaps would not have been included in similar samples of free-flying bats at the cave roost (Tables 2 and 3 in Constantine et al. 1968). Despite equivocal evidence comparing RABV infection between roost types, it is possible that the higher RABV seroprevalence detected among bridge colonies in this study may be associated with higher infection prevalence at bridge roosts. As the current study was nondestructive, there were insufficient data to test the effect of roost type on infection prevalence.
As noted in earlier studies, the massive presence of hematophagous parasites, high concentrations of ammonia, and respiratory pathogens (e.g., Histoplasma capsulatum) that flourish in cave roosts (Davis et al. 1962, Constantine et al. 1968) may contribute to energetic trade-offs in cave roosting bats, resulting in lower body condition and generally lower rabies VNA seroprevalence, except after parturition. Our model of immune competence, as it relates to rabies VNA seroprevalence, has been adapted from RABV infection studies in mice (Smith 1981, Smith et al. 1982). Recent studies suggest that parasitism and immunocompetence may vary during reproduction in other colonial bat species (Christe et al. 2000, Pearce and O'Shea 2007), and may be influenced by roosting ecology in Brazilian free-tailed bats (Allen et al. 2009). We presume that lower immune competence leads to lower VNA seroprevalence, but lower immune competence may lead to more a productive infection, thus causing induction of a VNA response that may not have been elicited in an immunocompetent animal. Additional experimental studies are needed to address alternative scenarios on the effects of body condition and immune competence on the humoral (VNA) response to RABV exposure.
Adult males
Contrary to our expectations, we detected significant fluctuations in RABV seroprevalence among colonies of adult male bats, with peak VNA seroprevalence during the Mid period (Fig. 5). Although we did not have robust sampling of adult male bats in cave roosts, three of the four colonies sampled exhibited a peak in VNA seroprevalence during the Mid period (EEB, FC, and MB). It is possible that males experience contact with developing young that could explain the increase in RABV seroprevalence, although the periodic variation in RABV seroprevalence among colonies of adult males contrasts uniform seroprevalence among cohorts of adult female bats in some of the same colonies (FC and MB). Evidence of sex-specific differences in susceptibility to RABV infection, and possible effects on immune response after RABV exposure, among Brazilian free-tailed bats has been lacking. An alternative explanation for the periodicity observed in the seroprevalence among colonies of adult male bats, but not females within the same roosts, may relate to the proportion of time spent in the roost during the Mid period. Male and nonreproductive female Brazilian free-tailed bats spend significantly more time in roosts after parturition when compared to lactating adult females, consistent with lactating females having higher energetic requirements that require longer or more frequent nightly foraging bouts (Kunz et al. 1995, Lee and McCracken 2001). Thus, although males have less direct contact with developing young compared to nursing females, they may experience similarly high RABV exposure due to significantly longer duration of time spent in the roost with young during the Mid period. The differences in activity patterns among classes of reproductive and nonreproductive bats may be influenced by environmental conditions that impact the availability of prey (e.g., wetter summers lead to higher prey abundance), in terms of energetic trade-offs between foraging behavior and predator avoidance. Periodicity in rabies VNA seroprevalence was not detected in colonies of adult male bats at Carlsbad Cavern, NM, between May and August 1956 (p = 0.91) (Table 4 in Constantine et al. 1968), although comparisons of environmental conditions and prey availability between these studies were not possible. The seasonal and year-to-year variation in activity budgets of different classes of reproductive and nonreproductive bats warrants further study for estimating contact rates and disease exposure in colonial species. Two of the sites (FC and MB) with increased VNA seroprevalence in adult males during the Mid period also had significant seasonal variation in male body condition, with peak values during the Mid period. As the energetic requirements of adult males are thought to be relatively uniform across the summer months, higher body condition of males during the Mid period may be due to increased prey availability during late June and July. This hypothesis is consistent with the timing of episodic prey emergence from agricultural fields within the area of study (Lee and McCracken 2005). Ongoing studies in this system may clarify the interactions of prey availability, distance to foraging grounds (i.e., ability to closely track fluctuations in prey availability), and activity budgets on the body condition of male and female bats in different roosts (LC Allen, unpublished data), and the relationship between body condition and VNA response to RABV exposure.
