SUMMARY
Current active immunotherapy trials have shown durable tumor regressions in a fraction of patients. However, clinical efficacy of current vaccines is limited, possibly because tumors skew the immune system by means of myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs), all of which prevent the generation of effector cells. To improve the clinical efficacy of cancer vaccines in patients with metastatic disease, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of the immunosuppressive tumor microenvironment. This can be achieved by exploiting the fast increasing knowledge about the dendritic cell (DC) system, including the existence of distinct DC subsets. Critical to the design of better vaccines is the concept of distinct DC subsets and distinct DC activation pathways, all contributing to the generation of unique adaptive immune responses. Such novel DC vaccines will be used as monotherapy in patients with resected disease and in combination with antibodies and/or drugs targeting suppressor pathways and modulation of the tumor environment in patients with metastatic disease.
Keywords: dendritic cells, cancer, vaccines, priming
1. INTRODUCTION
Vaccines against infectious agents demonstrate the power of manipulating the immune system. Vaccines have spared countless numbers of people from polio, measles, tetanus etc (Nossal 1997), even though they have not been designed according to immunological principles (Doherty et al. 2006). Immunology has the potential to identify vaccines, i.e., antigen-specific, durable, non-noxious preventions and therapies for infections, cancer, allergy, autoimmunity, transplantation. This has formed a conceptual basis for the development of therapeutic vaccines in cancer. Molecular identification of human cancer antigens has ushered in a new era of antigen specific cancer immunotherapy specifically targeting these antigens. Initial attempts (e.g. peptides, DNA vaccines, viral vectors and first generation DC-based vaccines) have thus far met with a limited success in the clinic. However, cancer vaccines are in a renaissance era due to recent clinical trials showing promising immunological data and some clinical benefit to the patients. For example, an active immunotherapy product, sipuleucel-T (APC8015) based on antigen-loaded and GM-CSF activated PBMCs, appears to contribute to prolonged median survival in phase III trials in patients with prostate cancer (Higano et al. 2009). Similarly, a randomized phase II trial of a poxviral-based vaccine targeting PSA (PROSTVAC) in men with metastatic castration-resistant prostate cancer showed improved overall survival in patients who received PROSTVAC compared to patients receiving control vectors (Kantoff et al. 2010). While these first generation positive randomized phase II/III clinical trials need further analysis and mechanistic studies, they underline the therapeutic potential of the immune system that can be tapped into. Vaccines act through DCs which induce, regulate and maintain T cell immunity. Here we summarize our recent studies aimed at a better understanding of the DC system to unravel the pathophysiology of cancer and to design novel cancer vaccines.
2. DENDRITIC CELLS
Generating the right type of immune response can be a matter of life and death. In leprosy, for instance, the tuberculoid form of the disease is characterized by a Type 1 response which keeps the disease in check, while the lepromatous form induces an often fatal Type 2 response (Yamamura et al. 1991). These responses are under the control of DCs (Banchereau and Steinman 1998; Steinman and Banchereau 2007). DCs reside in peripheral tissues and in lymph nodes where they are poised to capture antigens (Ags). DCs present processed protein and lipid Ags to T cells via both classical (MHC class I and class II) and non-classical (CD1 family) antigen presenting molecules (Heath and Carbone 2009) (Figure 1). In the steady state, non-activated (immature) DCs present self-antigens to T cells, which leads to tolerance (Hawiger et al. 2001; Steinman et al. 2003). DCs induce immune tolerance in a number of ways including i) T cell deletion (Fairchild and Austyn 1990; Zal et al. 1994; Volkmann et al. 1997); ii) induction of T cell unresponsiveness (Hawiger et al. 2004); and iii) activation of regulatory T cells (Tregs) (Jonuleit et al. 2000; Akbari et al. 2001; Wing and Sakaguchi 2010; Zheng et al. 2010). Once activated (mature), antigen-loaded DCs are geared towards the launching of antigen-specific immunity (Finkelman et al. 1996; Brimnes et al. 2003) leading to the T cell proliferation and differentiation into helper and effector cells. DCs are also important in launching humoral immunity partly due to their capacity to directly interact with B cells (Jego et al. 2005; Qi et al. 2006) and to present unprocessed antigens (Zhong et al. 1997; Wykes et al. 1998; Bergtold et al. 2005; Batista and Harwood 2009).
