Abstract
Hexavalent chromium [Cr (VI)] is a constituent of chromite ore. Although it is known to have several industrial and technological applications, its release into the aquatic environment as a result of chemical spill or inadequate waste discharge may hamper the health of aquatic organisms. In this study, we have investigated the effects of Cr (VI) on multiple biomarkers responses in goldfish under sub-chronic exposure conditions. Laboratory-acclimatized fish were exposed to 4.25 ppm and 8.57 ppm Cr (VI) for four weeks using a continuous flow-through system. During exposure, fish samples were collected on a weekly basis and analyzed for multiple biomarkers including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), metallothionein (MT), and total protein in liver and kidney. Study results indicated that the CAT activity and total protein levels in Cr (VI) – treated goldfish did not significantly differ (p>0.05) from their respective controls during experimentation. However, highly significant up-regulations (p<0.05) of SOD, GPx, and MT expression in Cr (VI) – treated goldfish were recorded at different exposure times depending on Cr (VI) concentration, test organ, and/or biomarker of interest. For example, significantly higher liver GPx levels were found at weeks 2 and 3 in the 4.25 ppm concentration, and at weeks 3 and 4 in the 8.57 ppm, while kidney GPx levels were significantly higher at weeks 1, 2 and 3 in the 4.25 ppm concentration, and at weeks 2, 3 and 4 in the 8.57 ppm concentration. In summary, Cr (VI)-induced oxidative stress was characterized by statistically significant increases in SOD, GPx, and MT expression in goldfish tissues; with the kidney showing a relatively higher sensitivity to Cr (VI) toxicity compared to the liver.
Keywords: hexavalent chromium, goldfish, sub-chronic exposure, catalase, superoxide dismutase, glutathione peroxidase, metallothionein, protein expression, oxidative stress
INTRODUCTION
Chromium is a ubiquitous element in the earth crust. It exhibits various oxidation states; among which, the trivalent (Cr (III)) and hexavalent (Cr (VI)) forms are most common. Cr (VI) has diverse applications in various industries including chemical, refractory and metallurgical industries. Inadequate treatment of effluents from these industries during the processes of leather tanning, electroplating, steel manufacturing, burning of fossil fuels and cooling towers releases Cr (VI) into the surrounding water bodies (Palmer et al., 1991). This release may hamper the health of aquatic organisms (Torrey et al., 1978; Hattingh et al., 1977; Elwood et al., 1980).
Cr (VI) has more potential to cross the cellular membranes and is reduced to Cr (III) in biological systems (Hneihen et al., 1993). During this chemical process, reactive oxygen species (ROS) are generated (Wilbur et al., 1988). As result, the biological system induces antioxidants such as catalase (CAT), superoxide dismutase (SOD) and glutathione-related enzymes to combat the increased levels of ROS. Failure to mitigate the attack of ROS leads to development of oxidative stress (Halliwell et al., 2007a; Halliwell et al., 2007b). Antioxidants enzymes serve as excellent biomarkers to study oxidative stress in aquatic organisms. Various studies have been conducted to measure the antioxidant levels as early biomarkers to assess the oxidative stress (Sridevi et al., 1998; Bagchi et al., 1997; Lopes et al., 2001). In a more recent study, Robert and Oris (2004) investigated a number of biomarkers including biochemical, transcriptional and histological markers in rainbow trout in response to chromium toxicity. Arillo et al., (1988) also pointed out that Cr (VI) induces alterations in oxidative function of mitochondria in trout fish at micro molar concentrations. In Tilapia sparrmanii it has been reported to cause thrombocytopenia by depleting thrombocytes in blood (Van Pittius et al., 1992). It has also been found to inhibit ion-transporting ATPases in gills, kidney and intestinal tissues of the coastal teleost, Periohthalmus dipes (Thaker et al., 1996). In vitro studies of goldfish hepatocytes have reported that acute Cr (VI) exposure significantly reduces cell viability and stimulates production of ROS to induce oxidative stress (Krumschnabel et al., 2004). Arunkumar et al. (2000) have reported that the Cr (VI) is more toxic and suppresses immune response more significantly compared to Cr (III).
Although oxidative stress has been linked to Cr (VI) toxicity, additional scientific data is needed to further understand the cellular and molecular mechanisms, as well as the toxico-kinetics and toxico-dynamics of its harmful effects to aquatic organisms. Therefore, this research provides valuable information on tissue-specific toxicity of Cr (VI) through assays that determine its ability to induce oxidative stress and alteration of protein expression in goldfish under sub-chronic exposure conditions.
MATERIALS AND METHODS
Chemicals
Potassium dichromate with a purity of ≥ 99.5%, ethanol, chloroform, formaldehyde, mannitol, ethylene glycol tetraacetic acid (EGTA), sucrose, 5,5′- dithiobis (2-nitrobenzoic acid) (DTNB), reduced glutathione, ethylenediaminetetraacetic acid (EDTA), 2-Phenoxyethanol, dimethyl sulfoxide, methanol, xylene, 10% formalin, Tris borate electrophoresis (TBE) buffer, protease inhibitor cocktail, dithiotreitol (DTT), b-mercaptoethanoln, sodium chloride (NaCl), hydro chloric acid (HCl) and human albumin were purchased from Sigma-Aldrich (St Louis, MO, USA). Phosphate buffered saline (PBS) was purchased from Trevigen Inc., (Gaithersburg, MD, USA). Heparin was purchased from Fisher Scientific (Suwanee, GA, USA). Catalase assay kit, superoxide dismutase assay kit, glutathione peroxidase assay kit, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, potassium phosphate and Tris-HCl were purchased from Calbiochem (San Diego, CA, USA).