Juveniles
After parturition in early June, pups become densely clustered on the ceiling and walls of caves (McCracken and Gustin 1991), and in expansion joints at bridge roosts (A.S.T. and L.C.A., personal observations). At 4 weeks postpartum, the density of roosting clusters may decrease, although clusters often remain separate from adult groups up to 6 weeks postpartum (McCracken and Gustin 1991). Although young pups were not observed in the crevices of two of the bridge sites (EEB and SCB), we did capture juvenile bats in flight during emergence from all colonies sampled. Our capture methods resulted in sampling of juveniles that were likely to be 4 weeks of age or older, and were engaging in practice flights or emerging to forage independently. Seropositive juvenile bats were detected from all colonies sampled except JRC, but samples sizes at JRC were low (n = 3) (Table 2). We did not find evidence for significant variation in juvenile RABV seroprevalence by period or sex, but do find a significant association with body condition. Juvenile bats with lower body condition were more likely to be seropositive. However, the significance of this result was sensitive to the inclusion of six juveniles sampled from DBC during the Mid period (Table 2). These six bats were the only juveniles sampled during the Mid period among all colonies in the study, and five were seropositive (80%). Without more robust and even sampling, we cannot conclude that there is a significant association of RABV seroprevalence and juvenile body condition, particularly with regard to evidence of prenatal maternal antibody transfer in this species (Constantine et al. 1968). Although separate analyses of body condition detected increasing values from Late to Premigratory periods, the body condition of juveniles is expected to steadily increase after parturition, initially due to the rapid development during lactation (Kunz and Robson 1995) and later as both adult and young of the year are preparing for migration.
Constantine (1986) reported RABV infection in 19% (76 of 395) of 5–11-day-old Brazilian free-tailed pups from FC in 1974, but no infection (0 of 284) in pups less than 5 days of age, evidence that many pups may be infected shortly after birth. Steece and Altenbach (1989) reported elevated levels of RABV infection in juvenile bats at Lava Cave, NM, after parturition (July and August). The data in this study are consistent with, but do not lend evidence to, elevated RABV infection in pups during the first month after parturition. RABV infection among juvenile bats during the early weeks after parturition may result from contact with infected adult females, or aerosol RABV exposure in cave roosts (Baer and Bales 1967, Constantine 1967b, Winkler 1968, Constantine et al. 1972, Davis et al. 2007). No controlled experiments have been conducted to compare the susceptibility of adult versus juvenile bats to RABV infection, nor are there controlled studies that document the significance of maternally transmitted rabies VNA in protecting pups against RABV infection. One study suggests that greater mouse-eared juvenile bats (Myotis myotis) harbor greater numbers of reproductive parasites compared to adults, perhaps due to lower immune competence (Christe et al. 2000), although another pair of studies found higher parasite intensities on adult big brown bats (Eptesicus fuscus) (Pearce and O'Shea 2007), and did not report any association of rabies VNA with parasite loads on adult or juvenile bats (Pearce et al. 2007). Experimental evidence is needed to evaluate whether pups and volant juveniles may be immunocompromised compared to adult bats, and may experience greater susceptibility to doses of RABV that produce abortive infections in immunocompetent adults (e.g., aerosol inoculation) (Baer and Bales 1967, Davis et al. 2007). The cohort of volant juveniles that were sampled from DBC in late July may have comprised individuals that successfully mounted a VNA response to RABV exposure, or had maternally transmitted rabies VNA. Further investigation into the comparative immune competence and disease susceptibility of adults, pups, and volant juvenile bats is needed, particularly with regard to the significance of maternally acquired immune protection.
Conclusions
Seasonality has recognized importance for epizootiological processes (Altizer et al. 2006). Using rabies VNA seroprevalence as an indicator of RABV exposure, infection dynamics were influenced by seasonal changes in reproductive activity and roost ecology. Further study is necessary to address the relationship between the activity budgets of different classes of reproductive and nonreproductive bats and potential for disease exposure, in the context of variation in prey availability and adverse weather conditions between years (Davis et al. 1962, Constantine 1967a, Lee and McCracken 2001). A study of several vespertilionid bat species found an increase in the prevalence of coronavirus infection in adult female bats during lactation (Gloza-Rausch et al. 2008), and an independent study found higher Hendra virus seroprevalence in pregnant and lactating female little red flying foxes (Pteropus scapulatus; Plowright et al. 2008), suggesting that epizootiology in colonial bats may be influenced by reproductive activity.
