Abstract
Increased oxidative stress is associated with type-2 diabetes and related cardiovascular diseases but oxidative modification of LDL has been partially characterized. Our aim was to compare the lipid and fatty acid composition as well as the redox status of LDL from diabetic patients and healthy subjects. First, to ensure that isolation of LDL by sequential ultracentrifugation did not result in lipid modifications, lipid composition and peroxide content were determined in LDL isolated either by ultracentrifugation or fast-protein liquid chromatography. Both methods resulted in similar concentrations of lipids, fatty acids, hydroxy-octadecadienoic acid (HODE) and malondialdehyde (MDA). Then, LDL were isolated by ultracentrifugation from 8 type-2 diabetic patients and 8 control subjects. Compared to control LDL, diabetic LDL contained decreased cholesteryl esters and increased triglyceride concentrations. Ethanolamine plasmalogens decreased by 49%. Proportions of linoleic acid decreased in all lipid classes while proportions of arachidonic acid increased in cholesteryl esters. Total HODE concentrations increased by 56%, 12- and 15-hydroxy-eicosatetraenoicacid by 161 and 86%, respectively, and MDA levels increased by 2-fold. α-tocopherol concentrations, expressed relative to triglycerides, were lower in LDL from patients compared to controls while γ-tocopherol did not differ. Overall, LDL from type-2 diabetic patients displayed increased oxidative stress. Determination of hydroxylated fatty acids and ethanolamine plasmalogen depletion could be especially relevant in diabetes.
Keywords: diabetes, fatty acids, peroxidation, plasmalogens, ultracentrifugation, fast-protein liquid chromatography
Introduction
Type-2 diabetes is associated with an increased risk for atherosclerosis. There is strong evidence that oxidative stress plays a key role in the onset of diabetes [1] and in the development of vascular complications of the disease [2]. Low-density lipoproteins (LDL) are important targets of oxidation, and oxidative modification of LDL is involved in the pathogenesis of atherosclerosis [3]. Oxidative stress has been mostly assessed in plasma from diabetic patients which showed increased concentrations of thiobarbituric acid-reactive species (TBARS) [4] and isoprostanes [5], both end-products of lipid peroxidation. Evidence for increased oxidative stress in LDL from type-2 diabetic patients is indirect, mostly based on increased plasma levels of autoantibodies against oxidatively modified LDL [6]. More data on specific parameters of LDL oxidation are therefore needed and could be useful to define predictive biomarkers for cardiovascular risk.
The aim of the present study was to assess oxidative stress specifically in LDL from type-2 diabetic patients compared to LDL from healthy subjects. First, we compared the lipid composition and redox status of LDL isolated by the conventional sequential flotation ultracentrifugation (UC) to that of LDL separated by fast-protein liquid chromatography (FPLC) in order to check that isolation of LDL by UC did not lead to artifactual oxidation of the particles as previously suggested [7]. Then, we performed detailed analysis of lipid classes including fatty acids and different complementary markers of lipid peroxidation in diabetic and control LDL.
Materials and methods
Blood collection
Regarding the comparative study of LDL separated by UC or FPLC, venous blood from three healthy blood donors (33 ± 13 years) was collected on Citrate Phosphate Dextrose (citrate 15.6mM, sodium citrate 89.4mM, monosodic phosphate 16.1 mM, dextrose 128.7 mM, pH 5.6, one volume for 7 volumes of blood) as an anticoagulant. Plasma was immediately separated by centrifugation at 1500 g for 10 min and spiked with 1mM EDTA and 10μM butylhydroxytoluene (BHT).
Regarding the clinical study, type-2 diabetic patients (8 men, aged 59 ± 2 yr) recruited in the Department of Endocrinology and Metabolic Diseases (HCL Lyon) were matched for sex and age to healthy subjects (8 men, aged 51 ± 4 yr). Exclusion criteria were smoking, antioxidant/vitamin supplementation, anti-aggregatory drugs and insulin treatment. Hypolipidemic drugs (statins and fibrates) treatment was suspended for 7 days before venipuncture. Control subjects were in good health as assessed by medical history and exclusion criteria were any pathology including diabetes. Written informed consent was obtained from all participants and the study was approved by the local ethics committee (CCP Sud Est IV). Plasma was separated by centrifugation at 1500 g for 10 min of blood collected on EDTA. 10 μM BHT was added to plasma which was immediately frozen at −80°C under nitrogen for further characterizations.
LDL isolation by ultracentrifugation
LDL (density 1.019–1.063) were separated from plasma by potassium bromide stepwise UC. UC was carried out in a TLA 100.3 fixed-angle rotor of a Beckman TL-100 table top UC (Beckman Coulter France, Roissy CDG, France). The LDL fraction was dialyzed extensively against phosphate buffered saline (NaCl 136.8 mM, KCl 2.6 mM, KH2PO4 1.71 mM, Na2HPO4, 12H2O 8 mM, pH 7.2) containing 1mM EDTA.
