Abstract
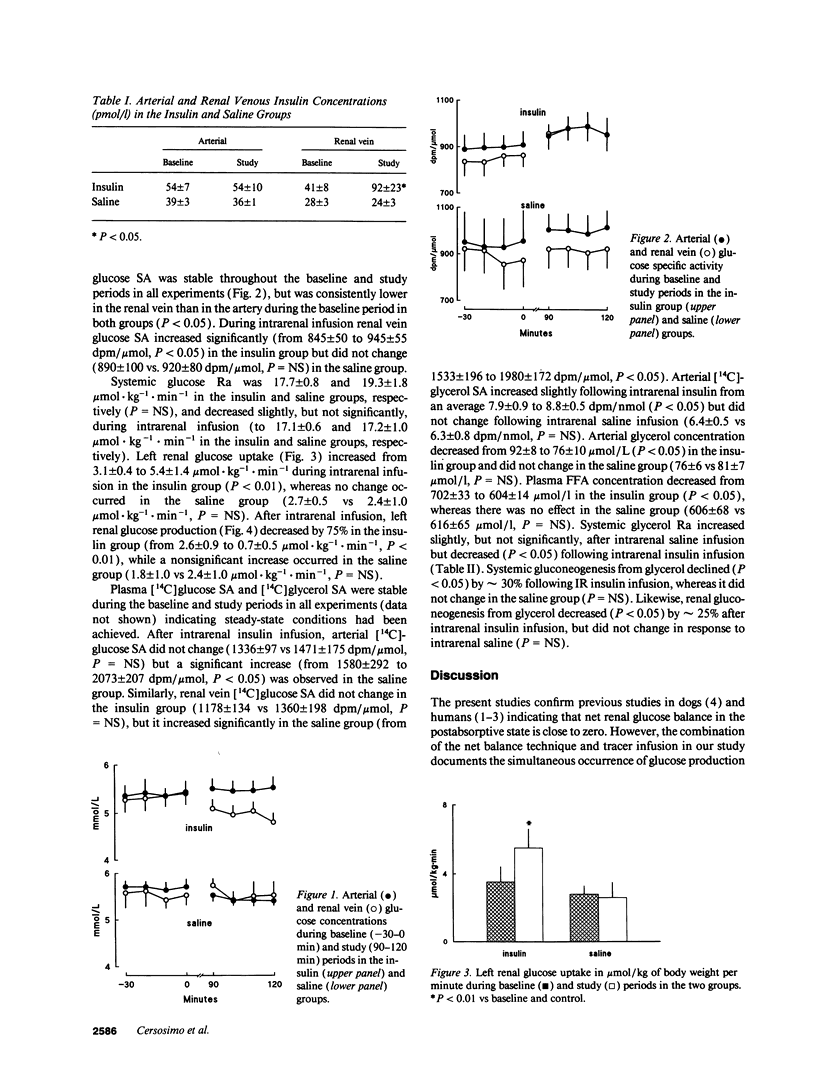
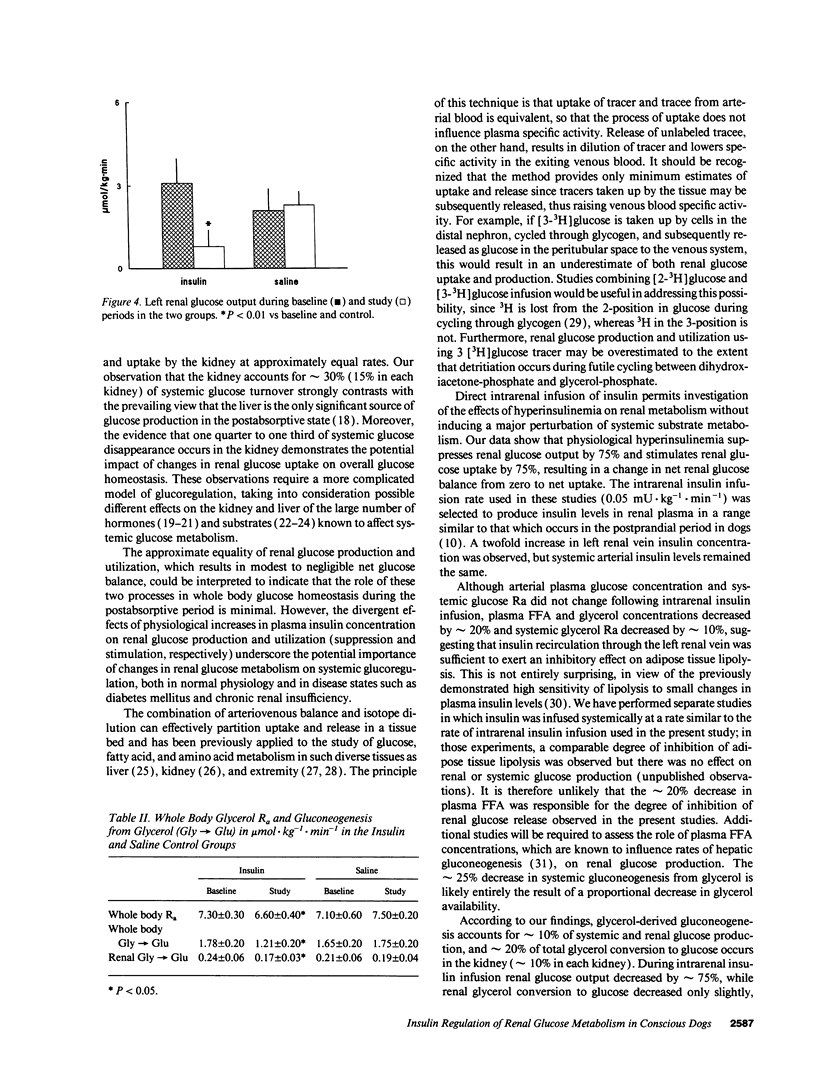
Previous studies indicating that postabsorptive renal glucose production is negligible used the net balance technique, which cannot partition simultaneous renal glucose production and glucose uptake. 10 d after surgical placement of sampling catheters in the left renal vein and femoral artery and a nonobstructive infusion catheter in the left renal artery of dogs, systemic and renal glucose and glycerol kinetics were measured with peripheral infusions of [3-3H]glucose and [2-14C]glycerol. After baseline measurements, animals received a 2-h intrarenal infusion of either insulin (n = 6) or saline (n = 6). Left renal vein insulin concentration increased from 41 +/- 8 to 92 +/- 23 pmol/l (P < 0.05) in the insulin group, but there was no change in either arterial insulin, (approximately 50 pmol/l), glucose concentrations (approximately 5.4 mmol/l), or glucose appearance (approximately 18 mumol.kg-1.min-1). Left renal glucose uptake increased from 3.1 +/- 0.4 to 5.4 +/- 1.4 mumol.kg-1.min-1 (P < 0.01) while left renal glucose production decreased from 2.6 +/- 0.9 to 0.7 +/- 0.5 mumol.kg-1.min-1 (P < 0.01) during insulin infusion. Renal gluconeogenesis from glycerol decreased from 0.23 +/- 0.06 to 0.17 +/- 0.04 mumol.kg-1.min-1 (P < 0.05) during insulin infusion. These results indicate that renal glucose production and utilization account for approximately 30% of glucose turnover in postabsorptive dogs. Physiological hyperinsulinemia suppresses renal glucose production and stimulates renal glucose uptake by approximately 75%. We conclude that the kidney makes a major contribution to systemic glucose metabolism in the postabsorptive state.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abodeely D. A., Lee J. B. Fuel of respiration of outer renal medulla. Am J Physiol. 1971 Jun;220(6):1693–1700. doi: 10.1152/ajplegacy.1971.220.6.1693. [DOI] [PubMed] [Google Scholar]
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumrad N. N., Wise K. L., Williams P. E., Abumrad N. A., Lacy W. W. Disposal of alpha-ketoisocaproate: roles of liver, gut, and kidneys. Am J Physiol. 1982 Aug;243(2):E123–E131. doi: 10.1152/ajpendo.1982.243.2.E123. [DOI] [PubMed] [Google Scholar]