Aside from active surveillance in highly colonial species, little is known about the ecology of RABV infection in bats (but see Constantine et al. 1967c). Most human rabies cases in the United States associated with bats have occurred during September and October, suggesting contact with bats during the late summer months, but variation in bat and human incubation periods preclude a conclusive temporal association with bat reproductive activity (Messenger et al. 2002). Nondestructive long-term sampling of natural colonies is needed to provide additional insight into how roosting and behavioral ecology affect enzootic RABV infection in colonial and solitary bats (Amengual et al. 2007), particularly in the context of variable ecological conditions across years. Individual and population-level models of bat rabies have been proposed using the results obtained in this and similar studies (Dimitrov et al. 2007, 2008), and have demonstrated that immunocompromised bats can contribute significantly to RABV infection dynamics within colonies. Models incorporating seasonal variation into the immunotypic structure of colonies may provide additional insight into RABV infection dynamics among colonial bats.
Acknowledgments
Funding for this research included support from the National Science Foundation–National Institutes of Health Ecology of Infectious Disease Program (#0430418 to G.F.M.). A.S.T. received support from a Science-To-Achieve-Results Fellowship from the U.S. Environmental Protection Agency. The research was also supported by a Theodore Roosevelt Memorial Fund Grant from the American Museum of Natural History, a Grant-in-Aid of Research from the American Society of Mammalogists, and a Summer Research Grant from the Department of Ecology and Evolutionary Biology at the University of Tennessee, to A.S.T. We express our thanks to members of the Rabies Program at the CDC (Centers for Disease Control) for invaluable advice and assistance, especially P. Yager, M. Niezgoda, and L. Orciari. We thank also S. Duncan, N. Hristov, J. Reichard, L. Borrelli, A. Frank, W. Reimer, and C. Schmaeman for their technical assistance in the field. We thank I. Marbach, D. Davis, and B. Cofer for allowing access to their property. Special thanks to P. Morton and M. Goodman (Texas Parks and Wildlife), M. Bloschock (Texas Department of Transportation), B. Walker (Hill Country Adventure Tours), and B. French (Bat Conservation International) for logistic and technical support in the field. Lastly, we thank A. Reed at the University of Tennessee for statistical advice and assistance.
Disclosure Statement
The use of trade names and commercial sources is for identification only and do not imply endorsement by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency. No competing financial interests exist.
References
- Allen LC. Turmelle AS. Mendonca MT. Navara KJ, et al. Roosting ecology and variation in adaptive and innate immune system function in the Brazilian free-tailed bat (Tadarida brasiliensis) J Comp Physiol [B] 2009;197:315–323. doi: 10.1007/s00360-008-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S. Dobson A. Hosseini P. Hudson P, et al. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Amengual B. Bourhy H. Lopez-Roig M. Serra-Cobo J. Temporal dynamics of European Bat Lyssavirus type 1 and survival of Myotis myotis bats in natural colonies. PLoS ONE. 2007;2:e566. doi: 10.1371/journal.pone.0000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony ELP. Age determination in bats. In: Kunz TH, editor. Ecological and Behavioral Methods for the Study of Bats. Washington, DC: Smithsonian Institution Press; 1988. pp. 47–58. [Google Scholar]
- Baer GM. Bales GL. Experimental rabies infection in the Mexican freetail bat. J Infect Dis. 1967;117:82–90. doi: 10.1093/infdis/117.1.82. [DOI] [PubMed] [Google Scholar]