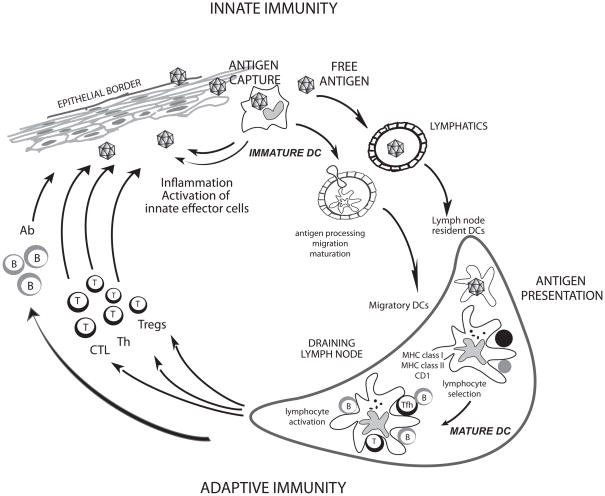
Figure 1. Dendritic cells.
DCs reside in the tissue where they are poised to capture antigens (Geissmann et al. 2010). During inflammation, circulating precursor DC enter tissues as immature DC (Geissmann et al. 2010). DCs can encounter pathogens (e.g.: viruses) directly, which induce secretion of cytokines (e.g.: IFN-α); or indirectly through the pathogen effect on stromal cells. Cytokines secreted by DCs in turn activate effector cells of innate immunity such as eosinophils, macrophages and NK cells. Microbe activation triggers DCs migration towards secondary lymphoid organs and simultaneous activation (maturation). These activated migratory DCs that enter lymphoid organs display antigens in the context of classical MHC class I and class II or non-classical CD1 molecules, which allow selection of rare circulating antigen-specific T lymphocytes. Activated T cells help drive DCs toward their terminal maturation, which allows lymphocyte expansion and differentiation. Activated T lymphocytes traverse inflamed epithelia and reach the injured tissue, where they eliminate microbes and/or microbe-infected cells. B cells, activated by DCs and T cells, migrate into various areas where they mature into plasma cells that produce antibodies that neutralize the initial pathogen. Antigen can also reach draining lymph nodes without involvement of peripheral tissue DCs and be captured and presented by lymph node resident DCs (Itano et al. 2003).
2.1 Human Dendritic Cell Subsets
To allow resistance to infection and tolerance to self, DCs are endowed with two critical features: subsets and functional plasticity (Steinman and Banchereau 2007). The two major subsets are the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs). The best studied human mDC subsets are those from skin, where three subsets can be identified. The epidermis hosts only Langerhans Cells (LCs) while the dermis displays two mDC subsets, CD1a+ DCs and CD14+ DCs, as well as macrophages (Zaba et al. 2007; Klechevsky et al. 2008; Merad et al. 2008; Nestle et al. 2009).
2.1.1 Dermal DCs, antibody responses and IL-12
In the mid 90, we observed that CD14+ DCs derived from CD34+ hematopoietic progenitor cells (HPCs) induce CD40-activated naïve B cells to differentiate into IgM-producing plasma cells through the secretion of IL-6 and IL-12 (Caux et al. 1997). A decade later, we found that CD14+ DCs, but not LCs, induce naïve CD4+ T cells to differentiate into cells with properties of T follicular helper cells (Tfh) (Klechevsky et al. 2008), a CD4+ T cell subset specialized in B cell help (King et al. 2008; Fazilleau et al. 2009). There, CD4+ T cells primed by CD14+ DCs help naïve B cells to produce large amounts of IgM, and switch isotypes towards IgG and IgA. Our recent studies in human indicate that acquisition of Tfh phenotype and function depends on IL-12p70 (Schmitt et al. 2009).
Thus, IL-12 appears to contribute to humoral immunity in humans through a direct path in DC-B interaction, and an indirect path in DC-T cell interaction and induction of Tfh cells. These findings might explain the modest clinical efficacy of systemic IL-12 administration in cancer patients (Motzer et al. 2001; Cheever 2008). Furthermore, the injection of IL-12 into tumor sites of head and neck cancer patients resulted in the activation of B cells in the draining lymph nodes, which was associated with their infiltration into tumor sites and tumor regression (van Herpen et al. 2008).