LDL isolation by FPLC
LDL were isolated from plasma by gel filtration chromatography at 4°C as previously described [8] and modified as follows [9]. Briefly, a BioRad System FPLC (Bio-Rad Laboratories, Marnes-la-Coquette, France) equipped with a Superose 6 HR 10/30 column (GE Healthcare Europe GmbH, Munich, Germany) was used with 10 mM Tris-HCl, 150 mM NaCl, pH 7.4 buffer containing 2 mM EDTA and 0.02% sodium azide as a running buffer. After loading filtered plasma, chromatography was carried out with a flow rate of 0.3 mL/min under a pressure of 150 psi. Fractions of 0.3 mL were collected and the concentrations of triglycerides (TG) and total cholesterol in the eluted fractions were measured (BioMérieux, Marcy l’Etoile, France). LDL fractions were concentrated on a Millipore filter 30,000 MW (10 min at 4000 g).
Protein, total LDL cholesterol and triacylglycerol determinations
Colorimetric determination of protein concentration was processed according to the modified Lowry method [10]. Enzymatic determination of total cholesterol and TG were processed using commercial kits (BioMérieux, Marcy l’Etoile, France).
LDL cholesterol determination
Lipid classes were separated by HPLC coupled to an Evaporative Light Scattering Detector (ELSD) according to the modified method of Seppanen-Laakso [11].
Fatty acid composition of LDL lipid classes
After the addition of appropriate internal standards (1,2-diheptadecanoyl-sn-glycero-3-phosphocholine, 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine, 1,2,3-triheptadecanoyl-sn-glycerol and heptadecanoyl cholesteryl ester), total LDL lipids were extracted twice by the addition of ethanol (3 vol) and chloroform (6 vol) in presence of BHT (50μM). Lipid classes were then separated by thin-layer chromatography. The first solvent mixture chloroform/methanol (80:8, v/v) allowed us to separate neutral lipids from phospholipids (PL). The second solvent mixture chloroform/methanol/water (63:27:4, by vol.) separated phosphatidylcholines (PC), ethanolamine phospholipids (Etn-PL) and sphingomyelins (SM). Neutral lipids were separated in TG and cholesteryl esters (CE) by a second thin-layer chromatography using hexane/diethylether/acetic acid (80:20:1, by vol.) as solvent. The different spots were scrapped off, treated with trifluoride boron/methanol (1:1, v/v) for 90 min at 100°C. The derivatized fatty acid methyl esters were then extracted twice with isooctane and separated by GLC, using an HP 6890 gas chromatograph equipped with a SP 2380 capillary column (0.25μm, 30m × 0.25mm, Supelco, Bellefonte, PA, USA).
Quantification of total LDL monohydroxylated fatty acids
Following the lipid extraction as described above, dried extracts were subjected to ethanolic hydrolysis with 10M KOH for 20 min at 60°C [12]. No reduction of lipid hydroperoxides was carried out. Non esterified hydroxylated fatty acids and fatty acids were first separated on Oasis Sep-Pak cartridge column (Waters, Milford, Massachusetts, USA). The column was activated with 6 mL of methanol and 6 mL of water before loading the sample. Then it was washed with 8 mL of 2% NH4OH water and 8 mL of methanol. Products were eluted with 8 mL of acetonitrile/2-propanol (60:40, v/v) containing 5% formic acid. Non esterified hydroxylated fatty acids were secondly separated by thin-layer chromatography with hexane/diethylether/acetic acid (60:40:1, by vol.) as mobile phase and extracted from silica with methanol. Then, hydroxylated fatty acids were separated by reverse phase-HPLC on X Bridge C18 column (3.5 μm, 4.6 × 150 mm column, Waters, Milford, Massachusetts, USA) using a gradient solvent of acetonitrile and water (pH 3) and detected at 235 nm.
Malondialdehyde determination in LDL
Malondialdehyde (MDA) concentration was determined by RP-HPLC according to the method of Therasse and Lemonnier [13]. LDL samples, mixed with thiobarbituric acid (TBA) (10 mM), acetic acid and BHT (5mM) were heated for 60 min at 95°C. The TBA-MDA adducts were then extracted with ethyl acetate and separated onto a Nucleosil C18 column (5μm, 4.6 × 250 mm, Macherey-Nagel, Hoerdt, France) using methanol/water (20:80, v/v) as mobile phase. Detection was performed by fluorimetry (excitation 515 nm, emission 553 nm).
Vitamin E determination in LDL and plasma
Tocopherol concentrations were determined by RP-HPLC [14]. Tocopherols were first extracted from LDL and plasma (1 vol) twice with hexane (4 vol) after the addition of ethanol (1 vol). Then, they were separated onto a Nucleosil C18 column (5μm, 4 × 150 mm, Macherey-Nagel, Hoerdt, France) using methanol/water (98:2, v/v) as mobile phase. Detection was performed by fluorimetry (excitation 295 nm, emission 340 nm) and quantification was allowed by the addition of tocol as an internal standard.
Statistical analysis
Results are expressed as the mean ± S.E.M. Comparisons between groups were performed using a non parameter Mann-Whitney test. Statistical significance was established at P ≤ 0.05. Correlation coefficients were determined using linear regression analysis.
Results
Comparison of LDL isolated by ultracentrifugation and fast-protein liquid chromatography
In order to determine whether isolation of LDL by UC generated LDL with a lipid composition and lipid redox status comparable to those issued from FPLC separation, fresh plasma samples from healthy volunteers were subjected in parallel to the two procedures. There were no differences between lipid composition in LDL either separated by UC or FPLC. As expected, CE was the major lipid class in LDL (51%) followed by cholesterol (29%) and few TG (3.4%). PC were the major phospholipids present in LDL (11%) (data not shown).