- Betke M. Hirsch DE. Makris NC. McCracken GF, et al. Thermal imaging reveals significantly smaller Brazilian free-tailed bat colonies than previously estimated. J Mammal. 2008;89:18–24. [Google Scholar]
- Blanton JD. Self J. Niezgoda M. Faber ML, et al. Oral vaccination of raccoons (Procyon lotor) with genetically modified rabies virus vaccines. Vaccine. 2007;25:7296–7300. doi: 10.1016/j.vaccine.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DJ. Smith JS. Mueller FL. Schwenke J, et al. A comparison of two serological methods for detecting the immune response after rabies vaccination in dogs and cats being exported to rabies-free areas. Biologicals. 1998;26:347–355. doi: 10.1006/biol.1998.0162. [DOI] [PubMed] [Google Scholar]
- Burns KF. Farinacci CJ. Rabies in nonsanguivorous bats of Texas. J Infect Dis. 1955;97:211–218. doi: 10.1093/infdis/97.2.211. [DOI] [PubMed] [Google Scholar]
- Burns KF. Farinacci CJ. Murnane TG. Rabies in insectivorous bats of Texas. J Am Vet Med Assoc. 1956a;128:27–31. [PubMed] [Google Scholar]
- Burns KF. Farinacci CJ. Murnane TG. Insectivorous bats naturally infected with rabies in Southwestern United States. Am J Public Health. 1956b;46:1089–1097. doi: 10.2105/ajph.46.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christe P. Arelettaz R. Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol Lett. 2000;3:207–212. [Google Scholar]
- Cleveland CJ. Betke M. Federico P. Frank JD, et al. Economic value of the pest control service provided by Brazilian free-tailed bats in south-central Texas. Front Ecol Environ. 2006;4:238–243. [Google Scholar]
- Cockrum EL. Migration in the guano bat Tadarida brasiliensis. Univ Kans Mus Nat Hist Misc Publ. 1969;51:303–336. [Google Scholar]
- Constantine DG. Activity patterns of the Mexican free-tailed bat. Univ New Mex Publ Biol. 1967a;7:1–79. [Google Scholar]
- Constantine DG. US Public Health Service Publication 1617. Georgia: National Communicable Disease Center; 1967b. Rabies transmission by air in bat caves; pp. 1–51. [Google Scholar]
- Constantine DG. Bat rabies in Southwestern United States. Public Health Rep. 1967c;82:867–888. [PMC free article] [PubMed] [Google Scholar]
- Constantine DG. Absence of prenatal infection of bats with rabies virus. J Wildl Dis. 1986;22:249–250. doi: 10.7589/0090-3558-22.2.249. [DOI] [PubMed] [Google Scholar]
- Constantine DG. Emmons RE. Woodie JD. Rabies virus in the nasal mucosa of naturally infected bats. Science. 1972;175:1255–1256. doi: 10.1126/science.175.4027.1255. [DOI] [PubMed] [Google Scholar]
- Constantine DG. Tierkel ES. Kleckner MD. Hawkins DM. Rabies in New Mexico cavern bats. Public Health Rep. 1968;83:303–316. [PMC free article] [PubMed] [Google Scholar]
- Davis AD. Rudd RJ. Bowen RA. Effects of aerosolized rabies virus exposure on bats and mice. J Infect Dis. 2007;195:1144–1150. doi: 10.1086/512616. [DOI] [PubMed] [Google Scholar]
- Davis RB. Herreid CF., II Short HL. Mexican free-tailed bats in Texas. Ecol Monogr. 1962;32:311–346. [Google Scholar]
- Dean WD. Maddy KT. Cockrum EL. Crecelius HG. Rabies in insectivorous bats of Arizona. Ariz Med. 1960;17:69–77. [PubMed] [Google Scholar]
- Dimitrov DT. Hallam TG. Rupprecht CE. McCracken GF. Adaptive modeling of viral diseases in bats with a focus on rabies. J Theor Biol. 2008;255:69–80. doi: 10.1016/j.jtbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DT. Hallam TG. Rupprecht CE. Turmelle AS, et al. Integrative models of bat rabies immunology, epizootiology, and disease demography. J Theor Biol. 2007;245:498–509. doi: 10.1016/j.jtbi.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav. 2005;70:967–971. [Google Scholar]
- Enright JB. Sadler WW. Moulton JE. Constantine DG. Isolation of rabies virus from an insectivorous bat (Tadarida mexicana) in California. Proc Soc Exp Biol Med. 1955;89:94–96. doi: 10.3181/00379727-89-21725. [DOI] [PubMed] [Google Scholar]
