Abstract
The cliff swallow (Petrochelidon pyrrhonota) could play an important role in the transmission of West Nile virus (WNV) because of its breeding ecology, reservoir competence status, and potentially high natural exposure rates. Cliff swallows nest within colonies and their nests are occupied year-round by swallow bugs (Oeciacus vicarius), hematophagus ectoparasites that feed primarily on cliff swallows. These parasites are likely exposed to WNV while feeding on infectious blood of nesting cliff swallow adults and nestlings and thus, if competent vectors, could contribute to seasonal elevations in WNV transmission. In addition, swallow bugs remain within nests year-round and therefore could provide a potential overwintering mechanism for WNV if persistently infected. To test the hypotheses that swallow bugs are competent vectors and become persistently infected with WNV, we experimentally inoculated cliff swallow nestlings, allowed swallow bugs to feed on these birds during the acute phase of infection, and then exposed naive cliff swallow nestlings to the same swallow bugs. In addition, a subset of swallow bugs that fed on infectious swallow nestlings was maintained through a simulated overwintering period. Although swallow bugs ingested infectious blood (up to 106.8 plaque-forming units of WNV/mL serum) and subsequently blood-fed on naive swallows, no WNV transmission was detected, and all bugs tested WNV negative after the simulated overwintering period. Although many ecologic scenarios exist beyond the present study, our results suggest that swallow bugs may be unlikely to serve as competent biological vectors for WNV during active transmission periods or to reinitiate seasonal transmission.
Key Words: Cliff swallow, flavivirus, nest, overwintering, swallow bug, West Nile virus
Introduction
Cliff swallows (Petrochelidon pyrrhonota) are abundant, widespread, and migratory; their breeding range includes most of North America (Brown and Brown 1995) and overlaps with much of the expanding geographic range of West Nile virus (WNV; family Flaviviridae and genus Flavivirus) (Gubler 2007). Cliff swallows are competent reservoir hosts of WNV (Oesterle et al. 2009), and their nesting habits provide multiple opportunities for arbovirus exposure. These birds often nest over water, including slow-moving canals and streams (Brown and Brown 1995), where some mosquito species are relatively abundant; for example, higher proportions of Culex tarsalis were found within swallow nesting colonies versus outside the colonies (Brown and Sethi 2002). In addition, cliff swallows coexist with swallow bugs (Oeciacus vicarius) in the nest; these hematophagus ectoparasites remain in nests year-round, and blood feed primarily on swallows during the nesting season (Loye 1985).
Although the transmission cycle of WNV involves birds as the principal reservoir host and mosquitoes as the primary vector (Work et al. 1955, Hayes 1989), some aspects of transmission remain unknown, such as the role of nontraditional vectors and overwintering mechanisms of the virus (Dohm and Turell 2001, Reisen et al. 2006). Evidence of overwintering of WNV in mosquitoes has been limited (Nasci et al. 2001, Farajollahi et al. 2005, Bolling et al. 2007), suggesting that this phenomenon may be rare in nature. Nonmosquito vectors (e.g., ticks) have proven competent WNV vectors within an experimental setting (Hutcheson et al. 2005), but their contribution to natural transmission remains unknown. These nontraditional vectors may contribute to maintaining virus throughout winter and re-establishing avian-mosquito amplification cycles the following spring.
Given the parasitic nature of swallow bugs and WNV reservoir competence of cliff swallows (Oesterle et al. 2009), swallow bugs likely ingest relatively high titers of WNV when feeding on infectious cliff swallows. However, there is limited information regarding the ability of swallow bugs to become infected with and transmit WNV (Sixl et al. 1989). To better understand the vector competence of swallow bugs and the likelihood that they transmit WNV to swallows in nature, we inoculated cliff swallow nestlings with WNV, allowed swallow bugs to feed on these birds during the acute phase of infection, and subsequently allowed the bugs feed on naive nestlings. The objectives of the present study were to assess the vector competence of swallow bugs when feeding on their natural host and to look for WNV dissemination, amplification, and persistence within these parasites.