The GLC analysis of fatty acid methylesters derived from Etn-PL allowed us to determine the fatty dimethylacetals (DMA) representative of alkeny, acyl-GPE (or PE plasmalogens), a subclass particularly sensitive to oxidative stress. No difference in total DMA concentrations was found between both methods of isolation (6.1 ± 1.7 nmol/mg protein in LDL isolated by UC vs 4.6 ± 2.9 nmol/mg protein in LDL isolated by FPLC) representing 15.8 ± 4.6 % vs 12.5 ± 8% total fatty acids in Etn-PL, respectively.
The concentrations of linoleic acid (18:2 n-6), the most abundant polyunsaturated fatty acid (PUFA) and substrate for lipid peroxidation, were similar in LDL either isolated by UC or FPLC (1638 ± 44 vs 1543 ± 17 nmol/mg protein). As shown in Table 1, the stable primary products issued from the peroxidation of 18:2 n-6, 9-hydroxy-octadecadienoic acid (HODE) and 13-HODE, represented 0.05% of the parent molecule and were present in similar concentrations regardless of the LDL isolation method. The ratio of total HODEs to its parent fatty acid was also similar in LDL isolated by UC or FPLC. One of the aldehydes arising from the decomposition of fatty acid hydroperoxides, MDA, was found to be formed in similar amounts in LDL either separated by UC or FPLC. Concerning the main antioxidants, α- and γ-tocopherol were present in similar concentrations in LDL isolated by either method. Overall, the lipid composition and the redox status were similar whatever the LDL separation method.
Table 1.
Redox status of LDL isolated by UC or FPLC.
| UC | FPLC | |
|---|---|---|
| 9-HODE (pmol/mg cholesterol) | 217 ± 140 | 196 ± 132 |
| 13-HODE (pmol/mg cholesterol) | 242 ± 47 | 228 ± 25 |
| HODE/18:2n-6 (μmol/mol) | 519 ± 162 | 499 ± 151 |
| MDA (pmol/mg cholesterol) | 118 ± 9 | 131 ± 32 |
| α-tocopherol (nmol/mg cholesterol) | 3.68 ± 0.58 | 3.68 ± 0.72 |
| γ-tocopherol (nmol/mg cholesterol) | 0.39 ± 0.14 | 0.31 ± 0.06 |
Values represent means ± S.D. of three experiments. UC, ultracentrifugation; FPLC, fast-protein liquid chromatography; HODE, hydroxy-octadecadienoic acid; MDA, malondialdehyde.
Lipid composition and redox status of LDL from diabetic patients compared to LDL from healthy subjects
Clinical characteristics
Type-2 diabetic patients (n=8) had poorly controlled diabetes as shown by elevated values of glycated hemoglobin HbA1c (10.6 ± 0.9%) and glycemia (9.2 ± 1.3 mM) compared to healthy volunteers (5.4 ± 0.1% and 5.6 ± 0.2 mM, respectively, n=8). They presented a metabolic syndrome as assessed by their large waist circumference of 118.1 ± 8.4 cm, elevated TG of2.5 ± 0.2 mM, and low HDL-cholesterol of 0.9 ± 0.1 mM [15]. Sex- and age-matched healthy volunteers had values of waist circumference (91.3 ± 1.7 cm), TG (1.3 ± 0.1 mM) and HDL-cholesterol (1.4 ± 0.1 mM) in the expected normal range.
Lipid and fatty acid compositions of diabetic LDL compared to control LDL
The concentrations of main lipid classes were determined in LDL from type-2 diabetic patients and healthy subjects (Table 2). In diabetic LDL, the concentration of CE, the main LDL lipid class, decreased by 21%, whereas concentrations of TG increased by 75%. No changes in cholesterol, PC and Etn-PL concentrations were observed between the two groups. The fatty acid composition of the main LDL lipid classes, namely CE and PC, showed several differences between diabetic patients and control subjects. As shown in Table 3, PUFA represented 62% of the total fatty acids in CE from control LDL and their proportion significantly decreased in diabetic LDL. In particular, 18:2 n-6, the main PUFA, decreased, whether expressed in mole percent (−11%) or in nmol/mg protein (−30%). Arachidonic acid (20:4 n-6) proportion significantly increased by 43% in diabetic LDL, unlike its concentration. An increase in saturated fatty acid (SFA) proportion was observed while monounsaturated fatty acid (MUFA) proportion was unchanged between the two groups (Table 3). Concerning PC, the main PL class, lower proportions and concentrations of the major fatty acid 18:2 n-6 (−12% and −17% respectively) were present in diabetic LDL compared to control LDL, while proportion of 20:4n-6 tended to increase. No significant changes of SFA and MUFA were observed (Table 4). In Etn-PL including the PE plasmalogen subclass, DMA represented 22.9% of the total fatty chains present in Etn-PL from control LDL and only 11.8% of those in PE from diabetic LDL. The 49% decrease reflected a significant decrease of each DMA species, namely 16:0, 18:0 and 18:1n-9 DMA produced by transmethylation of the sn-1 position of plasmalogens (Figure 1). The sum of DMA species negatively correlated with HbA1c % (r2=0.61, P<0.001).