Test Animals and Chromium Exposure
Goldfish, Carassius auratus, with an average weight of 17.4 ± 4.5 g and average length of 9.0 ± 0.6 cm were purchased from a local commercial store in Jackson, Mississippi. More than 210 fish were acclimatized to the laboratory conditions for 15 days in 2 glass aquaria of 150 L capacity each. During the acclimatization, the fish were fed with flakes food twice a day. A photoperiod of 12h light and 12h dark was maintained. The laboratory-acclimatized fish were used for chromium exposure.
Fish were exposed to Cr (VI) under sub-chronic conditions for a period of four weeks in a continuous flow through system. Chemical estimations were derived from a previous study in which we found that the 96h-LC50 of Cr (VI) for goldfish was 85.7 ± 4.8 mg/L (Velma and Tchounwou, 2008). During the sub-chronic exposure, the fish were exposed to 5% of 96h-LC50 (4.28 ppm) and 10% of 96h-LC50 (8.57 ppm). A control group of fish was also made without chromium exposure. Filtered and aerated tap water was used for control and treatment aquaria through out the experiment. The acclimatized fish were separated into 3 groups including control, 5% of 96h-LC50 and 10% of 96h-LC50. Each treatment had 70 fish in a glass aquarium of 150 L capacity. The fish were fed twice a day during the exposure and photoperiod was maintained. During the exposure, 3 fish were picked up randomly each week to analyze each parameter from the control, 5% and 10% treatment aquaria. In accordingly 15 fish were picked up per week for each treatment. The fish were anesthetized with 0.1% 2-phenoxyethanol (Fluka) and dissected livers and kidneys were stored at −80°C. We made 3 replicates of samples from each liver and kidney; in accordingly we have analyzed 9 samples for each organ and each parameter. All controls and Cr (VI)-exposed livers and kidneys were analyzed for selected antioxidants enzymes, metallothionein and total protein levels in liver and kidney tissues.
Catalase (EC 1.11.1.6 - CAT) Assay
The liver and kidney tissues were rinsed with phosphate buffer (pH 7.4) to remove red blood cells, and homogenized in cold buffer (1:8 w/v) containing 50 mM potassium phosphate, pH 7.0 and 1 mM EDTA per gram of tissue. The homogenate was centrifuged at 1500x g for 5 min at 4°C (Beckman XL-100K, USA). The supernatant from each tissue was used as the enzyme source and analyzed in a 96 well plate according to Johansson and Borg method using commercial catalase assay kit (Johansson and Borg, 1988). Three replicates of 20 μl samples were mixed with 100 μl of 100 mM potassium phosphate, pH 7.0 and 30 μl methanol. The reaction was initiated with 20 μl of 35 mM hydrogen peroxide and the mixture was incubated on shaker at room temperature for 20 min. The reaction was terminated by adding 30 μl of 10 M potassium hydroxide. Thirty μl of 4-amino-3-hydrazino-5-mercapto-1, 2, 4-triazole (chromogen) was added to the three replicates of each sample, and incubated on a shaker for 10 min at room temperature. After incubation, 10 μl of potassium periodate was added to the mixture and incubated for 5 min at room temperature. Finally, the reaction mixture's absorbance was recorded at 540 nm using 96 well plate reader (Multiskan Ascent, Lab systems USA). The principle of this assay is based on the reaction of the CAT with methanol in the presence of an optimal concentration of hydrogen peroxide, and the measurement of formaldehyde produced. One unit of CAT is defined as the amount of CAT that will cause the formation of 1.0 nM formaldehyde per min at 25°C. Reference standard curve was prepared with formaldehyde solution.
Superoxide Dismutase (EC 1.15.1.1 - SOD) Assay
The tissues were rinsed with phosphate buffer (pH 7.4) containing 0.16 mg/ml heparin, to remove any red blood cells. The tissues were homogenized (1:8, w/v) in cold 20mM HEPES buffer, pH 7.2, containing 1mM EGTA, 210 mM mannitol, and 70 mM sucrose per gram of tissue. Each tissue homogenate was centrifuged at 1500x g for 5 min at 4°C (Beckman XL-100K, USA). The supernatants were further analyzed in a 96 well plate according to Marklund and Marklund method using commercial SOD assay kit (Marklund and Marklund, 1974). Three replicates of 10 μl of each sample were mixed with 200 μl of radical detector. Radical detector was prepared with 50 μl of tetrazolium salt solution and 19.95 ml of 50 mM Tris-HCl (pH 8.0, containing 0.1 mM diethylene triamine pentaacetic acid and 0.1 mM hypoxanthine). The reaction was initiated by adding 20 μl of xanthine oxidase and the reaction mixture was incubated on a shaker for 20 min at room temperature. The xanthine oxidase was prepared with 50 μl of xanthine oxidase solution and 1.95 ml of 50 mM Tris-HCl, pH 8.0. After 20 min of incubation, the reaction mixture's absorbance was recorded at 450 nm using 96 well plate reader (Multiskan Ascent, Lab systems USA). The assay principle is based on the utilization of a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The standard reference curve was prepared with solution of bovine erythrocyte SOD.