Materials and Methods
Collection and husbandry of free-ranging swallow bugs and cliff swallows
Ten cliff swallow nests were collected from a colony near Fort Collins, Colorado, in March 2007. Methods used to collect swallow bugs were based on those of J. Loye (personal communication). The nests were individually placed in 2 L plastic storage containers and transported to the National Wildlife Research Center, U.S. Department of Agriculture, Fort Collins, Colorado. Within 24 h, bugs were removed from nests using Berlese funnels (Bioquip, Rancho Dominguez, CA) and transferred to a 4 L glass beaker. Seed germination paper (Anchor Paper Company, Saint Paul, MN) was folded accordion-style and placed in the beaker as a substrate for the bugs; the beakers were covered with a fine mesh cloth to prevent escape of bugs. The beakers were then placed in an insect growth chamber (BioCold Environmental, Fenton, MO) with environmental parameters of 12°C, 70% humidity, and 10 h photoperiod. Temperature and photoperiod were gradually increased to 27°C and 14 h of light/day over 3 months to bring bugs out of diapause. Bugs were not allowed to feed during this period (Loye 1985).
The first cohort of 20 cliff swallow nestlings (aged 10–14 days posthatch) (Stoner 1945) was collected on 6 June 2007 from two colonies near Fort Collins, Colorado. The nestlings were transported to a biosafety level-3 laboratory at Colorado State University. The birds were weighed, bled (0.1 mL via jugular venipuncture), thoroughly inspected to ensure that there were no ectoparasites, and then randomly assigned to 10 artificial nests (two birds/nest). Each nest was placed in an isolation cage within an environmentally controlled room maintained at 24–27°C and 40–50% humidity.
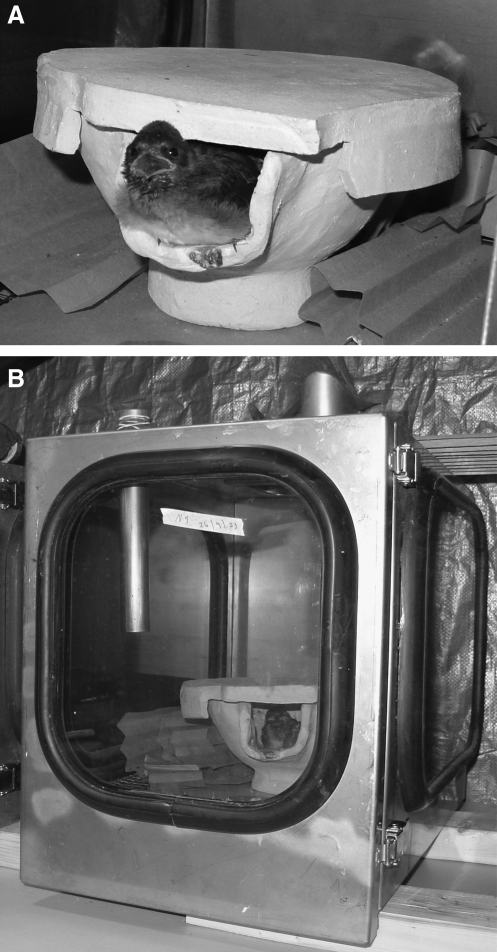
Handmade ceramic artificial nests were designed to mimic the natural mud nests of cliff swallows, with crevices in which swallow bugs could reside when inactive (Fig. 1A). The nests also provided a dark refuge for the swallow nestlings and allowed them to defecate outside of the nest via the nest entrance as they do in the wild. Each nest was placed within an isolation cage to ensure that nestlings and swallow bugs could not escape or move among nests (Fig. 1B). The air supply ports on top of the cages were covered with a fine mesh and doors opened only when feeding or removing birds. Once nestlings were placed in the artificial nests, they were hand-fed hourly for 12 h/day. Feedings consisted of a rotation of five food items (cricket abdomens, mealworm guts, wax worms, scrambled egg, and soaked kitten chow). Before daily feedings, birds were weighed to monitor weight gain and assess overall health status.