Table 2.
Lipid classes in LDL from healthy subjects and type-2 diabetic patients.
| Lipid class | Healthy subjects | Type-2 diabetic patients |
|---|---|---|
| nmol/mg protein | nmol/mg protein | |
| Cholesteryl esters | 2472 ± 152 | 1949 ± 110** |
| Cholesterol | 1112 ± 187 | 1199 ± 76 |
| Triacylglycerols | 154 ± 16 | 269 ± 49* |
| Phosphatidylcholine | 504 ± 15 | 473 ± 12 |
| Ethanolamine phospholipids | 17 ± 2 | 19 ± 2 |
Values represent means ± S.E.M. of at least six determinations per group.
p ≤ 0.05 and
p ≤ 0.01 compared with healthy subjects.
Table 3.
Fatty acid composition of LDL cholesteryl esters from healthy subjects and type-2 diabetic patients.
| Fatty acid | Healthy subjects | Type-2 diabetic patients | ||
|---|---|---|---|---|
| mol% | nmol/mg protein | mol% | nmol/mg protein | |
| 16:0 | 12.3 ± 0.5 | 325.7 ± 15.7 | 13.8 ± 0.4 | 288.4 ± 15.5 |
| 16:1n-7 | 2.5 ± 0.4 | 63.7 ± 7.8 | 2.7 ± 0.4 | 56.2 ± 9.0 |
| 16:1n-9 | 0.3 ± 0.1 | 7.4 ± 2.1 | 0.4 ± 0.1 | 8.7 ± 1.8 |
| 18:0 | 1.0 ± 0.1 | 23.3 ± 2.2 | 1.0 ± 0.1 | 18.9 ± 2.5 |
| 18:1n-7 | 1.1 ± 0.2 | 26.0 ± 4.8 | 0.9 ± 0.3 | 18.2 ± 5.4 |
| 18:1n-9 | 20.7 ± 0.9 | 500.5 ± 22.1 | 22.5 ± 0.8 | 430.5 ± 19.0 |
| 18:2n-6 | 52.4 ± 1.4 | 1295.5 ± 102.6 | 46.6 ± 1.4** | 909.3 ± 72.0** |
| 18:3n-3 | 0.4 ± 0.1 | 11.2 ± 2.0 | 0.5 ± 0.1 | 8.8 ± 2.3 |
| 18:3n-6 | 0.7 ± 0.2 | 18.3 ± 4.8 | 0.7 ± 0.2 | 13.4 ± 3.2 |
| 20:3n-6 | 0.7 ± 0.1 | 16.6 ± 1.7 | 0.8 ± 0.1 | 15.2 ± 2.2 |
| 20:4n-6 | 6.0 ± 0.5 | 140.7 ± 20.6 | 8.6 ± 0.7** | 151.8 ± 9.5 |
| 20:5n-3 | 1.2 ± 0.3 | 26.9 ± 5.9 | 0.9 ± 0.2 | 15.5 ± 2.6 |
| 22:6n-3 | 0.7 ± 0.1 | 13.6 ± 1.9 | 0.6 ± 0.1 | 10.0 ± 1.7 |
| SFA | 13.3 ± 0.6 | 350.5 ± 17.6 | 14.8 ± 0.3* | 308.4 ± 16.6 |
| MUFA | 24.5 ± 1.0 | 598.5 ± 22.0 | 26.6 ± 0.6 | 514.2 ± 19.4* |
| PUFA | 62.2 ± 1.4 | 1523.2 ± 125.4 | 58.6 ± 0.8* | 1124.2 ± 77.0* |
Values expressed as mole percent of main fatty acids and nmol/mg protein. are means ± S.E.M. of at least six subjects per group.
p ≤ 0.05 and
p ≤ 0.01 compared with LDL from healthy subjects.
MUFA. monounsaturated fatty acids; PUFA. polyunsaturated fatty acids; SFA. saturated fatty acids.
Table 4.
Fatty acid composition of LDL phosphatidylcholine from healthy subjects and type-2 diabetic patients.