Glutathione Peroxidase (EC 1.11.1.9 - GPx) Assay
The tissues were washed with phosphate buffer saline (PBS), pH 7.4, and containing 0.16 mg/ml heparin, to remove red blood cells. The tissues were homogenized (1:8, w/v) in cold buffer containing 50 mM Tris-HCl, pH 7.5, 5 mM EDTA and 1 mM DTT per gram tissue. The homogenates were centrifuged at 1500x g for 15 min at 4°C and each tissue supernatant was separated (Beckman XL-100K, USA). The supernatants were analyzed according to the method described by Paglia and Valentine using commercial GPx assay kit (Paglia and Valentine, 1967). Three replicates of 20 μl of each sample were mixed with 100 μl of 50 mM Tris-HCl, pH 7.6, containing 5 mM EDTA and 50 μl of co-substrate mixture (Calbiochem supplied NADPH, glutathione, and glutathione reductase). The reaction was initiated by adding 20 μl of cumene hydroperoxide solution. The reaction mixture was vortexed properly, and the absorbance was recorded at 340 nm wavelength (Multiskan Ascent, Lab systems USA). The assay indirectly measures the activity of GPx by a coupled reaction with glutathione reductase. One unit of GPx is defined as the amount of enzyme that will cause the oxidation of 1.0 nmol of NADPH to NADP+ per minute at 25°C.
Metallothionein (MT) Quantification
MT levels were quantified as described previously by Viarengo et al. (1997). Briefly, the tissues were homogenized separately (1:8, w/v) in a buffer containing 20 mM Tris-HCl, pH 8.6, 0.01% ß-mercaptoethanol, protease inhibitor cocktail and 0.5 M sucrose. The homogenates were centrifuged at 30000x g for 20 min (Beckman XL-100K, USA); and the supernatants were collected and resuspended in 49% ethanol containing 3.7% chloroform. The mixture was centrifuged at 30000x g for 20 min and again resuspended in 49% ethanol containing 3.7% chloroform, and centrifuged at 6000x g for 10 min. The supernatant was collected, acidified cold 87% ethanol was added and the mixture was incubated at −20°C for 1 hr. The mixture was centrifuged at 6000x g for 10 min, the pellet was resuspended in 20 mM Tris-HCl buffer, pH 8.6, containing 87% ethanol and 1% chloroform, and centrifuged at 6000x g for 10 min. The pellet was allowed to dry under a nitrogen gas stream and resuspended in a solution containing 0.16 M NaCl, 0.5N HCl, and 2 mM EDTA. The resuspended 0.3 ml aliquots were mixed with 4.2 ml of 0.43mM DTNB. The DTNB was prepared in 0.2 M phosphate buffer, pH 8.0, supplemented with 2M NaCl. The mixture was centrifuged at 3000x g for 5 min at room temperature and the absorbance of the supernatant was measured at 412 nm using reduced glutathione as reference standard.
Total Protein Quantification
The goldfish liver and kidney tissues were homogenized (1:8 w/v) in pre-chilled phosphate buffer (pH 7.4). The total protein levels were measured according to the Bradford method using bovine serum albumin as reference standard (Bradford, 1976).
Statistical Analysis
Each experiment was repeated three times, and the data were expressed as means ± SDs. Statistical analysis was performed using SAS software for Windows (SAS Institute, Cary, North Carolina, USA). Differences between controls and Cr (VI)-treated goldfish for all assessment endpoints were determined by ANOVA for global comparison, followed by Dunnett's test for identifying groups whose means are significantly different from the controls. The level of significant difference was considered at p<0.05.
RESULTS
Catalase (CAT) Activity
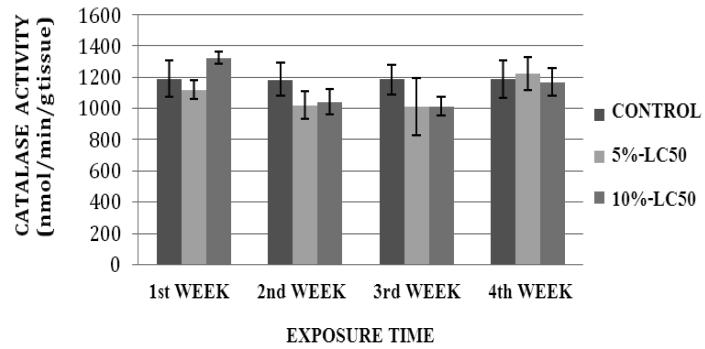
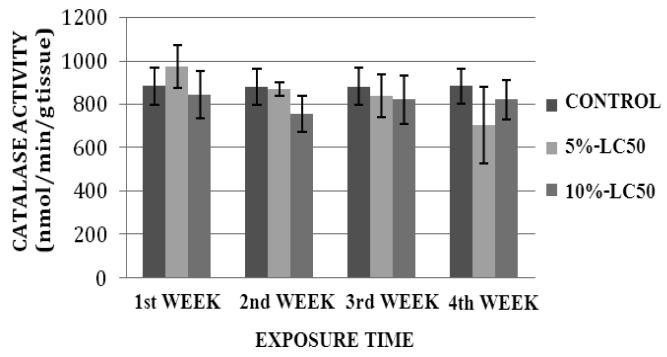
CAT activity levels in liver of goldfish after sub-chronic exposure to Cr (VI) for four weeks are summarized in Figure 1. In the 5% LC50 concentration (4.25 ppm), the CAT activity was decreased during the 1st, 2nd and 3rd week of exposure, and increased during the 4th week of exposure compared to the control. In the 10%-LC50 concentration (8.57 ppm), the CAT activity was increased during the 1st week and decreased during the 2nd, 3rd and 4th week compared to the controls. However, the increases or decreases in CAT activity were not significantly different (p>0.05) from the controls. CAT activity levels in kidney of goldfish after sub-chronic exposure to Cr (VI) for four weeks are summarized in Figure 2. The CAT activity levels were decreased in both 5%-LC50 and 10%-LC50 concentrations during the exposure period except for the first week in 5%-LC50. However, the decreases or increases in CAT activity were not significantly different (p>0.05) from the controls.
