Abstract
The regulatory elements of the Tie2/Tek promoter are commonly used in mouse models to direct transgene expression to endothelial cells. Tie2 is also expressed in hematopoietic cells, although this has not been fully characterized. We determine the lineages of adult hematopoietic cells derived from Tie2 expressing populations using Tie2-Cre;Rosa26R-EYFP mice. In Tie2-Cre;Rosa26R-EYFP mice, analysis of bone marrow cells showed Cre-mediated recombination in 85% of the population. In adult bone marrow and spleen, we analyzed sub-classes of early hematopoietic progenitors, T cells, monocytes, granulocytes, and B cells. We found that ~84% of each lineage was EYFP+, and nearly all cells that come from Tie2 expressing lineages are CD45+, confirming widespread contribution to definitive hematopoietic cells. In addition, more than 82% of blood cells within the embryonic yolk sac were of Tie2+ origin. Our findings of high levels of Tie2-Cre recombination in the hematopoietic lineage have implications for the use of the Tie2-Cre mouse as a lineage restricted driver strain.
Keywords: Tie2, Cre recombinase, hematopoietic lineage, mouse transgenic, ROSA reporter
There are two types of embryonic hematopoietic cells, primitive and definitive. Primitive hematopoietic cells arise in the yolk sac from blood islands from Flk1+ cells (Lugus et al., 2009), and contain erythroid and megakaryocyte progenitors. Primitive erythroid progenitors appear in the murine yolk sac between E7.25 and E9.0, and produce erythroid cells expressing both embryonic and adult hemoglobins. Primitive erythroid progenitors express the endothelial cell markers VE-cadherin, CD31, Tie2, and endoglin (Ema et al., 2006). Definitive hematopoiesis generates erythroid cells expressing adult hemoglobins (McGrath and Palis, 2005). Definitive hematopoietic cells expand in the yolk sac, and are in the embryo soon after E8.25 (Palis et al., 2001). In the mouse, hematopoietic stem cells are generated at E10.5 in the aorta-gonad-mesonephros (AGM) region (Palis et al., 2001), and migrate to the fetal liver, spleen, and bone marrow. All primitive and definitive hematopoietic cells emerging before E10 in the mouse embryo are from the yolk sac (Lux et al., 2008), which also contributes to adult hematopoiesis (Samokhvalov et al., 2007).
The contribution of endothelial cells to the adult hematopoietic lineage was shown using Cre/LoxP (Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009; Zovein et al., 2008), and the VE-cadherin-Cre strain, but it is not clear whether endothelial cells contribute to primitive hematopoiesis. Endothelial and hematopoietic cells share molecular determinants, and are postulated to arise from a shared hemangioblast. Previous studies of VE-cadherin expression in the yolk sac, and analysis of single yolk sac cells fromVEC-Cre;Rosa26R-EYFP mice at E9.5 indicated little contribution of VE-cadherin positive cells to primitive hematopoietic cells. A similar study has not been performed to test other endothelial lineage markers.
Tie2 (tunica intima endothelial kinase 2) is a receptor tyrosine kinase that binds angiopoietin-1 and angiopoietin-2. Tie2 is expressed in all endothelial cells (Kisanuki et al., 2001), and is also expressed in hematopoietic cells in the AGM region, fetal liver and adult bone marrow, as well as in several differentiated hematopoietic cells (Puri and Bernstein, 2003; Takakura et al., 1998). Tie2 null embryos die at E10.5 due to defects in the cardiovasculature and definitive hematopoiesis (Takakura et al., 1998) (Dumont et al., 1994; Sato et al., 1995). Mesodermal precursors undergoing primitive erythropoiesis express endothelial cell markers including Tie2 in the mouse embryo (Ema et al., 2006), although the lineages that derive from Tie2 expressing cells are not well defined. This is in part due to the transient Tie2 expression in developing hematopoietic cells. We utilized the Cre-loxP system to trace the progeny of Tie2+ cells in distinct lineages of the hematopoietic system, and found that almost all subtypes of hematopoietic cells are the progeny of Tie2+ cells.
Because Tie2 is expressed within both endothelium and hematopoietic cells (Dumont et al., 1994; Kisanuki et al., 2001; Takakura et al., 1998), we investigated whether both primitive and definitive hematopoietic cells can be traced using Tie2-Cre mice. Tie2-Cre mice were crossed to flox-STOP-flox-EYFP (Rosa26R-EYFP) Rosa reporter mice. In this system, cells expressing Tie2 will express Cre recombinase and recombine the floxed-STOP sequence. Such a cell and its progeny will permanently express EYFP after recombination based on the constitutively active nature of the Rosa26 locus.