| Fatty acid | Healthy subjects | Type-2 diabetic patients | ||
|---|---|---|---|---|
| mol% | nmol/mg protein | mol% | nmol/mg | |
| 16:0 | 33.0 ± 0.9 | 358.7 ± 16.7 | 34.2 ± 0.4 | 347.6 ± 9.5 |
| 16:1n-7 | 0.3 ± 0.1 | 3.7 ± 0.9 | 0.2 ± 0.1 | 2.3 ± 0.7 |
| 18:0 | 15.8 ± 0.5 | 154.8 ± 4.9 | 15.4 ± 0.8 | 142.9 ± 9.6 |
| 18:1n-7 | 1.4 ± 0.3 | 14.0 ± 2.3 | 1.3 ± 0.3 | 11.8 ± 2.5 |
| 18:1n-9 | 10.9 ± 0.5 | 108.4 ± 6.0 | 11.6 ± 0.4 | 108.0 ± 5.0 |
| 18:2n-6 | 22.0 ± 0.9 | 220.0 ± 14.1 | 19.4 ± 0.7* | 181.9 ± 10.3* |
| 18:3n-3 | 0.2 ± 0.0 | 1.5 ± 0.2 | 0.2 ± 0.1 | 2.0 ± 0.7 |
| 18:3n-6 | 0.1 ± 0.0 | 0.6 ± 0.2 | 0.0 ± 0.0 | 0.3 ± 0.2 |
| 20:3n-6 | 2.9 ± 0.2 | 26.7 ± 2.2 | 3.4 ± 0.3 | 29.3 ± 2.5 |
| 20:4n-6 | 7.8 ± 0.7 | 71.4 ± 5.6 | 9.1 ± 1.0 | 78.0 ± 7.7 |
| 20:5n-3 | 1.2 ± 0.4 | 10.3 ± 2.8 | 0.8 ± 0.2 | 7.0 ± 1.6 |
| 22:5n-3 | 0.3 ± 0.2 | 2.9 ± 1.4 | 0.3 ± 0.1 | 2.7 ± 0.9 |
| 22:6n-3 | 3.3 ± 0.5 | 27.6 ± 3.3 | 3.0 ± 0.2 | 24.3 ± 2.1 |
| SFA | 49.1 ± 1.0 | 516.7 ± 20.1 | 50.0 ± 0.8 | 493.7 ± 15.7 |
| MUFA | 13.2 ± 0.6 | 130.4 ± 7.4 | 13.5 ± 0.4 | 125.3 ± 4.5 |
| PUFA | 37.7 ± 0.8 | 361.1 ± 11.6 | 36.4 ± 0.8 | 326.1 ± 9.7 |
Values expressed as mole percent of main fatty acids and nmol/mg protein. are means ± S.E.M. of at least six subjects per group.
p ≤ 0.05 compared with LDL from healthy subjects. MUFA. monounsaturated fatty acids; PUFA. polyunsaturated fatty acids; SFA. saturated fatty acids.
Figure 1.
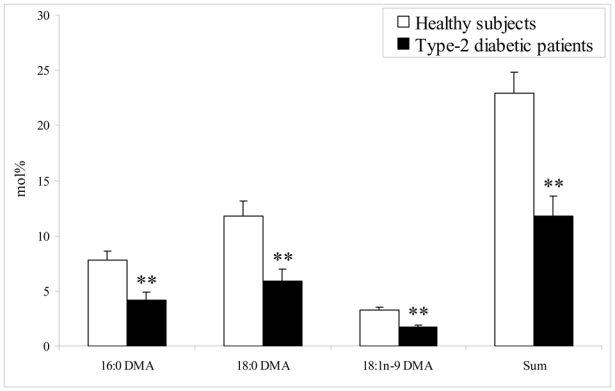
Plasmalogen alkenyl-chain composition of LDL ethanolamine phospholipids from healthy subjects and type-2 diabetic patients. Values, expressed as mole percent of main fatty acids, are means ± S.E.M. of at least seven subjects per group. **: p ≤ 0.01 compared with LDL from healthy subjects. DMA, dimethylacetal.
Lipid peroxide and antioxidant content of diabetic LDL compared to controls
The concentrations of total (both free and esterified) hydroxylated fatty acids, the stable primary products of non enzymatic peroxidation of PUFA, were assessed in LDL from patients and control groups. As shown in Table 5, 9-HODE and 13-HODE, derived from 18:2n-6, were detected and their concentrations significantly increased by 49 and 44%, respectively. In addition, the ratio of total HODE to its parent fatty acid, increased by 58% in diabetic LDL. Amongst hydroxy-eicosatetraenoic acids (HETE), derived from 20:4n-6, 8-, 11-, 12- and 15-HETE were detected in LDL from each volunteer but not 5- and 9-HETE. The concentrations of 12-HETE and 15-HETE increased by 161 and 86%, respectively, whereas the ratio of HETE to 20:4n-6 did not increase significantly. Concentrations of MDA significantly increased by 2-fold in diabetic LDL compared to control LDL (102 ± 18 vs 49 ± 5 pmol/mg cholesterol, respectively, n=7 to 8). They positively correlated with HbA1c % (r2=0.62, P<0.001).
Table 5.
Monohydroxylated fatty acids in LDL from healthy subjects and type-2 diabetic patients.
| Hydroxylated fatty acid | Healthy subjects | Type-2 diabetic patients |
|---|---|---|
| 9-HODE (pmol/mg cholesterol) | 148 ± 17 | 235 ± 21* |
| 13-HODE (pmol/mg cholesterol) | 133 ± 18 | 205 ± 17* |
| 8-HETE (pmol/mg cholesterol) | 26 ± 8 | 44 ± 8 |
| 11-HETE (pmol/mg cholesterol) | 23 ± 9 | 43 ± 11 |
| 12-HETE (pmol/mg cholesterol) | 13 ± 6 | 34 ± 7* |
| 15-HETE (pmol/mg cholesterol) | 29 ± 5 | 54 ± 8* |
| HODEs/18:2n-6 (μmol/mol) | 394 ± 39 | 624 ± 42** |
| HETEs/20:4n-6 (μmol/mol) | 822 ± 188 | 1222 ± 185 |
Values are means ± S.E.M. of seven subjects per group.
p ≤ 0.05 and
p ≤ 0.01 compared with LDL from healthy subjects.
HETE. hydroxy-eicosatetraenoic acid; HODE. hydroxy-octadecadienoic acid.