Fig.1.
Catalase (CAT) activity in Carassius auratus liver exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates.
Fig. 2.
Catalase (CAT) activity in Carassius auratus kidney exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates.
Superoxide Dismutase (SOD) Activity
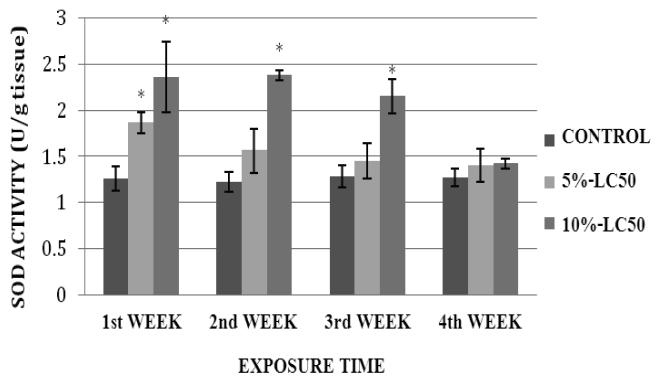
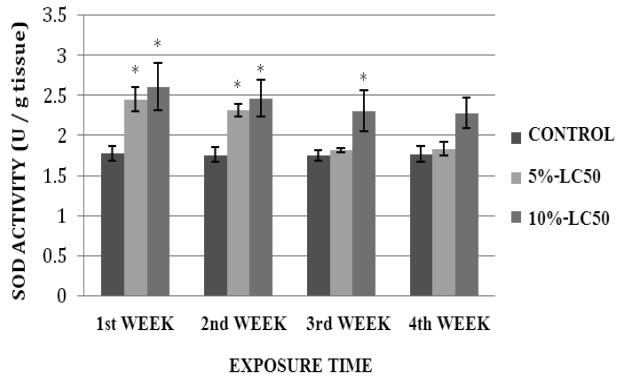
SOD activity in goldfish liver exposed to 5% and 10%-LC50 for four weeks is presented in Figure 3. Activity levels in goldfish liver exposed to 5%-LC50 were increased compared to controls throughout the exposure period; showing a significant difference during the 1st week of exposure, and no significant differences (p>0.05) at weeks 2, 3 and 4. In the 10%-LC50 concentration, SOD activity levels were increased; showing statistically significant differences (p<0.05) over the controls at weeks 1, 2 and 3, and no significant difference (p>0.05) at week 4. SOD activity levels in goldfish kidney after sub-chronic exposure to Cr (VI) are summarized in Figure 4. In the 5%-LC50 concentration, kidney SOD activity was increased. This increase was significantly different (p<0.05) from the control at weeks 1 and 2. In the 10%-LC50 concentration, there was also an increase in SOD activity; showing statistically significant differences (p<0.05) over the controls at weeks 1, 2 and 3.
Fig. 3.
Superoxide dismutase (SOD) activity in Carassius auratus liver exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
Fig. 4.