FIG. 1.
(A) Custom-made ceramic artificial nest with a cliff swallow nestling. (B) Isolation cage used to contain cliff swallow nestlings and swallow bugs.
Needle inoculation of swallow nestlings (first cohort)
At 5 days postarrival, 14 nestlings (divided equally among 7 nests) designated as first cohort were inoculated subcutaneously over the breast muscle with approximately 103.8 plaque-forming units (PFU) of WNV strain NY99-4132 in 0.1 mL BA-1 (M199, 0.05 M Tris pH 7.6, 1% bovine serum albumin, 0.35 g/L sodium bicarbonate, 100 units/mL penicillin, 100 μg/mL streptomycin, and 1 μg/mL fungizone). Six nestlings (divided equally among three nests) were administered a sham-inoculation of 0.1 mL of BA-1.
Swallow bug exposure to WNV-infected nestlings
Beakers containing 300 swallow bugs were covered with a fine mesh cloth to allow the bugs to live in proximity but prevent direct contact with nestlings.
Because of the potential negative health impacts of serial blood collection concurrent with blood feeding of swallow bugs on nestling swallows, the latter were bled (0.1 mL) only on 2 days postinoculation (DPI) to verify infection status through detection of viremia. Past studies indicate that at this time point, WNV-inoculated birds are likely at near-peak viremia titers (Komar et al. 2003). In addition, for all stages of the experiment, noninoculated nestlings were handled before inoculates, and handlers' gloves were replaced between cages.
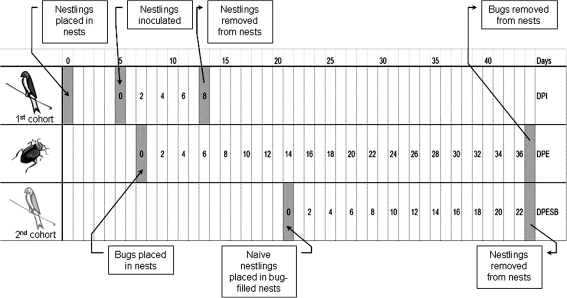
Swallow bugs (n = 300) were released from the beakers and introduced into the artificial nests with the first cohort of nestlings on 2 DPI (Fig. 2); this represented a similar bug density per nest as has been observed among natural cliff swallow colonies (Brown and Brown 1986). Bugs were allowed to feed freely upon nestlings for 6 days (between 2 and 8 DPI). Daily estimates of the number of bugs that had recently fed in each nest were made through direct observation of bugs with bright red, distended abdomens. In addition, the numbers of engorged bugs accidentally crushed during nestling manipulation were counted but not collected.
FIG. 2.
Experimental design of transmission study evaluating the capability of swallow bugs to transmit West Nile virus from experimentally inoculated cliff swallows to naive cliff swallow nestlings. DPI, days postinoculation; DPE, days postexposure; DPESB, days postexposure to swallow bugs.
All nestlings in the first cohort (i.e., needle inoculated) were bled (0.2 mL) and euthanized via intravenous sodium pentobarbital overdose on 8 DPI. Bugs remained in isolation cages without access to nestlings, hence without feeding opportunities, for the subsequent 8 days.
Exposure of WNV-exposed bugs to naive nestlings (second cohort)
A second cohort of 20 cliff swallows (aged 9–12 day posthatch) (Stoner 1945) was collected from a single site near Fort Collins, Colorado, and transported to a biosafety level-3 facility at Colorado State University. The birds were weighed, bled (0.1 mL) within 24 h of arrival, thoroughly inspected to ensure that there were no ectoparasites, and placed among 10 new artificial nests (two birds/nest), each within a temporary cage. Two days later, these nestlings were placed within the isolation cages already occupied by potentially infectious swallow bugs (bugs were transferred directly into nests containing the nestlings). Nestlings were bled (0.1 mL) at 2 days postexposure to swallow bugs (DPESB) and every 3 days thereafter. The second cohort was bled and euthanized after 23 days of contact with the potentially infectious swallow bugs (i.e., 23 DPESB). The number of bugs that had recently fed on the second cohort of nestlings was estimated daily.