α-tocopherol concentrations in LDL did not differ between patients and controls when expressed per mg cholesterol (3.47 ± 0.43 vs 2.79 ± 0.24 nmol/mg cholesterol). However, when expressed relative to TG, α-tocopherol levels significantly decreased by 32% in LDL from diabetic patients compared to control subjects (14.3 ± 1.1 vs 21.2 ± 2.5 nmol/mg TG, p<0.05). γ-tocopherol did not significantly change in diabetic LDL compared to control LDL when expressed per mg cholesterol (0.46 ± 0.08 vs 0.29 ± 0.06 nmol/mg cholesterol) or per mg TG (1.8 ± 0.3 vs 2.3 ± 0.5 nmol/mg TG). In plasma, α-tocopherol concentrations did not differ between diabetic patients and control subjects (12.2 ± 1.2 vs 11.9 ± 1.2 μmol/L). The concentrations of plasma α-tocopherol in patients (5.4 ± 0.5 mmol/mol TG) were significantly lower than concentrations in controls (9.8 ± 0.7 mmol/mol TG) when expressed relative to TG but not when expressed relative to cholesterol (2.8 ± 0.5 vs 2.0 ± 0.2 mmol/mol cholesterol).γ-tocopherol concentrations were similar in plasma from diabetic patients and controls (1.7 ± 0.3 vs 1.6 ± 0.4 μmol/L) and whether expressed relative to cholesterol (0.41 ± 0.10 vs 0.25 ± 0.06 mmol/mol cholesterol) or TG (0.68 ± 0.09 vs 1.22 ± 0.21 mmol/mol TG).
Discussion
In the present study, we first compared LDL isolated by UC to those isolated by FPLC, in order to use a method expected to minimize the artifactual oxidative modification of the particles during processing. It has indeed been suggested that conventional UC may cause structural changes in lipoproteins whereas FPLC does not affect lipoprotein structure [7]. Our results show that the isolation of lipoproteins by UC or FPLC results in similar concentrations of lipids, fatty acid proportions, and lipid peroxide content in LDL. Proportions of lipid classes were equivalent in LDL isolated by either method and close to those reported in the literature [16, 17]. Concentrations of PE plasmalogens, particularly prone to oxidation [18] were similar in LDL, whatever the method of isolation, suggesting that the procedure of UC and dialysis was not more damaging than FPLC.
Regarding the substrates for lipid peroxidation in LDL isolated by UC, total concentrations of PUFA were 2074 nmol/mg protein, including 1638 nmol/mg protein of 18:2 n-6, in good agreement with published data [19, 20]. Similar concentrations were obtained after FPLC isolation of LDL (1961 nmol/mg protein PUFA including 1543 nmol/mg protein 18:2 n-6), indicating that PUFA were not more autooxidized in LDL separated by UC and further dialyzed. In accordance with PUFA composition of LDL, very little peroxidation of PUFA occurred in LDL from healthy volunteers since hydroxylated derivatives of 18:2 n-6 (9- and13-HODE) represented 0.05% of the parent molecule, whatever the method of LDL isolation. Finally, concentrations of MDA, commonly used as an index of overall lipid peroxidation, and of tocopherol isomers, the main LDL antioxidants, were similar in LDL either isolated by UC and FPLC. This rules out a loss of MDA and vitamin E during the dialysis process following UC, as previously reported [21, 22]. As separation of lipoproteins by UC led to LDL particles with lipid and lipid peroxide contents comparable to those issued from FPLC, UC was used to determine the profile of LDL from type-2 diabetic patients compared to that of LDL from healthy subjects.
Although there is evidence of increased oxidative stress in type-2 diabetes, it has been mainly based to date on the measurement of a single biomarker, not reflecting the overall oxidative damage. In addition, oxidative stress has been mostly assessed in plasma [23] and not in LDL. We provide evidence that LDL from type-2 diabetic patients displayed increased oxidative stress compared to LDL from healthy volunteers. First, diabetic LDL showed increased TG and decreased CE. Such a modified lipid composition may reflect a higher percentage of small dense LDL which are known to be more prevalent in type-2 diabetes [24, 25]. It has indeed been shown that small dense TG-rich LDL particles are subjected to exchange processes that remove CE from the core and replace it with TG [26, 27] and are more susceptible to oxidation [28]. To identify original markers of oxidative stress in LDL from diabetic patients, different complementary indices representative of different steps in the lipid peroxidation process were assessed. We show that PE plasmalogens decreased by 49% in diabetic LDL compared to control LDL. Plasmalogens were shown to contribute to the oxidation resistance of LDL and can serve as endogenous antioxidants in situ and in vitro [18, 29]. Loss of plasmalogens in LDL could represent a marker of oxidative stress in diabetic patients. In addition, plasmalogens have been involved in many diseases [30] but to our knowledge, this is the first evidence for decreased concentrations of plasmalogens in type 2 diabetes.