Superoxide dismutase (SOD) activity in Carassius auratus kidney exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
Glutathione Peroxidase (GPx) Activity
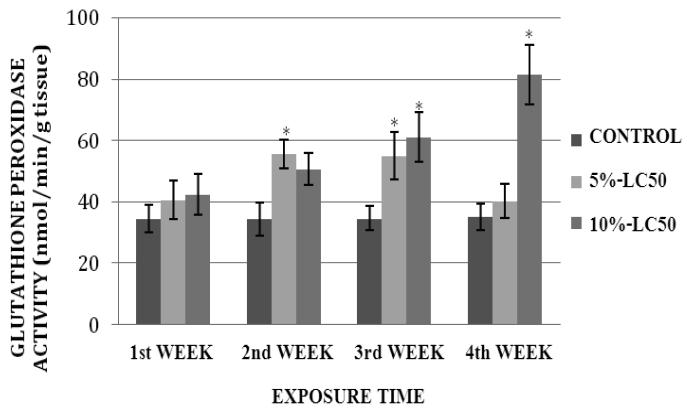
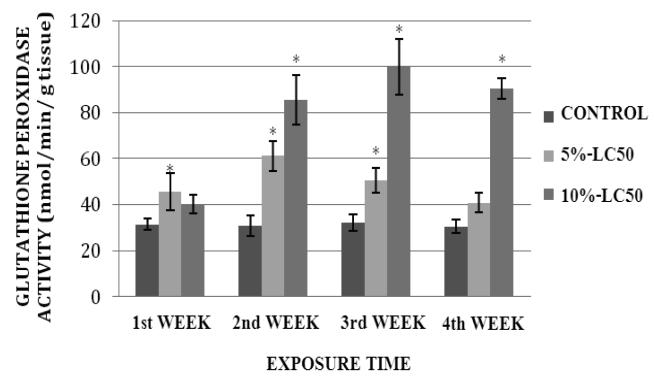
GPx activity levels in goldfish liver exposed to 5%- and 10%-LC50 concentrations are presented in Figure 5. As shown on this graph, GPx activity in goldfish liver was increased at exposure weeks 1, 2, 3 and 4. This increase was significantly different (p<0.05) from the control at weeks 2 and 3. In the 10%-LC50 concentration, the increase in GPx activity in goldfish liver was significantly different (p<0.05) from the control at weeks 3 and 4. GPx activity levels in goldfish kidney after sub-chronic exposure to Cr (VI) are shown in Figure 6. In the 5%-LC50 concentration, GPx activity in goldfish kidney was increased, and this increase was statistically significant (p<0.05) at weeks 1, 2 and 3. In the 10%-LC50 the increase in GPx activity in goldfish kidney was statistically significant (p<0.05) at weeks 2, 3 and 4 compared to the control.
Fig. 5.
Glutathione peroxidase (GPx) activity in Carassius auratus liver exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
Fig. 6.
Glutathione peroxidase (GPx) activity in Carassius auratus kidney exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
Metallothionein Expression
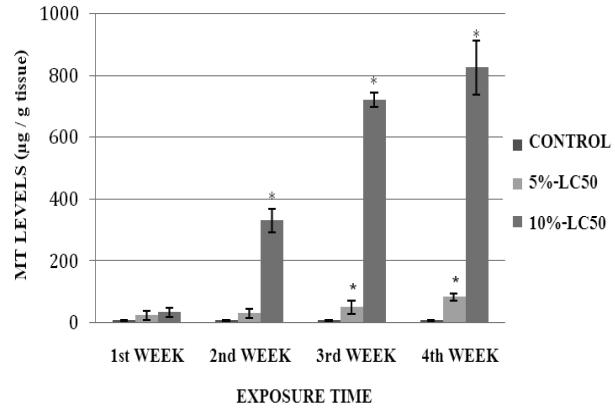
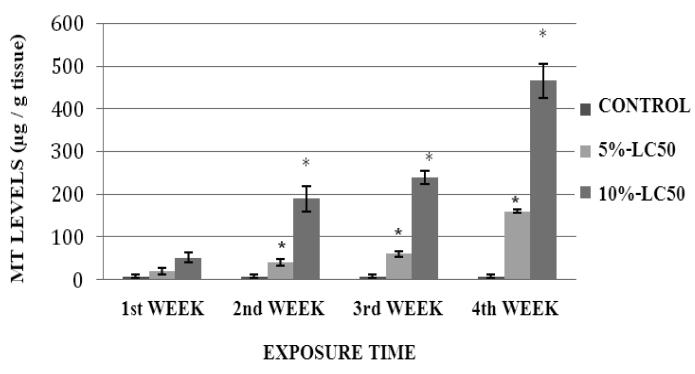
MT levels in goldfish liver exposed to Cr (VI) at 5%- and 10%-LC50 concentrations are presented in Figure 7. As shown in the figure, MT levels in goldfish liver were elevated during the exposure, in both 5% and 10%-LC50 concentrations. However, the increase over the control level was statistically significant (p<0.05) at weeks 3 and 4 in the 5%-LC50 concentration, and at weeks 2, 3 and 4 in the 10%-LC50 concentration. The MT levels in goldfish kidney exposed to 5% and 10%-LC50 Cr (VI) are presented in Figure 8. MT levels in kidney of exposed fish were elevated at all tested concentrations; showing statistically significant differences (p<0.05) over the controls at weeks 2, 3 and 4.
Fig. 7.
Metallothionein levels in Carassius auratus liver exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
Fig. 8.
Metallothionein levels in Carassius auratus kidney exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates. *Significantly different from the control according to Dunnett's multiple comparison test.