Swallow bug collection and simulated overwintering
Between three and five engorged swallow bugs were collected from each nest at 1, 3, and 5 days postexposure (DPE) to the first cohort of nestlings (corresponding to 3, 5, and 7 DPI of nestlings; Fig. 2). Bugs collected on 1 DPE were maintained (without feeding) until 17, 24, or 31 DPE to assess virus persistence in bugs that fed on known viremic swallows. To accomplish this, these bugs were placed in 40 mL glass vials (with seed germination paper and covered with mesh cloth) within an empty isolation in the same climate-controlled room as the nestlings. Bugs collected on 3 and 5 DPE were immediately frozen to −80°C for subsequent testing.
Once the second cohort of nestlings was removed (on 23 DPESB), all remaining swallow bugs were collected. Live bugs with a partial to full blood meal (n = 499) were separated by nest, placed in beakers within an insect growth chamber, and maintained through a simulated overwintering period designed to represent a shortened winter in northern Colorado and intended to induce diapause. The temperature inside the insect growth chamber was gradually reduced from 27°C to 10°C, and the photoperiod from 14 to 10 h of light/day over 45 days. The bugs remained in this overwintering period for 30 days, after which temperature and light cycles were gradually increased to 27°C and 14 h of light over an additional 45 days. Once the chamber had returned to nonwinter parameters, live bugs (n = 308) were placed individually in vials, whereas dead bugs (n = 191) were pooled in the same-nest groups (five bugs/pool); all were frozen to −80°C.
Sample collection and processing
Blood collected from nestlings was immediately transferred to serum separator tubes (Microtainer®; Benton Dickinson, Franklin Lakes, NJ), centrifuged (10,000 g for 5 min) within 1 h of collection to separate serum from packed cells, and frozen to −80°C.
Live bugs collected at 1, 3, and 5 DPE were dissected into three samples per bug (i.e., head with salivary glands, legs, and abdomen/thorax). Because blood-engorged bugs were fragile, their abdomens occasionally ruptured during handling; these bugs were tested whole as a single sample. Bugs collected at the end of the study were also left whole, with live bugs processed individually and dead bugs pooled (five bugs/pool).
Bug samples were processed by placing the bug (dissected, whole-bodied, or pooled) into 2 mL microcentrifuge tubes (Fisher Scientific, Pittsburgh, PA), each tube having a copper-coated ball bearing (4.5 mm; Crosman, East Bloomfield, NY) and either 0.5 (dissected parts or single bug) or 1.0 mL (pooled bugs) of BA-1 medium. The vials were placed in chilled racks (TissueLyser Adapter Set; Qiagen, Valencia, CA), agitated for 10 min at 25 cycles/s using a mixer mill (model MM 301; Retsch, Newton, PA), centrifuged at 10,000 g for 3 min, and stored at −80°C until testing. Viral dissemination within bugs was defined as any detection of infectious WNV in homogenates of legs or heads of dissected bugs.
Laboratory assays
Sera collected from swallows before inoculation and on 8 DPI and 23 DPESB were screened for anti-WNV antibodies at a 1:10 serum dilution by plaque reduction neutralization test as described in Beaty et al. (1995). The plaque reduction neutralization test challenge dose was approximately 100 PFU of the same WNV strain used for inoculation. Samples with <60% neutralization were considered negative for anti-WNV antibodies, and samples with >90% were considered positive (no samples neutralized between 60% and 90% of virus). In addition, preinoculation sera were tested for WNV RNA by reverse transcriptase–polymerase chain reaction (RT-PCR) (Lanciotti et al. 2000) and infectious WNV by Vero cell plaque assay (VCPA) (Komar et al. 2003).