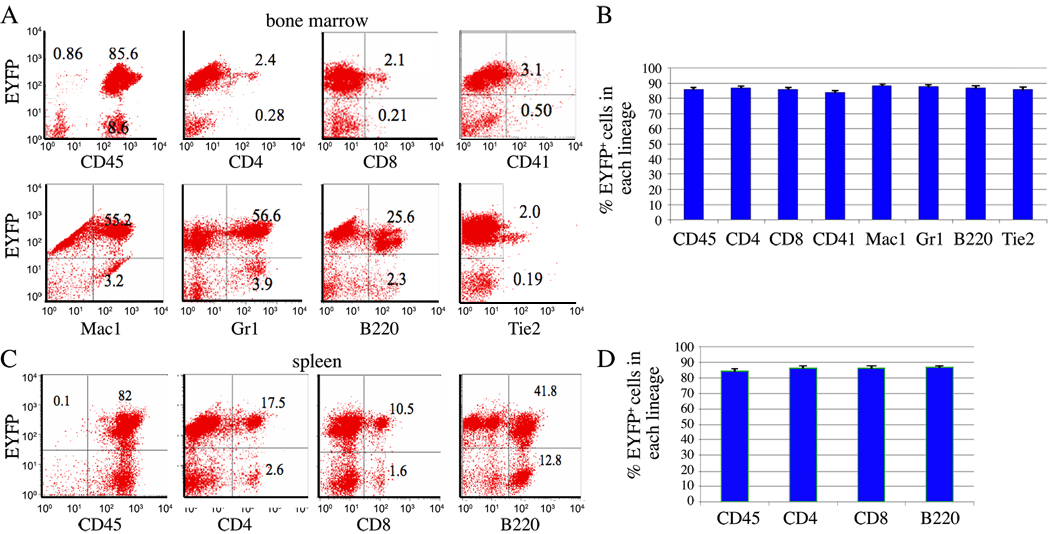
To trace the contribution of Tie2+ cells to definitive hematopoiesis, we analyzed the adult mouse hematopoietic tissues, bone marrow and spleen. In the bone marrow, about 85% (average, 85.9%± 5.1%; n = 8) cells were EYFP+, and more than 98% (average, 98.3%± 0.4%; n = 8) of EYFP+ cells were positive for CD45 (Fig. 1A–B). More importantly, approximately 85% (n = 8) of each lineage including CD4, CD8, B220, Mac1, CD41, and Gr1 positive cells are EYFP+. In the spleen, we also observed that about 84% (average, 84.6 %± 4.7%; n = 8) cells were positive for EYFP, and nearly all (average, 98.7%± 0.4%; n = 8) EYFP+ cells are positive for CD45 (Fig, 1C–D). Moreover, we also found about 84% (n = 8) of CD4, CD8, and B220 positive spleen cells were EYFP+ (Fig. 1C–D). These data indicated that Tie2 is expressed in the progeny of the majority of adult hematopoietic cells, within multiple lineages.
Figure 1. The contribution of Tie2+ lineage cells to adult blood cells.
A) Representative FACS plots of staining of bone marrow cells for the hematopoietic markers CD45, CD4, CD8, CD41, Gr1, Mac1, B220, and Tie2 (x-axis) and EYFP (y-axis) from Tie2-Cre;Rosa26R-EYFP mice at 2 months of age. B) Summary data of multiple experiments (n=8) analyzing bone marrow cells from Tie2-Cre;Rosa26R-EYFP mice. Data are presented as the percentage of each lineage that was EYFP+ ± S.D. of the mean percentage of each lineage. C) Representative FACS plots of staining of spleen cells for the hematopoietic markers CD45, CD4, CD8, and B220 (x-axis) and EYFP (y-axis). Hematopoietic lineages analyzed represent T cells (CD4 and CD8), B cells (B220), granulocytes (Gr1), megakaryocytes (CD41), and macrophages (Mac1). D) Summary data of multiple experiments (n = 8) analyzing spleens from Tie2-Cre;Rosa26R-EYFP mice. Data are presented as the percentage of each lineage that are EYFP+ ± S.D. of the mean percentage of each lineage.
A previous study suggested that all adult hematopoietic cells developed from Tie2 positive precursors (Liakhovitskaia et al., 2009), although specific adult lineages were not tested. Indeed, this study focused primarily on Cre-mediated recombination in mid-gestation embryos between E9.5–11.5, whereas our study focuses on adult bone marrow and spleen populations. Although we had a high proportion of positive cells in each hematopoietic lineage (we observed ~84% EYFP+ in adult hematopoietic cells), there was a significant population in each lineage that was not lineage marked. To test whether it is possible that we experienced incomplete recombination efficiency, single bone marrow cells from Tie2-Cre;Rosa26R-EYFP mice were stained with anti-Tie2 antibody and analyzed by FACS for Tie2 expression and EYFP expression (Fig. 1A). Out of the total cells positive for Tie2 based on anti-Tie2 staining, about 85% (average, 85.7%± 2.4%; n = 6) were also positive for EYFP. These data show that 10–15% of the Tie-2 expressing cells were not marked by EYFP, suggesting a high, but not complete level of Cre-mediated recombination and activation of the EYFP transgene. However, we cannot disregard the potential that there are small hematopoietic lineage subpopulations that derive from precursors independent of the Tie2 lineage.