2.1.2 LCs and CD8+ T cell responses
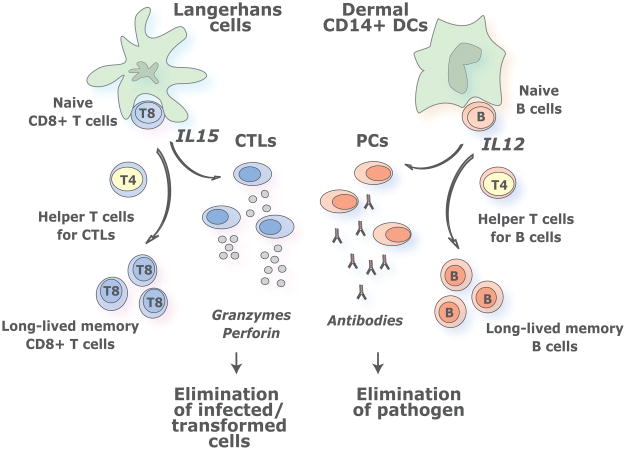
LCs induce a robust proliferation of naïve allogeneic CD8+ T cells when compared to CD14+ DCs (Klechevsky et al. 2008). Furthermore, when pulsed with MHC class I peptides derived from tumor or viral antigens, LCs are far more efficient than CD14+ DCs in the priming of antigen-specific CD8+ T cells. LCs are also efficient in cross-presenting peptides from protein antigens to CD8+ T cells. CD8+ T cells primed by LCs show high avidity in tetramer binding assays and express higher levels of cytotoxic molecules, such as granzymes and perforin. Accordingly, they are remarkably more efficient in killing target cells; in particular tumor cells that express low level of peptide/HLA complexes (Klechevsky et al. 2008). IL-15 might explain the remarkable effects of LCs on the development of Cytotoxic T Lymphocyte (CTL) responses (Mohamadzadeh et al. 2001; Dubsky et al. 2007; Klechevsky et al. 2009). Thus, the two different arms of adaptive immunity, i.e., humoral and cellular arms, might be differentially regulated by the two skin mDC subsets (Figure 2). Such framework might be of capital importance for the understanding of the immune alteration in malignancy and for development of novel and improved vaccination strategies against cancer, as well as chronic infections.
Figure 2.
DC s as tools for vaccination. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient cancer vaccines might be those that will target LCs thereby allowing the maximal stimulation of cellular immune responses and the generation of long-term memory protection.
2.1.3 Plasmacytoid DCs
Plasmacytoid DCs (pDCs) are considered as the front line in anti-viral immunity owning to their capacity to rapidly produce high amounts of type I interferon (Siegal et al. 1999; Liu 2005). Similar to mDCs, pDCs display a remarkable functional plasticity. Thus, pDCs exposed to viruses, such as live influenza virus, are able to launch memory responses by inducing the expansion and differentiation of antigen-specific memory B and T lymphocytes into plasma cells (Jego et al. 2003), and CTLs (Fonteneau et al. 2003; Di Pucchio et al. 2008), respectively. On the contrary, pDCs activated with CpG or IL-3/CD40L induce in vitro IL-10-secreting regulatory CD4+ T cells (Ito et al. 2007) as well as suppressor CD8+ T cells through the expression of ICOS ligand (Gilliet and Liu 2002).
Human pDCs, in fact, are composed of two subsets, distinguished by the expression of CD2 (Matsui et al. 2009). CD2high pDCs are more potent than the CD2low pDCs to induce allogeneic T cell proliferation. These different functional properties of CD2high pDCs and CD2low pDCs are associated to distinct transcription profiles, differential secretion of IL12 p40 and differential expression of co-stimulatory molecule CD80 on activation. Additional studies will be necessary to understand the biological role of these two pDC subsets.
2.2 DCs in tumor environment
Numerous studies in humans have concluded that DCs can infiltrate tumors. We found that breast cancer tumor beds are infiltrated with immature DCs. In contrast, mature DCs are found in the peri-tumoral areas in ~60% of cases (Bell et al. 1999). A number of studies have suggested that DCs can contribute to tumor development. Our studies in breast cancer indicate that tumor cells polarize mDCs into a state that drives the differentiation of naïve CD4+ T cells into IL-13-secreting T cells (Aspord et al. 2007). These Type 2 T cells in turn facilitate breast tumor development in xenograft model as it can be partly inhibited by administration of IL-13 antagonists (Figure 3). The role of Th2 cells was further established in a spontaneous mouse breast cancer model, where Th2 cells facilitate the development of lung metastasis through macrophage activation (DeNardo et al. 2009). In several other mouse tumor models, IL-13 produced by NKT cells induces myeloid cells to make TGF-β that inhibits CTL functions (Berzofsky and Terabe 2008). Thus, type 2 cytokines are involved in tumorigenesis through various mechanisms. mDCs can also have direct interactions with tumor cells as shown in multiple myeloma where they directly promote the survival and clonogenicity of tumor cells (Kukreja et al. 2006; Bahlis et al. 2007).
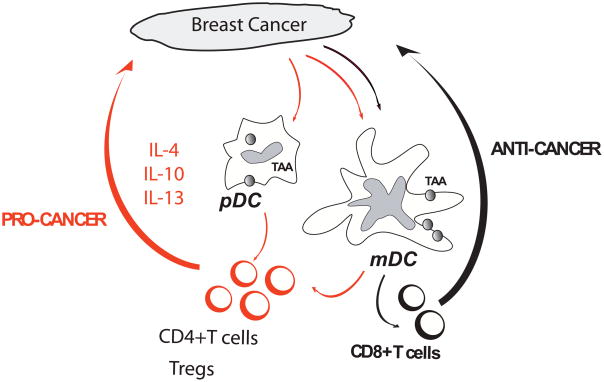
Figure 3.