All sera collected from swallows postinoculation and postexposure to potentially infectious bugs were tested by VCPA (detection threshold 101.7 PFU/mL serum). Viral plaques were confirmed as WNV by RT-PCR, whereas WNV-negative plaques were tested by RT-PCR for Buggy Creek virus (BCRV; family Togaviridae and genus Alphavirus) as described by Moore et al. (2006).
Homogenates of bugs that fed upon the first cohort of nestlings were titrated by VCPA to determine WNV endpoint titers (detection threshold 100.4–0.7 PFU/mL bug homogenate), whereas bugs collected at the end of the study were screened by RT-PCR.
Results
Needle inoculation of swallow nestlings (first cohort)
All nestlings in the first cohort were seronegative for WNV before needle inoculation, and all needle-inoculated nestlings (n = 14) had detectable viremia on 2 DPI (average titer, 105.9 PFU/mL of serum; range, 103.6–6.8; SEM, 105.7). All needle inoculates survived to 8 DPI, at which time none had detectable viremia and all had anti-WNV antibodies (≥95% neutralization at a 1:10 serum dilution). The six noninoculated control nestlings had no evidence of viremia or antibodies throughout the study.
Swallow bug exposure to WNV-infected nestlings
Approximately 10% of bugs in contact with the first cohort of nestlings fed within the first 24 h (i.e., between 2 and 3 DPI), and this feeding rate continued to 6 DPI. The daily feeding rate declined to approximately 5% of bugs on 7 and 8 DPI; this was also the approximate feeding rate during the 23 days bugs had access to the second cohort of nestlings. An average of 26 engorged bugs (range 21–30) per nest (n = 300/nest) were inadvertently killed during husbandry.
Exposure of WNV-exposed bugs to naive nestlings (second cohort)
All nestlings in the second cohort were seronegative for WNV upon arrival, and the nestlings failed to develop detectable West Nile viremia during the 23-day period that they were cohoused with the potentially infectious bugs. In addition, none of these nestlings demonstrated evidence of anti-WNV antibodies by the end of the 23-day period. BCRV was isolated from the serum of one nestling collected on 20 DPESB.
Detection of WNV in swallow bugs
WNV was detected in bug homogenates at early time points (i.e., 3 and 5 DPE); titers were lower at the latter time point. The proportion of WNV-positive bugs also decreased between these time points. All bugs known to have consumed infectious blood (i.e., collected on 1 DPE) and maintained alive until 17, 24, and 31 DPE were WNV negative. In addition, all bugs collected at the termination of the study, including those sent through a simulated overwintering period, were WNV negative (Table 1). No virus was detected in head/salivary glands or legs.
Table 1.
Swallow Bugs Collected Postexposure to Infectious Cliff Swallows
| Number of bugs tested | WNV positive (%) | DPEabugs removed from birds | DPE bugs sacrificed for testing | Average viral titer/bug (PFU/mL) |
|---|---|---|---|---|
| 45 | 20 | 3 | 3 | 102.8 |
| 35 | 14 | 5 | 5 | 102.2 |
| 20 | 0 | 1 | 17 | — |
| 15 | 0 | 1 | 24 | — |
| 15 | 0 | 1 | 31 | — |
| 499 | 0 | 37 | 148b | — |
Average WNV titer of cliff swallows at 0 DPE was 105.9 PFU/mL serum.
DPE to potentially infectious cliff swallows.
Bugs sent through a simulated winter period lasting 120 days.
DPE, days postexposure; PFU, plaque-forming units; WNV, West Nile virus.