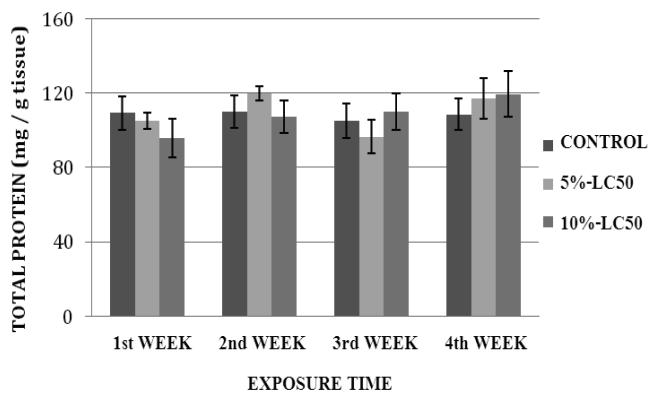
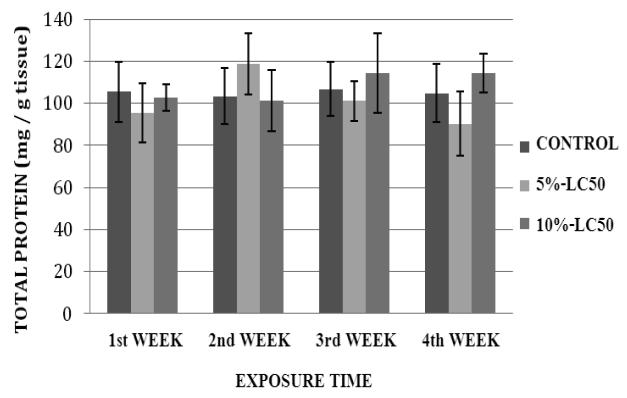
Total Protein Expression
Total protein levels in goldfish liver exposed to Cr (VI) at 5%- and 10%-LC50 concentrations are presented in Figure 9. The total protein levels in goldfish liver exposed to 5%-LC50 were decreased at weeks 1 and 3, and increased at weeks 2 and 4 compared to the controls. In the 10%-LC50, the total protein levels in goldfish liver were decreased at weeks 1 and 2, and increased at weeks 3 and 4 compared to the controls. However, the increases and decreases in total proteins were not significantly different (p>0.05) from the controls for both 5% and 10%-LC50 concentrations. Total protein levels in kidney of goldfish exposed to Cr (VI) at 5% and 10%-LC50 are presented in Figure 10. The protein levels in goldfish kidney exposed to 5%-LC50 were decreased at weeks 1, 3 and 4 and increased at week 2 compared to the controls. However, the decreases or increases in protein levels were not significantly different (p>0.05) from the controls. In the 10%-LC50 concentration, the total protein levels in goldfish kidney were decreased at weeks 1 and 2, and increased at weeks 3 and 4. However, the decreases or increases in protein levels were not significantly different (p>0.05) from the controls.
Fig. 9.
Total protein levels in Carassius auratus liver exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates.
Fig.10.
Total protein levels in Carassius auratus kidney exposed to Cr (VI) (5% and 10% of 96hr-LC50) for a four week time period. Each point represents a mean value ± SD of three replicates.
DISCUSSION
Catalase (CAT) is one of the antioxidant enzymes used in the present research to study oxidative stress in goldfish. CAT is one of the sensitive enzyme biomarkers and its activity is modulated by various factors including over production of superoxide radicals (Kono and Fridovich, 1982; Pandey et al., 2002), and carbonylation of proteins (Stadtman and Oliver, 1991). In the present study, our results indicated some alterations in CAT activity in liver and kidney of Cr (VI) exposed fish during experimentation. However, the increase or decrease in CAT activity was not significantly different from control. It has been reported that CAT is a sensitive antioxidant, and its protein function can be influenced by the products of lipid peroxidation, thereby modulating the enzymatic activity level (Stadtman and Levine, 2000; Bagnyukova et al., 2006). Hence, the lack of a significant up-regulation of CAT activity in the present study might be attributed to a number of factors that may include the test conditions (sub-chronic exposure) and test species (goldfish).
Superoxide dismutase (SOD) plays a significant role in cellular antioxidant defense mechanism. Modulation of the SOD activity is an early indicator of oxidative stress in biological systems. Our data indicated that SOD activity was elevated in liver and kidney of exposed fish at all tested concentrations. Similar findings have been previously reported from a study evaluating the enzymatic activity of SOD in gills and liver of rainbow trout fish exposed to ozone and oxygen-supersaturated water (Ritola et al., 2002). Several other studies have also demonstrated the potential of Cr (VI) to up-regulate SOD activity. In an investigation of rainbow trout exposed to sub lethal concentrations of Cr (VI) for 28 days, Roberts and Oris (2002) reported a significant increase in liver SOD activity. Similarly, a significant induction of SOD activity has been observed in liver fractions of Oreochromis niloticus (Tilapia) and Wistar rats administered with intra-peritoneal doses of Cr (VI) (Tagliari et al., 2004). The significant up-regulation of SOD activity observed in our study indicates the high potential of Cr (VI) to induce oxidative stress in goldfish.
Glutathione peroxidase (GPx) is one of the antioxidant-associated enzymes. It catalyzes the reduction of hydroperoxides (including hydrogen peroxides) by reducing glutathione. In our study, the GPx levels were significantly increased in Cr (VI)-treated fish, in a time-dependent manner. This result is in agreement with previous studies reporting that Cr (VI) is a strong inducer of GPx activity because of its high sensitivity to hydro-peroxides concentrations (Pandey et al., 2002; Ahmad et al., 2006). Geetha et al. (2002) studied the antioxidants and immunomodulatory mechanisms of seabuckthorn in response to Cr (VI) exposure, and reported an increased cytotoxicity, apoptosis, free radical production and decreased glutathione levels. Results from the present study also indicated that the induction of GPx was more pronounced in the kidney tissue; indicating that this organ may be more sensitive to Cr (VI) toxicity compared to the liver. Moreover, data from this study indicates that GPx appears to be a more sensitive biomarker of Cr (VI) – induced lipid peroxidation in goldfish compared to CAT.