DC s as targets for therapy. Cancer cells attract immature DC possibly through chemokines such as MIP3 alpha and/or SDF-1. The DC can then be either blocked or skewed in their maturation, for example by VEGF, leading to induction of polarized CD4+T cells that promote the expansion of cancer cells (pro-cancer) at the expense of CD8+T cells that can cause tumor regression (anti-cancer). An interesting strategy would be to rewire their molecular pathways from “pro-cancer” DCs into “anti-cancer” DCs for example with antibodies or DC activators.
pDCs have been found in approximately 10% of breast carcinomas and are associated with poor prognosis (Treilleux et al. 2004). The infiltrating pDCs produce little type I IFN upon TLR ligation (Hartmann et al. 2003). This inhibition appears to depend on the ligation of ILT7 on pDCs binding by BST2 expressed on tumor cells (Cao et al. 2009). Likewise, in ovarian carcinoma, tumor-infiltrating pDCs do not induce effector CD8+ T cell responses, but rather promote the differentiation of IL10+ CCR7+ CD8+ Tregs (Wei et al. 2005). Finally, pDCs may promote tumor angiogenesis by the secretion of proangiogenic cytokines (Curiel et al. 2004; Coukos et al. 2005).
DC can fight back tumors at least through two pathways: an indirect one with the induction of potent CTL responses, and a direct one through DC-dependent tumor cytotoxicity. For example, pDCs appear to directly contribute to the anti-tumor activity of in vivo-administered Imiquimod (TLR7 ligand), which is used for the treatment of basal cell carcinoma (Urosevic et al. 2005; Panelli et al. 2007; Stary et al. 2007).
Clearly, understanding the functions of DCs in the tumor bed represents an important area of future investigations and exploitation for therapy. An interesting strategy would be to rewire their molecular pathways from “pro-tumor” DCs into “anti-tumor” DCs.
3. DENDRITIC CELLS IN VACCINATION AGAINST CANCER
3.1 Outcomes of current DC vaccination trials
Ex vivo-generated DCs have been used as therapeutic vaccines in patients with metastatic cancer for over a decade and early studies have been discussed in detail elsewhere (Palucka et al. 2007). While a fraction of patients can experience durable tumor regressions (Palucka et al. 2006), the most common outcome of the current DC vaccination protocols is a demonstration of expanded antigen-specific immunity, most often using IFN-γ ELISPOT, but no durable objective tumor regression.
Altogether, three outcomes emerge from our studies:
1) No immune response. Patients of this group usually progress quickly. These patients mount immune responses to control antigens such as KLH or viral peptides (Flu-M1 or CMV). In vitro experiments indicated that T cells of several patients can be primed to differentiate into CTLs with specificity for multiple melanoma antigens (Berard et al. 2000). Thus, tumor antigen-specific CD8+ T cells are kept anergic rather than deleted. This inability to mount immune responses to tumor antigens in vivo might be at least partly related to the presence of tumor antigen-specific Tregs (Vence et al. 2007; Andrews et al. 2008). Tregs limit the onset of protective immunity through several mechanisms, for example by eliminating DCs in lymph nodes (Boissonnas et al. 2010). As discussed later, the control of Tregs becomes a key target to address fir the coming vaccination trials. 2) Immune response without clinical response. The most common outcome of current DC vaccination protocols is the induction of immune responses in the absence of clinical responses. This might in part be explained by the quality of the elicited T cells including their capacity to migrate into tumors and penetrate tumor stroma (Gajewski 2007). Improved immunomonitoring is expected to provide insights into the mechanisms of immune efficacy as discussed hereunder (Butterfield et al. 2008; Tahara et al. 2009). 3) Immune response and clinical response. Vaccination with DCs can elicit therapeutic immunity. These patients represent a formidable opportunity for the development of cancer immunotherapy. The challenge is two-fold. First, to establish the immunological mechanism that allowed tumor eradication. Second, we need to find ways to increase the fraction of patients experiencing durable tumor regression and/or prolonged survival.
3.2 The quality of elicited antigen-specific immune responses
Establishing causative links in clinical studies is a difficult task which often requires large patient cohorts. The current data suggest an association between the tumor-specific CD8+ T cell responses and clinical outcomes. In our view, four critical components will determine whether the induced immune response will be therapeutic: 1) the quality of elicited CTLs; 2) the quality of induced CD4+ helper T cells; 3) the elimination and/or non-activation of Tregs; and 4) the breakdown of immunosuppressive tumor microenvironment.