Discussion
The cliff swallow has a unique ecology that could potentially contribute to regional elevations in WNV transmission. Their breeding behavior may contribute to high rates of WNV exposure as these birds nest synchronously in large colonies (Brown and Brown 1995) often over water, where some mosquito species (e.g., Cx. tarsalis) are in relatively high abundance (Brown and Sethi 2002). For example, high rates of natural WNV infection (40% seropositive) were observed in adult breeding swallows in northern Colorado in June and July 2004 (Clark, unpublished data). In addition, cliff swallows likely reach viremia titers capable of infecting mosquitoes and have a reservoir competence index of 0.34 (Oesterle et al. 2009), similar to that of the northern mockingbird (Mimus polyglottos) and northern cardinal (Cardinalis cardinalis (Kilpatrick et al. 2007). Swallows feed upon mosquitoes and other insects, which represent an additional potential infection route, as oral WNV infection (ingesting an aqueous virus solution or infected mosquitoes) has been demonstrated in several avian species (Komar et al. 2003). Further, cliff swallows coexist with swallow bugs, hematophagus parasites that feed on both adults and nestlings; the potential for swallow bugs to transmit WNV is not known.
Similar to cliff swallows, swallow bugs have life history traits that could support WNV transmission cycles. As hemimetabolous wingless parasites, these bugs remain in swallow colonies throughout the year. Within a colony, swallow bugs move freely among nests; as cliff swallow breeding activity declines, bugs are translocated via birds to more active colonies for continued access to blood meal sources (Brown and Brown 2005). When swallows are absent, swallow bugs opportunistically feed on house sparrows (Passer domesticus) and other vertebrates (Loye 1985); house sparrows are highly competent reservoir hosts of WNV and are likely important in transmission (Komar et al. 2003, 2005). Finally, swallow bugs regularly endure extended periods (e.g., 10 months) without feeding while vertebrate hosts are absent, and can survive more than 3 years without a blood meal (Loye 1985). The interactive ecologies of cliff swallows, swallow bugs, and other avian hosts could offer additional opportunities for transmission and a potential overwintering mechanism for WNV if these bugs are competent vectors.
The symbiotic relationship of swallow bugs and cliff swallows, with the former being dependent upon its vertebrate host for survival, lends itself to arbovirus transmission and persistence. For example, BCRV transmission is continually perpetuated between swallow bugs and cliff swallows. Swallow bugs are the primary vector of BCRV, transmitting this virus to free-ranging cliff swallows, which are the primary amplifying host and resistant to clinical disease (Scott et al. 1984, Strauss and Strauss 1994). Swallow bugs also serve to overwinter BCRV, and there is evidence of vertical transmission to eggs (Brown et al. 2009a, 2009b). Similar to BCRV, swallow bugs are likely exposed to WNV while blood feeding on viremic swallows. Evidence for this includes WNV RT-PCR–positive swallow bugs from nests in northern Colorado in January and February of 2004, but the presence of infectious virus was not assessed (Clark, unpublished data). In addition, Sixl et al. (1989) evaluated the hypothesis that swallow bugs transmit WNV to mice; although mice cohoused with swallow bugs seroconverted during the study, it was not determined whether swallow bugs were the source of infection.
As the primary vectors of WNV, mosquitoes have provided a model for dissemination and transmission, and have been examined for their potential to overwinter the virus. In competent mosquito species, WNV replicates to high titers in the midgut and disseminates to the salivary glands, after which the virus can be transmitted to new hosts (Turell et al. 2001). In addition, WNV RNA has been detected in free-ranging overwintering mosquitoes (Nasci et al. 2001, Anderson and Main 2006, Phillips and Christensen 2006), but these detections have not been definitively linked to reinitiation of natural transmission cycles (Farajollahi et al. 2005, Reisen et al. 2006, Bolling et al. 2007). In the present study, only abdomens of bugs that fed on recently inoculated (e.g., 5 and 7 DPI) swallow nestlings tested positive, with no evidence of virus dissemination to head or legs; additionally, bugs that fed on viremic birds (2–3 DPI) and kept alive for 17–31 days showed no evidence of viral dissemination or persistence. These data suggest that swallow bugs are not competent vectors of WNV. Further, the lack of detection of WNV in bugs that had fed upon viremic swallows and maintained over time, including over a simulated overwintering period, suggests that swallow bugs are not likely to provide an overwintering mechanism for WNV.