Metallothionein (MT) is a family of cystein-rich, low molecular weight proteins that play an important role in neutralizing the toxicity of heavy metals and protect the cell from stress (Kägi and Schäffer, 1988). In the present study, MT levels were elevated in all the Cr (VI)-treated goldfish compared to the controls. This increase in MT expression was time-dependent. Our result is in agreement with previous reports indicating a concentration-dependent MT induction in rat liver and kidney cells exposed to Cr (VI) (Solis-Heredia et al., 1999). Another important study has also demonstrated the Cr (VI) potential to induce the MT mRNA levels in fish. Treatment of zebra fish liver and caudal fin cell-line models with Cr (VI) resulted in an induction of MT mRNA (Cheuk et al., 2008). Induction of MT mRNA in liver and kidney could be the result of direct activation of metal responsive elements of MT gene (Roberts and Oris, 2004).
Data obtained from the protein analysis experiment indicated some alterations in total protein levels in goldfish liver and kidney. However, these alterations were not statistically significant. Our findings are in agreement with a recent study that did not find any significant alterations in total protein levels in freshwater fish Oreochromis niloticus exposed for 30 days to various heavy metals including chromium (Oner et al., 2009). In another study, Vutukuru (2003) reported the acute toxicity of chromium to Labeo rohita following 24 h and 96 h exposure times. He pointed out a decrease of total proteins in gill, muscle and liver as a result of chromium exposure. It has also been reported that metallic stress may impair or decrease the rate of protein synthesis (Nanda and Behera, 1996). Moreover, total protein levels can be depleted because of the utilization of proteins for energy needs and in cell repair (Nagai and Ikeda, 1971).
CONCLUSION
Hexavalent chromium is a known human carcinogen that has also been reported to be highly toxic to aquatic organisms. However, its mechanisms of toxicity as well as its toxicokinetics and toxicodynamics have not been fully elucidated. Our research investigated its oxidative stress potential in both liver and kidney of goldfish exposed under subchronic conditions; using catalase (CAT), superoxide dismutase (SOD), glutathione peroxidate (GPx), metallothionein (MT), and total protein as specific biomarkers and toxicological end-points. Study results indicated a significant modulation SOD, GPx and MT activities in liver and kidney in response to Cr (VI) toxicity, and adaptability of goldfish to chromium-induced stress. The kidney appeared to be more sensitive to Cr (VI) toxicity than the liver. CAT activity modulation and total protein expression in Cr (VI)-exposed fish were not significantly different compared to controls.
Acknowledgments
This research was supported in by the National Institutes of Health RCMI Grant No. 2G12RR013459, and in part by the National Oceanic and Atmospheric Administration ECSC Grant No. NA06OAR4810164 & Subcontract No. 000953.
REFERENCES
- Ahmad I, Maria VL, Oliveria M, Pacheco M, Santos MA. Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to α-naphthoflavone. Mutational Research. 2006;608:16–28. doi: 10.1016/j.mrgentox.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Arillo A, Melodia F. Effect of hexavalent chromium on trout mitochondria. Toxicol Letters. 1988;44:71–76. doi: 10.1016/0378-4274(88)90131-2. [DOI] [PubMed] [Google Scholar]
- Arunkumar RI, Rajashekaran P, Michael RD. Differential effect of chromium compounds on the immune response of the African mouth breeder Oreochromis mossambicus (Peter) Fish & shellfish immunol. 2000;10:667–676. doi: 10.1006/fsim.2000.0281. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ. Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic Biol Med. 1997;22(3):471–478. doi: 10.1016/s0891-5849(96)00352-8. [DOI] [PubMed] [Google Scholar]
- Bagnyukova TV, Chahrak OI, Lushchak VI. Coordinated response of goldfish antioxidant defenses to environmental stress. Aquatic Toxicol. 2006;78:325–331. doi: 10.1016/j.aquatox.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheuk WK, Chan PC, Chan KM. Cytotoxicities and induction of metallothionein (MT) and metal regulatory element (MRE)-binding transcription factor-1 (MTF-1) messenger RNA levels in the zebrafish (Danio rerio) ZFL and SJD cell lines after exposure to various metal ions. Aquatic Toxicol. 2008;89(2):103–112. doi: 10.1016/j.aquatox.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Elwood JW, Beuchamp JJ, Allen CP. Chromium levels in fish from a lake chronically contaminated with chromate from cooling towers. Int J Environ Stud. 1980;14:289–298. [Google Scholar]
- Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides): an in vitro study. J of Ethnopharmacol. 2002;79:373–378. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007a;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th edition Clarendon Press; Oxford: 2007b. [Google Scholar]
- Hattingh WHJ. Reclaimed water: a health hazard. Vol. 3. Water SA: 1977. pp. 104–112. [Google Scholar]
- Hneihen AS, Standeven M, Wetterhahn EK. Differential binding of chromium (VI) and chromium (III) complexes to salmon sperm nuclei and nuclear DNA and isolated calf thymus DNA. Carcinogenesis. 1993;14:1795–1803. doi: 10.1093/carcin/14.9.1795. [DOI] [PubMed] [Google Scholar]