Indeed, the immune responses elicited by the first generation DC vaccines might not be of the quality required to allow the rejection of bulky tumors. For example, the induced T cells might not migrate into the tumor lesions (Appay et al. 2008; Harlin et al. 2009). Furthermore, low avidity T cells might be unable to recognize peptide-MHC class I complexes on tumor cells and/or to kill them (Appay et al. 2008). Finally, the tumor micro-environment might inhibit effector T cell functions, for example by action of myeloid derived suppressor cells and Tregs as summarized in recent reviews, respectively (Gabrilovich and Nagaraj 2009; Menetrier-Caux et al. 2009).
The recent progresses in immunomonitoring of specific immune responses in the blood and at the tumor site should help us address these questions (Palucka et al. 2006; Vence et al. 2007; Butterfield et al. 2008; Janetzki et al. 2009; Tahara et al. 2009). Modern approaches including polychromatic flow cytometry rather than the analysis of a single cytokine (e.g., IFN-γ ELISPOT) and/or frequency of tetramer positive cells will contribute to a better assessment of the quality of the immune responses elicited in the patients (Kammula et al. 1999; Lee et al. 1999). Indeed, several studies, mostly performed in the context of HIV vaccines, have led to the conclusion that a mere measurement of the frequency of IFN-γ secreting CD8+ T cells is insufficient to evaluate the quality of vaccine-elicited immunity (Wille-Reece et al. 2006; Appay et al. 2008; Seder et al. 2008).
4. BUILDING ON DENDRITIC CELL SUBSETS TO IMPROVE CANCER VACCINES
4. 1 Optimal DCs
The results summarized above prompted us to hypothesize that DCs with the properties of LCs might prove to be the best ones for the generation of strong cellular immunity (Figure 2). In line with this, the combination of cytokines used to differentiate monocytes into DCs play a critical role in determining the quality of the elicited T cell responses. For example, DCs generated with GM-CSF and IL-15 display the phenotype and characteristics of LCs. In particular, they are more efficient in priming melanoma-antigen specific CD8+ T cells in vitro than DCs derived with GM-CSF and IL-4 (Mohamadzadeh et al. 2001; Dubsky et al. 2007). Thus, vaccination with IL15-DCs might elicit stronger CD8+ T cell responses that might lead to improved clinical responses. We are currently initiating such a clinical trial in patients with malignant melanoma. The selected method for activating DCs also represents a critical parameter is the DC activation pathway. First, immature (non-activated) DCs induce antigen specific IL-10 producing T cells (Dhodapkar et al. 2001; Dhodapkar and Steinman 2002). Second, IL-4 DCs activated with a cocktail of IFN-α, polyI:C, IL-1β, TNF, and IFN-γ induce up to 40 times more melanoma-specific CTLs in vitro than DCs matured with the “standard” cocktail of IL-1β/TNF/IL-6/prostaglandin E2 (PGE2)(Mailliard et al. 2004; Fujita et al. 2009; Giermasz et al. 2009). Additional studies will be necessary to establish the therapeutic value of these newer generation DC vaccines in patients. These studies are critical to the understanding of the human immune system because they permit us to assess in vivo the type of immune responses elicited by human DCs generated in different cytokine environments.
This in turn is essential for building a novel approach to vaccination that is based on the delivery of antigens directly to DCs in vivo using chimeric proteins that are made of an anti-DC receptor antibody molecularly fused to a selected antigen (DC targeting). Studies in mice demonstrate that the specific targeting of antigen to DCs in vivo results in considerable potentiation of antigen-specific CD4+ and CD8+ T cell immunity if the DC maturation signal is provided (Hawiger et al. 2001; Bonifaz et al. 2002; Bonifaz et al. 2004). Otherwise, tolerance ensues (Hawiger et al. 2001). Thus, selection of appropriate adjuvant is also a critical parameter for the induction of the immunity of the desired type. Although TLR-ligands are widely considered to promote protective immunity against infectious agents, selecting the appropriate ligand will be critical. For instance, TLR2 ligation, which promotes the induction of Tregs rather than Th1 or Th17 cells (Manicassamy et al. 2009), does not appear to be a preferred option for cancer vaccines.
These pioneering studies have been already extended to demonstrate the targeting of tumor antigens to DCs (Caminschi et al. 2009) and Langerhans cells (LCs) in animal models (Flacher et al. 2008; Flacher et al. 2009) and the generation of anti-tumor immunity (Wei et al. 2009). The therapeutic success of these vaccines will build on the recent knowledge and progress in our understanding of the biology of human DC subsets, cutaneous myeloid DCs (mDCs) in particular.
4. 2 “Ideal” antigens
Assuming that appropriate solutions are identified to reverse immunosuppression, there is a need for an “ideal” set of target antigens. An “ideal” antigen is one which is necessary for cancer cells to survive and/or for which strong immunity able to reject the tumor and prevent its growth can be elicited.