This study design had several limitations that may have affected the likelihood of detecting WNV transmission via swallow bugs. Bird sample sizes were small due to the difficulty of hand-rearing cliff swallow nestlings and the number of bugs cohoused with swallows was limited because of the potential blood volume depletion among individual swallows. Low bug feeding rates on swallow nestlings decreased the probability of detecting a transmission event and also contributed to low bug survival throughout the experiment. In addition, engorged swallow bugs occasionally ruptured during bird husbandry, further decreasing bug sample sizes. Collectively, these factors decreased our ability to detect potential WNV transmission via swallow bugs. Finally, the study timeline was aimed at assessing biological transmission; mechanical transmission of WNV via swallow bugs may occur soon after ingestion of an infectious blood meal and cannot be ruled out by the present study.
In conclusion, while we were unable to implicate swallow bugs in the transmission or overwintering of WNV, further exploration of this concept is warranted based on lack of definitively documented overwintering mechanisms for WNV and many other arboviruses that reinitiate seasonal transmission cycles, and because much remains unknown about the complex ecological interactions that could impact transmission dynamics. These interactions are likely influenced by numerous environmental factors that vary spatiotemporally. Investigations of WNV dynamics within swallow bugs after ingestion of infectious blood meals using advanced techniques (e.g., immunofluorescence and green fluorescent protein expression), as well as continued testing of field-collected bugs, would help further assess their possible role in WNV transmission.
Acknowledgments
This study was supported by National Institutes of Health Grant 1RO1CI000219-01 (L.C.), the Centers for Disease Control and Prevention, and the National Wildlife Research Center (USDA/APHIS/WS). We especially thank Jenella Loye for guidance on husbandry methods for swallow bugs, Amy Moore for identification of BCRV, and Darren Steege for nest construction. This study was conducted in partial fulfillment of the requirements for the Masters of Science degree at Colorado State University.
Disclosure Statement
No competing financial interests exist.
References
- Anderson JF. Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- Beaty BJ. Calisher CH. Shope RE. Diagnostic procedures for viral, rickettsial, and chlamydial infections. In: Lennette EH, editor; Lennette DA, editor; Lennette ET, editor. Arboviruses. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- Bolling BG. Moore CG. Anderson SL. Blair CD. Beaty BJ. Entomological studies along the Colorado front range during a period of intense West Nile virus activity. J Am Mosq Control Assoc. 2007;23:37–46. doi: 10.2987/8756-971X(2007)23[37:ESATCF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Brown CR. Brown MB. Between-group transmission dynamics of the swallow bug, Oeciacus vicarius. J Vector Ecol. 2005;30:137–143. [PubMed] [Google Scholar]
- Brown CR. Brown MB. Cliff swallow (Hurundo pyrrhonota) In: Poole A, editor; Gill F, editor. The Birds of North America. The Academy of Natural Sciences; Philadelphia, PA: 1995. pp. 1–28. [Google Scholar]
- Brown CR. Brown MB. Ectoparasitism as a cost of coloniality in cliff swallows (Hirundo pyrrhonota) Ecology. 1986;67:1206–1218. [Google Scholar]
- Brown CR. Moore AT. Knutie SA. Komar N. Overwintering of infectious Buggy Creek virus (Togaviridae: Alphavirus) in Oeciacus vicarius (Hemiptera: Cimicidae) in North Dakota. J Med Entomol. 2009a;46:391–394. doi: 10.1603/033.046.0227. [DOI] [PubMed] [Google Scholar]