Since 18:2 n-6 and 20:4 n-6 are the main PUFA in LDL, their stable primary products of peroxidation, HODE and HETE, were determined as well as the ratio of oxidation product to parent fatty acids, described as a biomarker of oxidation status of LDL [31]. We show for the first time that both free and esterified HODE, representing 0.04% of the parent molecule in control LDL, increased by 49% in LDL from diabetic patients. This reflects free radical peroxidation of 18:2 n-6 and is in line with the decrease of 18:2 n-6 in PUFA-rich lipid classes, CE and PC. Consequently, HODE to 18:2n-6 ratio was significantly higher (+58%) in diabetic LDL than in control LDL. Because of the lack of appropriate standards, we could not confirm the presence of trans, trans-HODE stereoisomers as shown in plasma [32, 33], but cannot exclude them. Total HETE represented 0.08% of the parent molecule in control LDL, indicating that 20:4n-6 is more susceptible to oxidation. 12- and 15-HETE increased by 161 and 86% in diabetic LDL while 8- and 11-HETE tended to increase. However, there was no significant increase of HETE to 20:4n-6 ratio due in part to increased proportion of 20:4n-6 in CE. This paradoxical increase of 20:4n-6 cannot be explained from the present investigation but might result from exacerbation of Δ6 and Δ5 desaturase activities in type-2 diabetes leading to increased production of 20:4n-6 from 18:2n-6. As insulin activates both enzymes [34], this situation may occur in type-2 diabetes which is associated with chronic hyperinsulinemia periods. Finally, increased lipid peroxidation in LDL from diabetic patients compared to control LDL was confirmed by 2-fold increased concentrations of MDA. This is in agreement with previously reported increased levels of serum lipid peroxides, measured as thiobarbituric acid - reactive substances, in plasma from patients suffering from diabetes [4, 35]. Regarding the main lipid-soluble antioxidant in LDL, α-tocopherol, its concentration was significantly lower in LDL from diabetic patients compared to control LDL when expressed relative to TG, but not when expressed relative to cholesterol. Similar results were obtained in plasma. While most studies did not show any difference in α-tocopherol between diabetic patients and control subjects [4], a previous study also reported decreased α-tocopherol-to-TG ratios but unchanged α-tocopherol-to-total cholesterol ratio in LDL [36]. Although these results need to be confirmed in a larger population, the decrease of plasma and LDL α-tocopherol relative to TG in patients could be simply due to increased triglycerides levels in diabetic patients.
Altogether, our results show higher levels of lipid peroxidation markers in LDL from poorly controlled type 2 diabetic patients compared to control LDL. Since significant negative and positive correlations between HbA1C % and plasmalogens and MDA, respectively, were observed, it can be then hypothesized that the increased non enzymatic glycation of LDL, as assessed by HbA1C, could have contributed to the increased non enzymatic lipid peroxidation of LDL in patients [37]. It remains to establish the role of these modified parameters in the biological properties of oxidatively modified LDL in atherosclerosis.
In conclusion, we show that isolation of LDL by UC is no more damaging than FPLC separation, as far as oxidative modification of lipids is concerned. We give new information on the baseline values of different and complementary lipid peroxides present in LDL from healthy subjects, which may serve as references to unravel lipid abnormalities in LDL from diabetic patients. We also show that increased lipid peroxidation is present in LDL from type-2 diabetic patients and may be evidenced by the measurement of hydroxylated fatty acids and PE plasmalogens.
Acknowledgments
We thank the subject participants for their contribution. We also thank the nursing staff at the Hospices Civils de Lyon for their help with blood sample collection and some analyses. We acknowledge the French Ministry of Education and Research for RC’s grant. CC is supported by CNRS.
List of abbreviations
- BHT
butylhydroxytoluene
- CE
cholesteryl ester
- DMA
dimethylacetal
- ELSD
evaporative light scattering detector
- Etn-PL
ethanolamine phospholipids
- FPLC
fast-protein liquid chromatography
- GPE
glycerophosphoethanolamine
- HETE
hydroxy-eicosatetraenoic acid
- HODE
hydroxy-octadecadienoic acid
- MDA
malondialdehyde
- MUFA
monounsaturated fatty acids
- PC
phosphatidylcholine
- PL
phospholipid
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- SM
sphingomyelin
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive species
- TG
triacylglycerols
- UC
ultracentrifugation
References
- 1.Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol. 2006;38:794–803. doi: 10.1016/j.biocel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griesmacher A, Kindhauser M, Andert SE, et al. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:469–475. doi: 10.1016/s0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- 5.Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo G, Maggi E, Poli M, Agosta FG, Bollati P, Finardi G. Autoantibodies against oxidatively modified low-density lipoproteins in NIDDM. Diabetes. 1995;44:60–66. doi: 10.2337/diab.44.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Napoli C, Mancini FP, Corso G, et al. A simple and rapid purification procedure minimizes spontaneous oxidative modifications of low density lipoprotein and lipoprotein (a) J Biochem. 1997;121:1096–1101. doi: 10.1093/oxfordjournals.jbchem.a021700. [DOI] [PubMed] [Google Scholar]