Candidate tumor antigens include: i) unique (mutated) antigens; and ii) shared self-antigens including cancer/testis antigens and tissue differentiation antigens (Gilboa 1999; Vlad et al. 2004; Boon et al. 2006; Parmiani et al. 2007). The choice between these types of antigens for vaccination could be viewed as a choice between inducing immunity (mutated antigens) or breaking tolerance and inducing autoimmunity (self antigens). The debate about which type of antigen will be more efficient is still open. Mutated antigens are postulated to present several advantages, for example their specific T cell repertoire should not be deleted as they are not recognized as “self” by immune cells (Parmiani et al. 2007). Shared antigens are attractive as they might allow us to establish “generic” vaccines, however the enthusiasm for these antigens might be dampened because of their: i) relatively weak immunogenicity due to the negative selection of high affinity auto-reactive cells; and ii) the existence of antigen specific T regs (Hoos et al. 2007).
Perhaps the most compelling evidence of active in vivo tumor antigen-specific immune responses arises from the study of paraneoplastic neurologic disorders (PNDs) that led to the discovery of onconeural antigens (Darnell 1996). PNDs develop as remote effects of systemic malignancies. The discovery of onconeural antibodies led to the proposal that paraneoplastic cerebellar degeneration (PCD), associated to breast and ovarian cancer, is an autoimmune disorder mediated by the humoral arm of the immune system. These antibodies permitted the cloning of the cdr2 antigen, a protein with a coil/leucine zipper domain. It has now been shown that the disease is due to the development of cdr-2 specific CD8+ CTL (Albert et al. 1998). The list of onconeural antigens is growing and, besides cdr2, two other antigens such as Nova and amphiphysin appear as potential targets of the immune system (Floyd et al. 1998; Rosin et al. 1998).
An important shift in the selection of antigen targets might be brought about by the identification of cancer stem cells (Jordan et al. 2006; Polyak and Hahn 2006; Rossi et al. 2008). While a majority of studies have focused on eliminating mature cancer cells with limited proliferation capacity, it seems more efficient to target the self-renewing cancer stem cells. The importance of stem cell associated antigens in malignancy can be best illustrated by the presence of SOX-2-specific immunity in patients with monoclonal gammopathy (Spisek et al. 2007). This immunity is lost in patients who developed multiple myeloma suggesting differential antigenic targets at pre-malignant and malignant stages. In fact, the major factor from the immunization point of view is the linkage between expression of genes associated with pluripotency and those expressed in cancer. Ideal target genes would be those shared between cancer cells and embryonal cells, which are necessary for cancer cell survival but not expressed in adult stem cells (Dhodapkar 2010).
Thus far, all antigenic targets are protein antigens whose peptides can be presented on the cell surface in the form of complexes with classical MHC molecules (Townsend et al. 1985). However, tumors express altered lipids and sugars that can be bound by CD1 molecules on APCs and presented to NKT cells as well as T cells (Beckman et al. 1994; Fujii et al. 2002; Hava et al. 2005). These lipid antigens might possibly be harnessed for improved vaccination.
4. 3 Combining DC vaccines with other therapies
In view of the remarkable diversity of regulatory/suppressive pathways present in patients with metastatic cancer, any durable clinical response elicited by vaccination with DCs is already a remarkable achievement. However, to improve the outcomes in metastatic disease, DC vaccines need to be combined with other therapies that offset the suppressive environment created by the tumor (Dougan and Dranoff 2009). Such combination regimens will involve several drugs that target different pathways (Figure 4).
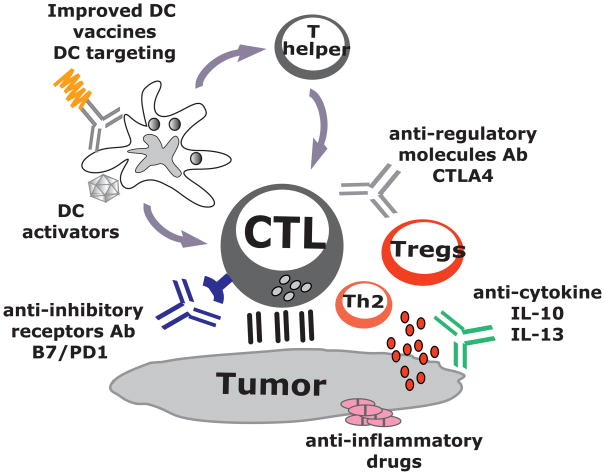
Figure 4.