- Brown CR. Moore AT. Young GR. Padhi A. Komar N. Isolation of Buggy Creek virus (Togaviridae: Alphavirus) from field-collected eggs of Oeciacus vicarius (Hemiptera: Cimicidae) J Med Entomol. 2009b;46:375–379. doi: 10.1603/033.046.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR. Sethi RA. Mosquito abundance is correlated with cliff swallow (Petrochelidon pyrrhonota) colony size. J Med Entomol. 2002;39:115–120. doi: 10.1603/0022-2585-39.1.115. [DOI] [PubMed] [Google Scholar]
- Dohm DJ. Turell MJ. Effect of incubation at overwintering temperatures on the replication of West Nile virus in New York Culex pipiens (Diptera: Culicidae) J Med Entomol. 2001;38:462–464. doi: 10.1603/0022-2585-38.3.462. [DOI] [PubMed] [Google Scholar]
- Farajollahi A. Crans WJ. Bryant P. Wolf B, et al. Detection of West Nile viral RNA from an overwintering pool of Culex pipens pipiens (Diptera: Culicidae) in New Jersey, 2003. J Med Entomol. 2005;42:490–494. doi: 10.1093/jmedent/42.3.490. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The continuing spread of West Nile virus in the Western Hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- Hayes CG. West Nile fever. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1989. pp. 59–88. [Google Scholar]
- Hutcheson HJ. Gorham CH. Machain-Williams C. Lorono-Pino MA, et al. Experimental transmission of West Nile virus (Flaviviridae: Flavivirus) by Carios capensis ticks from North America. Vector Borne Zoonot Dis. 2005;5:293–295. doi: 10.1089/vbz.2005.5.293. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM. LaDeau SL. Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Panella NA. Langevin SA. Brault AC, et al. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loye J. The life history and ecology of the cliff swallow bug, Oeciacus vicarius (Hemiptera: Cimicidae) Cah ORSTOM Ser Entomol Med Parasitol. 1985;23:133–139. [Google Scholar]
- Moore A. Edwards EA. Brown MB. Komar N. Brown CR. Ecological correlates of Buggy Creek virus infection in Cimicid swallow bugs Oeciacus vicarius, Southwestern Nebraska, 2004. Am J Trop Med Hyg. 2006;75:81–82. doi: 10.1603/0022-2585(2007)44[42:ecobcv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nasci RS. Savage HM. White DJ. Miller JR, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle PT. Nemeth NM. VanDalen K. Sullivan H, et al. Experimental infection of cliff swallows (Petrochelidon pyrrhonota) with varying doses of West Nile virus. Am J Trop Med Hyg. 2009;81 doi: 10.4269/ajtmh.2009.09-0136. (In press). [DOI] [PubMed] [Google Scholar]
- Phillips RA. Christensen K. Field-caught Culex erythrothorax larvae found naturally infected with West Nile virus in Grand County, Utah. J Am Mosq Control Assoc. 2006;22:561–562. doi: 10.2987/8756-971X(2006)22[561:FCELFN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Lothrop HD. Martinez VM, et al. Overwintering of West Nile virus in southern California. J Med Entomol. 2006;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Scott TW. Bowen GS. Monath TP. A field-study on the effects of Fort Morgan virus, an arbovirus transmitted by swallow bugs, on the reproductive success of cliff swallows and symbiotic house sparrows in Morgan County, Colorado, 1976. Am J Trop Med Hyg. 1984;33:981–991. doi: 10.4269/ajtmh.1984.33.981. [DOI] [PubMed] [Google Scholar]
- Sixl W. Stunzner D. Withalm H. Serological examinations for antibodies against West Nile virus, Semlikivirus and Chikungunyavirus in laboratory mice, parasitized by nidicole fauna from swallow's nests. Geogr Med Suppl. 1989;1:51–55. [PubMed] [Google Scholar]
- Stoner D. Temperature and growth studies of the northern cliff swallow. Auk. 1945;62:207–216. [Google Scholar]
- Strauss JH. Strauss EG. The alphaviruses: gene-expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. O'Guinn ML. Dohm DJ. Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Work TH. Hurlbut HS. Taylor RM. Indigenous wild birds of the Nile Delta as potential West Nile virus circulating reservoirs. Am J Trop Med Hyg. 1955;4:872–888. doi: 10.4269/ajtmh.1955.4.872. [DOI] [PubMed] [Google Scholar]