- Barrett E. J., Revkin J. H., Young L. H., Zaret B. L., Jacob R., Gelfand R. A. An isotopic method for measurement of muscle protein synthesis and degradation in vivo. Biochem J. 1987 Jul 1;245(1):223–228. doi: 10.1042/bj2450223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Assessment of the postprandial pattern of glucose metabolism in nondiabetic subjects and patients with non-insulin-dependent diabetes mellitus using a simultaneous infusion of [2(3)H] and [3(3)H] glucose. Metabolism. 1989 Jan;38(1):38–45. doi: 10.1016/0026-0495(89)90177-7. [DOI] [PubMed] [Google Scholar]
- Biava C., Grossman A., West M. Ultrastructural observations on renal glycogen in normal and pathologic human kidneys. Lab Invest. 1966 Jan;15(1 Pt 2):330–356. [PubMed] [Google Scholar]
- Bleiberg B., Beers T. R., Persson M., Miles J. M. Systemic and regional acetate kinetics in dogs. Am J Physiol. 1992 Feb;262(2 Pt 1):E197–E202. doi: 10.1152/ajpendo.1992.262.2.E197. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Shulman G. I., Lacy W. W. Differential time course of glucagon's effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes. 1981 Mar;30(3):180–187. doi: 10.2337/diab.30.3.180. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Effect of sodium linoleate infusion on plasma free fatty acids, glucose, insulin, and ketones in unanesthetized dogs. Diabetes. 1972 Dec;21(12):1179–1184. doi: 10.2337/diab.21.12.1179. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K. F., Lees J. A., Newman J. H. Hypoglycemia in hospitalized patients. Causes and outcomes. N Engl J Med. 1986 Nov 13;315(20):1245–1250. doi: 10.1056/NEJM198611133152002. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Bonadonna R. C., DelPrato S., Ratheiser K., Zyck K., Ferrannini E., DeFronzo R. A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989 Jul;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder W. G., Ross B. D. Enzyme distribution along the nephron. Kidney Int. 1984 Aug;26(2):101–111. doi: 10.1038/ki.1984.143. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest. 1968;21(3):263–276. doi: 10.3109/00365516809076994. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Holck P., Rasch R. Structure and segmental localization of glycogen in the diabetic rat kidney. Diabetes. 