DC vaccines in combination therapies. Current active immunotherapy trials have shown durable tumor regressions in a fraction of patients. However, clinical efficacy of current approaches is limited, possibly because tumors invade the immune system by means of myeloid-derived suppressor cells, inflammatory type 2 T cells and regulatory T cells (Tregs). To improve the clinical efficacy of immunotherapies, we need to design novel and improved strategies that can boost adaptive immunity to cancer, help overcome Tregs and allow the breakdown of an immunosuppressive tumor microenvironment. This can be achieved by developing combination therapies targeting these three major components.
In particular antibodies, or other soluble antagonists such as engineered receptors, can be exploited for the blockade of suppressive cytokines in the tumor microenvironment such as IL-10 (Moore et al. 2001), IL-13 (Terabe et al. 2000), TGF-β (Li et al. 2005; Terabe et al. 2009) and VEGF (Gabrilovich 2004; Rabinovich et al. 2007). They can also be used to block inhibitory ligand:receptor interactions (Melero et al. 2007) by acting on antigen presenting cells such as tumor or DCs (for example anti-PD-L1) or on lymphocytes as illustrated by anti-CTLA-4 (Peggs et al. 2006; Peggs et al. 2009) and/or anti-PD1 (Day et al. 2006; Curran et al. 2010; Pilon-Thomas et al. 2010). In contrast, agonistic antibodies (Gabrilovich 2004; Rabinovich et al. 2007) might further promote co-stimulation of effector T cells as for example with anti-CD137 (Watts 2005), a ligand for 4-1BB (Maus et al. 2002). Just as different tumors are treated with different combinations of cytostatic drugs and targeted therapies, we foresee development of clinical protocols combining DC vaccines with individualized adjunct therapies.
CONCLUDING REMARKS
The considerable progresses made in the knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of vastly improved clinical protocols. These, optimized vaccines eliciting strong and long-lived antigens-specific CD8+ T cell immunity will be offered to patients with early stage disease. For patients with late stage disease strategies that combine novel highly immunogenic vaccines and immunomodulatory antibodies will have high impact on enhancing therapeutic immunity in cancer by simultaneously increasing the potency of beneficial immune arms and offsetting immunoregulatory pathways (Figure 4). These optimized therapeutic strategies will be tailored to the patient and to the specific suppressive pathways that the patient displays (Figure 5).
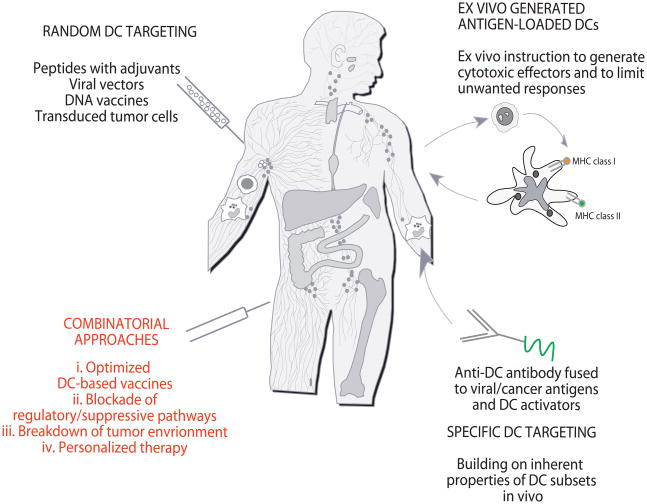
Figure 5. Approaches to DC-based immune intervention in cancer.
1) Vaccines based on antigen with or without adjuvant that target DCs randomly. That might result in vaccine antigens being taken up by a “wrong” type of DCs in the periphery which might lead to “unwanted” type of immune response. Vaccine antigens could also flow to draining lymph nodes where they can be captured by resident DCs; 2) Vaccines based on ex-vivo generated tumor antigen-loaded DCs that are injected back into patients; and 3) specific in vivo DC targeting with anti-DC antibodies fused with antigens and with DC activators. 4) Next generation clinical trials will test optimized DC vaccines combined with patient-adjusted approaches to block Tregs and to breakdown the tumor environment. These therapies will be tested in pre-selected patients thereby leading to personalized therapy.