- Federico P. Hallam TG. McCracken GF. Purucker ST, et al. Brazilian free-tailed bats as insect pest regulators in transgenic and conventional cotton crops. Ecol Appl. 2008;18:826–837. doi: 10.1890/07-0556.1. [DOI] [PubMed] [Google Scholar]
- Gannon WL. Sikes RS. the Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2007;88:809–823. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass BP. Recovery of rabies virus from the Mexican freetail bat in Oklahoma. Proc Okla Acad Sci. 1959;39:83–84. [Google Scholar]
- Gloza-Rausch F. Ipsen A. Seebens A. Göttsche M, et al. Detection and prevalence patterns of group 1 coronaviruses in bats, Northern Germany. Emerg Infect Dis. 2008;14:626–631. doi: 10.3201/eid1404.071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW. Lemeshow S. Applied Logistic Regression. 2nd. New Jersey: John Wiley and Sons Inc.; 2000. Assessing the fit of the model; pp. 143–202. [Google Scholar]
- Hughes GJ. Orciari LA. Rupprecht CE. Evolutionary timescale of rabies virus adaptation to North American bats inferred from the substitution rate of the nucleoprotein gene. J Gen Virol. 2005;86:1467–1474. doi: 10.1099/vir.0.80710-0. [DOI] [PubMed] [Google Scholar]
- Jackson FR. Turmelle AS. Farino DM. Franka R, et al. Experimental rabies virus infection of big brown bats (Eptesicus fuscus) J Wildl Dis. 2008;44:612–621. doi: 10.7589/0090-3558-44.3.612. [DOI] [PubMed] [Google Scholar]
- Keeley ATH. Keeley BW. The mating system of Tadarida brasiliensis (Chiroptera: Molossidae) in a large highway bridge colony. J Mammal. 2004;84:113–119. [Google Scholar]
- Keeley BW. Tuttle MD. Resource Publication 4. Texas: Bat Conservation International Inc.; 1999. Bats in American bridges; pp. 1–6. (Pamphlet) [Google Scholar]
- Kunz TH. Kurta A. Capture methods and holding devices. In: Kunz TH, editor. Ecological and Behavioral Methods for the Study of Bats. Washington, DC: Smithsonian Institution Press; 1988. pp. 491–528. [Google Scholar]
- Kunz TH. Nagy KA. Energy budget analysis. In: Kunz TH, editor. Ecological and Behavioral Methods for the Study of Bats. Washington, DC: Smithsonian Institution Press; 1988. pp. 283–285. [Google Scholar]
- Kunz TH. Robson SK. Postnatal growth and development in the Mexican free-tailed bat (Tadarida brasiliensis mexicana): birth size, growth rates, and age estimation. J Mammal. 1995;76:769–783. [Google Scholar]
- Kunz TH. Whitaker JO. Wadanoli MD. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia. 1995;101:407–415. doi: 10.1007/BF00329419. [DOI] [PubMed] [Google Scholar]
- Lee YF. McCracken GF. Timing and variation in the emergence and return of Mexican free-tailed bats, Tadarida brasiliensis mexicana. Zool Stud. 2001;40:309–316. [Google Scholar]
- Lee YF. McCracken GF. Dietary variation of Brazilian free-tailed bats links to migratory populations of pest insects. J Mammal. 2005;86:67–76. [Google Scholar]
- Lumlertdacha B. Boongird K. Wanghongsa S. Wacharapluesadee S, et al. Survey for bat Lyssaviruses, Thailand. Emerg Infect Dis. 2005;11:232–236. doi: 10.3201/eid1102.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddy KT. Cockrum EL. Crecelius HG. Bat rabies in Arizona. Ariz Med. 1958;15:344–349. [PubMed] [Google Scholar]
- Manning SE. Rupprecht CE. Fishbein D. Hanlon CA, et al. Human rabies prevention-United States, 2008: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-3):1–28. [PubMed] [Google Scholar]
- McCracken GF. O'Shea TJ, editor; Bogan MA, editor. Estimates of population sizes in summer colonies of Brazilian free-tailed bats (Tadarida brasiliensis) Monitoring Trends in Bat Populations of the United States and Territories: Problems and Prospects. US Geological Survey Information and Technology Report, ITR-2003-003. 2003. pp. 21–30.