1993 Jun;42(6):891–900. doi: 10.2337/diab.42.6.891. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Caruso M., Heiling V., Miles J. M. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes. 1989 Dec;38(12):1595–1601. doi: 10.2337/diab.38.12.1595. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R., GASCOYNE T. RENAL GLUCONEOGENESIS. IV. GLUCONEOGENESIS FROM SUBSTRATE COMBINATIONS. Acta Biol Med Ger. 1963;11:607–615. [PubMed] [Google Scholar]
- Kida K., Nakajo S., Kamiya F., Toyama Y., Nishio T., Nakagawa H. Renal net glucose release in vivo and its contribution to blood glucose in rats. J Clin Invest. 1978 Oct;62(4):721–726. doi: 10.1172/JCI109182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K. L., Wang M. S., Torikai S., Davidson W. D., Kurokawa K. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int. 1981 Jul;20(1):29–35. doi: 10.1038/ki.1981.100. [DOI] [PubMed] [Google Scholar]
- LEE J. B., VANCE V. K., CAHILL G. F., Jr Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am J Physiol. 1962 Jul;203:27–36. doi: 10.1152/ajplegacy.1962.203.1.27. [DOI] [PubMed] [Google Scholar]
- LEEVY C. M., MENDENHALL C. L., LESKO W., HOWARD M. M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962 May;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J. M., Ellman M. G., McClean K. L., Jensen M. D. Validation of a new method for determination of free fatty acid turnover. Am J Physiol. 1987 Mar;252(3 Pt 1):E431–E438. doi: 10.1152/ajpendo.1987.252.3.E431. [DOI] [PubMed] [Google Scholar]
- Nurjhan N., Consoli A., Gerich J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Jan;89(1):169–175. doi: 10.1172/JCI115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisters P. W., Restifo N. P., Cersosimo E., Brennan M. F. The effects of euglycemic hyperinsulinemia and amino acid infusion on regional and whole body glucose disposal in man. Metabolism. 1991 Jan;40(1):59–65. doi: 10.1016/0026-0495(91)90193-z. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981 Jun;52(6):1235–1241. doi: 10.1210/jcem-52-6-1235. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirthensohn G., Guder W. G. Renal substrate metabolism. Physiol Rev. 1986 Apr;66(2):469–497. doi: 10.1152/physrev.1986.66.2.469. [DOI] [PubMed] [Google Scholar]