- 8.Zambon A, Schmidt I, Beisiegel U, Brunzell JD. Dimeric lipoprotein lipase is bound to triglyceride-rich plasma lipoproteins. J Lipid Res. 1996;37:2394–2404. [PubMed] [Google Scholar]
- 9.Pruneta V, Autran D, Ponsin G, et al. Ex vivo measurement of lipoprotein lipase-dependent very low density lipoprotein (VLDL)-triglyceride hydrolysis in human VLDL: an alternative to the postheparin assay of lipoprotein lipase activity? J Clin Endocrinol Metab. 2001;86:797–803. doi: 10.1210/jcem.86.2.7261. [DOI] [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 11.Seppanen-Laakso T, Laakso I, Vanhanen H, Kiviranta K, Lehtimaki T, Hiltunen R. Major human plasma lipid classes determined by quantitative high-performance liquid chromatography, their variation and associations with phospholipid fatty acids. J Chromatogr B Biomed Sci Appl. 2001;754:437–445. doi: 10.1016/s0378-4347(01)00031-7. [DOI] [PubMed] [Google Scholar]
- 12.Browne RW, Armstrong D. HPLC analysis of lipid-derived polyunsaturated fatty acid peroxidation products in oxidatively modified human plasma. Clin Chem. 2000;46:829–836. [PubMed] [Google Scholar]
- 13.Therasse J, Lemonnier F. Determination of plasma lipoperoxides by high-performance liquid chromatography. J Chromatogr. 1987;413:237–241. doi: 10.1016/0378-4347(87)80232-3. [DOI] [PubMed] [Google Scholar]
- 14.Calzada C, Coulon L, Halimi D, et al. In vitro glycoxidized low-density lipoproteins and low-density lipoproteins isolated from type 2 diabetic patients activate platelets via p38 mitogen-activated protein kinase. J Clin Endocrinol Metab. 2007;92:1961–1964. doi: 10.1210/jc.2006-2045. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Kuksis A, Myher JJ, Geher K, Breckenridge WC, Jones GJ, Little JA. Lipid class and molecular species interrelationships among plasma lipoproteins of normolipemic subjects. J Chromatogr. 1981;224:1–23. doi: 10.1016/s0378-4347(00)80133-4. [DOI] [PubMed] [Google Scholar]
- 18.Engelmann B, Brautigam C, Thiery J. Plasmalogen phospholipids as potential protectors against lipid peroxidation of low density lipoproteins. Biochem Biophys Res Commun. 1994;204:1235–1242. doi: 10.1006/bbrc.1994.2595. [DOI] [PubMed] [Google Scholar]
- 19.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 20.Barre E. A more detailed fatty acid composition of human lipoprotein(a)--a comparison with low density lipoprotein. Chem Phys Lipids. 2003;123:99–105. doi: 10.1016/s0009-3084(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Jurgens G, Quehenberger O, Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987;28:495–509. [PubMed] [Google Scholar]
- 22.Scheek LM, Wiseman SA, Tijburg LB, van Tol A. Dialysis of isolated low density lipoprotein induces a loss of lipophilic antioxidants and increases the susceptibility to oxidation in vitro. Atherosclerosis. 1995;117:139–144. doi: 10.1016/0021-9150(95)05555-b. [DOI] [PubMed] [Google Scholar]
- 23.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feingold KR, Grunfeld C, Pang M, Doerrler W, Krauss RM. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler Thromb. 1992;12:1496–1502. doi: 10.1161/01.atv.12.12.1496. [DOI] [PubMed] [Google Scholar]
- 26.Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. 2003;31:1066–1069. doi: 10.1042/bst0311066. [DOI] [PubMed] [Google Scholar]
- 27.Verges B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundam Clin Pharmacol. 2009;23:681–685. doi: 10.1111/j.1472-8206.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 28.Tribble DL, Holl LG, Wood PD, Krauss RM. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93:189–199. doi: 10.1016/0021-9150(92)90255-f. [DOI] [PubMed] [Google Scholar]
- 29.Jurgens G, Fell A, Ledinski G, Chen Q, Paltauf F. Delay of copper-catalyzed oxidation of low density lipoprotein by in vitro enrichment with choline or ethanolamine plasmalogens. Chem Phys Lipids. 1995;77:25–31. doi: 10.1016/0009-3084(95)02451-n. [DOI] [PubMed] [Google Scholar]
- 30.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 31.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Mashima R, Onodera K, Yamamoto Y. Regioisomeric distribution of cholesteryl linoleate hydroperoxides and hydroxides in plasma from healthy humans provides evidence for free radical-mediated lipid peroxidation in vivo. J Lipid Res. 2000;41:109–115. [PubMed] [Google Scholar]
- 33.Liu W, Yin H, Akazawa YO, Yoshida Y, Niki E, Porter NA. Ex vivo oxidation in tissue and plasma assays of hydroxyoctadecadienoates: Z,E/E,E stereoisomer ratios. Chem Res Toxicol. 2010;23:986–995. doi: 10.1021/tx1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner RR. Hormonal modulation of delta6 and delta5 desaturases: case of diabetes. Prostaglandins Leukot Essent Fatty Acids. 2003;68:151–162. doi: 10.1016/s0952-3278(02)00265-x. [DOI] [PubMed] [Google Scholar]
- 35.Kesavulu MM, Giri R, Kameswara Rao B, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26:387–392. [PubMed] [Google Scholar]
- 36.Schneider M, Verges B, Klein A, et al. Alterations in plasma vitamin E distribution in type 2 diabetic patients with elevated plasma phospholipid transfer protein activity. Diabetes. 2004;53:2633–2639. doi: 10.2337/diabetes.53.10.2633. [DOI] [PubMed] [Google Scholar]
- 37.Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–1424. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]