- Johansson LH, Borg LAH. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988;174:331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- Kägi JHR, Schäffer A. Biochemistry of metallothionein. Biochem. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Kono Y, Fridovich I. Superoxide radicals inhibit catalase. J Biol Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- Krumschnabel G, Nawaz M. Acute toxicity of hexavalent chromium in isolated teleost hepatocytes. Aquatic Toxicol. 2004;70:159–167. doi: 10.1016/j.aquatox.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Lopes PA, Pinheiro T, Santos MC, Mathias ML, Collares-Pereira MJ, Viegas-Crespo AM. Response of antioxidant enzymes in freshwater fish populations (Leuciscus alburnoides complex) to inorganic pollutants exposure. The Science of the Total Environ. 2001;280(1-3):153–163. doi: 10.1016/s0048-9697(01)00822-1. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Nagai M, Ikeda S. Carbohydrate metabolism in fish. I. Effects of starvation and dietary composition on the blood glucose level and the hepatopancreatic glycogen and lipid contents in carp. Bull Jap Soc Sci Fish. 1971;37:404–409. [Google Scholar]
- Nanda P, Behera MK. Nickel induced changes in some hemato-biochemical parameters of a catfish, Heteropneustes fossilis (Bloch.) Environ Ecol. 1996;14:82–85. [Google Scholar]
- Oner M, Atli G, Canli M. Effects of metal (Ag, Cd, Cr, Cu, Zn) exposures on some enzymatic and non-enzymatic indicators in the liver of Oreochromis niloticus. Bull Environ Contam Toxicol. 2009;82:317–321. doi: 10.1007/s00128-008-9577-4. [DOI] [PubMed] [Google Scholar]
- Paglia E, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Cm Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Palmer CD, Wittbrodt PR. Processes affecting the remediation of chromium-contaminated sites. Environ Health Prospectives. 1991;92:25–40. doi: 10.1289/ehp.919225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Ahmad I, Pervez S, Hafeez B, Haque R, Raisuddin S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch. 1. Protection against lipid peroxidation in liver by copper pre-exposure. Arch Environ Contam Toxicol. 2001;41:345–352. doi: 10.1007/s002440010258. [DOI] [PubMed] [Google Scholar]
- Ritola O, Livingstone DR, Peters LD, Lindstrom-Seppa P. Antioxidant processes are affected in juvenile rainbow trout (Oncorhynchus mykiss) exposed to ozone and oxygen-supersaturated water. Aquaculture. 2002;210:1–19. [Google Scholar]
- Roberts AP, Oris JT. Multiple biomarker response in rainbow trout during exposure to hexavalent chromium. Comparative Biochem and Physiol Part C. 2004;138:221–228. doi: 10.1016/j.cca.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Solis-Heredia MJ, Quintanilla-Vega B, Sierra-Santoyo A, Hernández JM, Brambila E, Cebrián ME, Albores A. Chromium increases pancreatic metallothionein in the rat. Toxicol. 1999;142(2):111–117. doi: 10.1016/s0300-483x(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Sridevi B, Reddy KV, Reddy SL. Effect of trivalent and hexavalent chromium on antioxidant enzyme activities and lipid peroxidation in a freshwater field crab, Barytelphusa guerini. Bull Environ Contam Toxicol. 1998;61(3):384–390. doi: 10.1007/s001289900774. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Protein oxidation. Annals of the NY Academy of Science. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Oliver CN. Metal-catalyzed oxidation of protein. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Tagliari KC, Vargas VF, Zimiani K, Cecchini R. Oxidative stress damage in the liver of fish and rats receiving an intraperitoneal injection of hexavalent chromium as evaluated by chemiluminescence. Environ Toxicol and Pharmacol. 2004;17(3):149–157. doi: 10.1016/j.etap.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thaker K, chhaya J, Nuzhat S, Mittal R, Mansuri AP, Kundu R. Effects of chromium (6+) on some ion-dependent ATPases in gills, kidney and intestine of a coastal teleost Periophthalmus dipes. Toxicol. 1996;112:237–244. doi: 10.1016/0300-483x(96)86481-x. [DOI] [PubMed] [Google Scholar]
- Torrey S. Trace contaminants from coal. Noyes Data Corporation; New Jersey: 1978. Ecological effects; pp. 143–208. [Google Scholar]
- Van Pittius MG, Van Vuren JHJ, du Preez HH. Effects of chromium during pH change on blood coagulation in Tilapia sparrmanii. Comp Biochem Physiol. 1992;101c(2):371–374. doi: 10.1016/0742-8413(92)90289-j. [DOI] [PubMed] [Google Scholar]
- Velma V, Tchounwou PB. Acute toxicity of sodium dichromate to gold fish, Carassius auratus. Metal Ions Biol Med. 2008;10:642–646. [Google Scholar]
- Viarengo A, Ponzano E, Dondero F, Fabbri RA. Simple spectrophotometric method for metallothionein evaluation in marine organism: an application to mediterranean and antarctic molluscs. Mar Environ Res. 1997;44:68–84. [Google Scholar]
- Vutukuru SS. Chromium induced alterations in some biochemical profiles of the Indian major carp, Labeo rohita (Hamilton) Bull Environ Contamn Toxicol. 2003;70:118–123. doi: 10.1007/s00128-002-0164-9. [DOI] [PubMed] [Google Scholar]
- Wilbur S, Voytek P. Centers for Disease Control and Prevention. Atlanta, Georgia, USA: 1988. Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry; p. 376. [Google Scholar]