Acknowledgments
Dedicated to patients and volunteers who participated in our studies. We thank former and current members of the Institute for their contributions. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
References
- Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, Darnell RB. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nat Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Maraskovsky E, Smyth MJ. Cancer vaccines for established cancer: how to make them better? Immunol Rev. 2008;222:242–255. doi: 10.1111/j.1600-065X.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlis NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA, Terebelo HR, Byrne GE, Jr, Levine BL, Boise LH, Lee KP. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-Priming of Naive CD8 T Cells against Melanoma Antigens Using Dendritic Cells Loaded with Killed Allogeneic Melanoma Cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol Immunother. 2008;57:1679–1683. doi: 10.1007/s00262-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3(+) T Cells Induce Perforin-Dependent Dendritic Cell Death in Tumor-Draining Lymph Nodes. Immunity. 2010;32:266–278. doi: 10.1016/j.immuni.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Thurin M, Trinchieri G, Wang E, Wigginton J, Chaussabel D, Coukos G, Dhodapkar M, Hakansson L, Janetzki S, Kleen TO, Kirkwood JM, Maccalli C, Maecker H, Maio M, Malyguine A, Masucci G, Palucka AK, Potter DM, Ribas A, Rivoltini L, Schendel D, Seliger B, Selvan S, Slingluff CL, Jr, Stroncek DF, Streicher H, Wu X, Zeskind B, Zhao Y, Zocca MB, Zwierzina H, Marincola FM. A systematic approach to biomarker discovery; preamble to “the iSBTc-FDA taskforce on immunotherapy biomarkers”. J Transl Med. 2008;6:81. doi: 10.1186/1479-5876-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci U S A. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV. Immunity to stemness genes in human cancer. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, Mellman I, Banchereau J, Connolly JE. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- Fairchild PJ, Austyn JM. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6:187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–1414. [PubMed] [Google Scholar]
- Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, Saeland S. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S, Butler MH, Cremona O, David C, Freyberg Z, Zhang X, Solimena M, Tokunaga A, Ishizu H, Tsutsui K, De Camilli P. Expression of amphiphysin I, an autoantigen of paraneoplastic neurological syndromes, in breast cancer. Mol Med. 1998;4:29–39. [PMC free article] [PubMed] [Google Scholar]
- Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, McDonald HA, Gibson GA, Watkins SC, Muthuswamy R, Kalinski P, Okada H. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells--significant roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Liu Y-J. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. CD1 assembly and the formation of CD1-antigen complexes. Curr Opin Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, Old LJ, Romero P, Hoos A, Davis MM. “MIATA”-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer Stem Cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–6875. [PubMed] [Google Scholar]
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Klechevsky E, Liu M, Morita R, Banchereau R, Thompson-Snipes L, Palucka AK, Ueno H, Banchereau J. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70:281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S, Dhodapkar MV. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming Growth Factor-beta Regulation of Immune Responses. Annu Rev Immunol. 2005 doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu CI, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B, Banchereau J, Palucka AK. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nat Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, Schwartz LH, Berg WJ, Bukowski RM. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal GJ. Host immunobiology and vaccine development. Lancet. 1997;350:1316–1319. doi: 10.1016/S0140-6736(97)03257-1. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- Panelli MC, Stashower ME, Slade HB, Smith K, Norwood C, Abati A, Fetsch P, Filie A, Walters SA, Astry C, Arico E, Zhao Y, Selleri S, Wang E, Marincola FM. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol. 2007;8:R8. doi: 10.1186/gb-2007-8-1-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and usein clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of Programmed Death Ligand 1 Enhances the Therapeutic Efficacy of Combination Immunotherapy against Melanoma. J Immunol. 2010 doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin L, DeCamilli P, Butler M, Solimena M, Schmitt HP, Morgenthaler N, Meinck HM. Stiff-man syndrome in a woman with breast cancer: an uncommon central nervous system paraneoplastic syndrome. Neurology. 1998;50:94–98. doi: 10.1212/wnl.50.1.94. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD, Jr, Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Tahara H, Sato M, Thurin M, Wang E, Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Wigginton JM, Ambs S, Akutsu Y, Chaussabel D, Doki Y, Eremin O, Fridman WH, Hirohashi Y, Imai K, Jacobson J, Jinushi M, Kanamoto A, Kashani-Sabet M, Kato K, Kawakami Y, Kirkwood JM, Kleen TO, Lehmann PV, Liotta L, Lotze MT, Maio M, Malyguine A, Masucci G, Matsubara H, Mayrand-Chung S, Nakamura K, Nishikawa H, Palucka AK, Petricoin EF, Pos Z, Ribas A, Rivoltini L, Sato N, Shiku H, Slingluff CA, Streicher H, Stroncek DF, Takeuchi H, Toyota M, Wada H, Wu X, Wulfkuhle J, Yaguchi T, Zeskind B, Zhao Y, Zocca MB, Marincola FM. Emerging concepts in biomarker discovery; The US-Japan workshop on immunological molecular markers in oncology. J Transl Med. 2009;7:45. doi: 10.1186/1479-5876-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, McPherson JM, Berzofsky JA. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- Townsend AR, Gotch FM, Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42:457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, Lebecque S. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- Urosevic M, Dummer R, Conrad C, Beyeler M, Laine E, Burg G, Gilliet M. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC, de Vries IJ, Boer TD, Dolstra H, Torensma R, van Krieken JH, Adema GJ, De Mulder PH. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Wei H, Wang S, Zhang D, Hou S, Qian W, Li B, Guo H, Kou G, He J, Wang H, Guo Y. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]