- McCracken GF. Gustin MK. Nursing behavior in Mexican free-tailed bat maternity colonies. Ethology. 1991;89:305–321. [Google Scholar]
- Messenger SL. Smith JS. Rupprecht CE. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin Infect Dis. 2002;35:738–747. doi: 10.1086/342387. [DOI] [PubMed] [Google Scholar]
- Pearce RD. O'Shea TJ. Ectoparasites in an urban population of big brown bats (Eptesicus fuscus) in Colorado. J Parasitol. 2007;93:518–530. doi: 10.1645/GE-973R.1. [DOI] [PubMed] [Google Scholar]
- Pearce RD. O'Shea TJ. Shankar V. Rupprecht CE. Lack of association between ectoparasite intensities and rabies virus neutralizing antibody seroprevalence in wild big brown bats (Eptesicus fuscus), Fort Collins, Colorado. Vector Borne Zoonot Dis. 2007;7:489–495. doi: 10.1089/vbz.2007.0572. [DOI] [PubMed] [Google Scholar]
- Plowright RK. Field HE. Smith C. Divljan A, et al. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus) Proc R Soc B. 2008;275:861–869. doi: 10.1098/rspb.2007.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racey P. Reproductive assessment in bats. In: Kunz TH, editor. Ecological and Behavioral Methods for the Study of Bats. Washington, DC: Smithsonian Institution Press; 1988. pp. 31–43. [Google Scholar]
- Reed LJ. Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Richardson JH. Ramsey RL. Starr LE. Bat rabies in Georgia 1956–65. Public Health Rep. 1966;81:1031–1035. [PMC free article] [PubMed] [Google Scholar]
- Rupprecht CE. Hanlon CA. Blanton J. Manangan J, et al. Oral vaccination of dogs with recombinant rabies virus vaccines. Virus Res. 2005;111:101–105. doi: 10.1016/j.virusres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Scales JA. Wilkins KT. Seasonality and fidelity in roost use of the Mexican free-tailed bat, Tadarida brasiliensis, in an urban setting. West N Am Nat. 2007;67:402–408. [Google Scholar]
- Schneider NJ. Scatterday JE. Lewis AL. Jennings WL, et al. Rabies in bats in Florida. Am J Public Health. 1957;47:983–989. doi: 10.2105/ajph.47.8.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar V. Bowen RA. Davis AD. Rupprecht CE, et al. Rabies in a captive colony of big brown bats (Eptesicus fuscus) J Wildl Dis. 2004;40:403–413. doi: 10.7589/0090-3558-40.3.403. [DOI] [PubMed] [Google Scholar]
- Smith JS. Mouse model for abortive rabies infection of the central nervous system. Infect Immun. 1981;31:297–308. doi: 10.1128/iai.31.1.297-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS. McCelland CL. Reid FL. Baer GM. Dual role of the immune response in street rabies virus infection of mice. Infect Immun. 1982;35:213–221. doi: 10.1128/iai.35.1.213-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS. Yager PA. Baer GM. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. In: Meslin FX, editor; Kaplan MM, editor; Koprowski H, editor. Laboratory Techniques in Rabies. 4th. Geneva, Switzerland: World Health Organization; 1996. pp. 181–192. [Google Scholar]
- Steece RS. Altenbach JS. Prevalence of rabies specific antibodies in the Mexican free-tailed bat (Tadarida brasiliensis mexicana) at Lava Cave, New Mexico. J Wildl Dis. 1989;25:490–496. doi: 10.7589/0090-3558-25.4.490. [DOI] [PubMed] [Google Scholar]
- Sullivan TD. Grimes JE. Eads RB. Menzies GC, et al. Recovery of rabies virus from colonial bats in Texas. Public Health Rep. 1954;69:766–768. [PMC free article] [PubMed] [Google Scholar]
- Villa RB. Cockrum EL. Migration in the guano bat Tadarida brasiliensis mexicana (Saussure) J Mammal. 1962;43:43–64. [Google Scholar]
- Winkler WG. Airborne rabies virus isolation. Bull Wildl Dis Assoc. 1968;4:37–40. doi: 10.7589/0090-3558-4.2.37. [DOI] [PubMed] [Google Scholar]