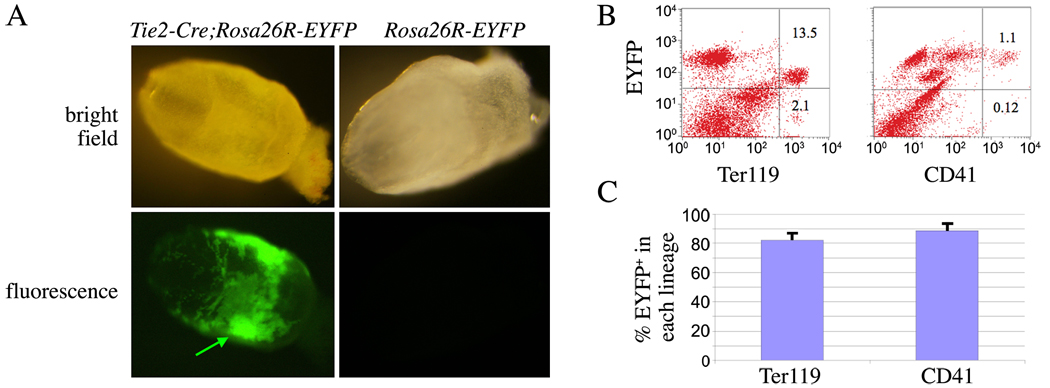
There are two types of hematopoiesis during embryogenesis, primitive and definitive. Several studies indicate the contribution of VE-cadherin positive endothelial cells to definitive hematopoietic cells (Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009; Zovein et al., 2008). To trace the contribution of VE-cadherin-expressing cells to primitive hematopoietic cells, yolk sacs from VE-cadherin-Cre;Rosa26R-EYFP embryos at E9.5 were collected and analyzed for EYFP+ cells. Very few EYFP+ cells could be identified (data not shown). Tie2 is expressed significantly earlier than VE-cadherin, starting at E6.5, and reporter activity from Tie2-Cre;CAG-CAT-Z double transgenic embryos at E7.5 and E8.5 indicated positive hematopoietic cells in yolk sacs and the dorsal aorta (Kisanuki et al., 2001), suggesting that Tie2-Cre could be used to trace primitive hematopoiesis. To address this, we collected yolk sacs of Tie2-Cre;Rosa26R-EYFP at different embryonic stages. At E7.5, there was abundant expression of EYFP in cells of the extraembryonic mesoderm of the visceral yolk sac, corresponding to aggregations that are precursors of blood islands (Fig. 2A). We collected yolk sacs at E9.5 for single cell analysis, and found that about 88% (average, 88.7%± 5.7%; n = 10) of primitive hematopoietic progenitors (CD41) (Ferkowicz et al., 2003; Mitjavila-Garcia et al., 2002) from yolk sacs and 82% (average, 82.1%± 6.4%; n = 10) of the primitive erythroid cells (Ter119) were positive for EYFP. Notably, Tie2-Cre does not label all primitive hematopoietic cells, again suggesting a non-Tie2+ hematopoietic source or incomplete recombination mediated by the Tie2-Cre transgene. Recent studies indicate that both definitive and primitive hematopoietic cells developed from Flk1+ cells (Lugus et al., 2009). The expression pattern of Flk1 and Tie2 in the yolk sac may support the idea that a very small population of primitive hematopoietic cells develop from Flk1+Tie2− cells (Ema et al., 2006; Lugus et al., 2009). Our data indicate that almost all primitive hematopoietic cells can be traced to Tie2+ cells.
Figure 2. A Tie2+ origin for embryonic blood cells from Tie2-Cre;Rosa26R-EYFP embryos.
A) Tie2-Cre;Rosa26R-EYFP or control Rosa26R-EYFP embryos were collected at E7.5. Arrow shows EYFP+ mesodermal aggregations in visceral yolk sac in regions of forming blood islands. B) Representative FACS plots of E9.5 Tie2-Cre;Rosa26R-EYFP yolk sacs stained for the primitive hematopoietic progenitor marker CD41 or erythroid cell marker Ter119 (x-axis) and EYFP (y-axis) to assess the origin of these lineages within the E9.5 yolk sac. C) Summary data of multiple experiments (n = 10) analyzing the yolk sacs of Tie2-Cre;Rosa26R-EYFP mice. Data are presented as the percentage of Ter119+ and CD41+ that are also EYFP+ ± S.D. of the mean percentage of each lineage.
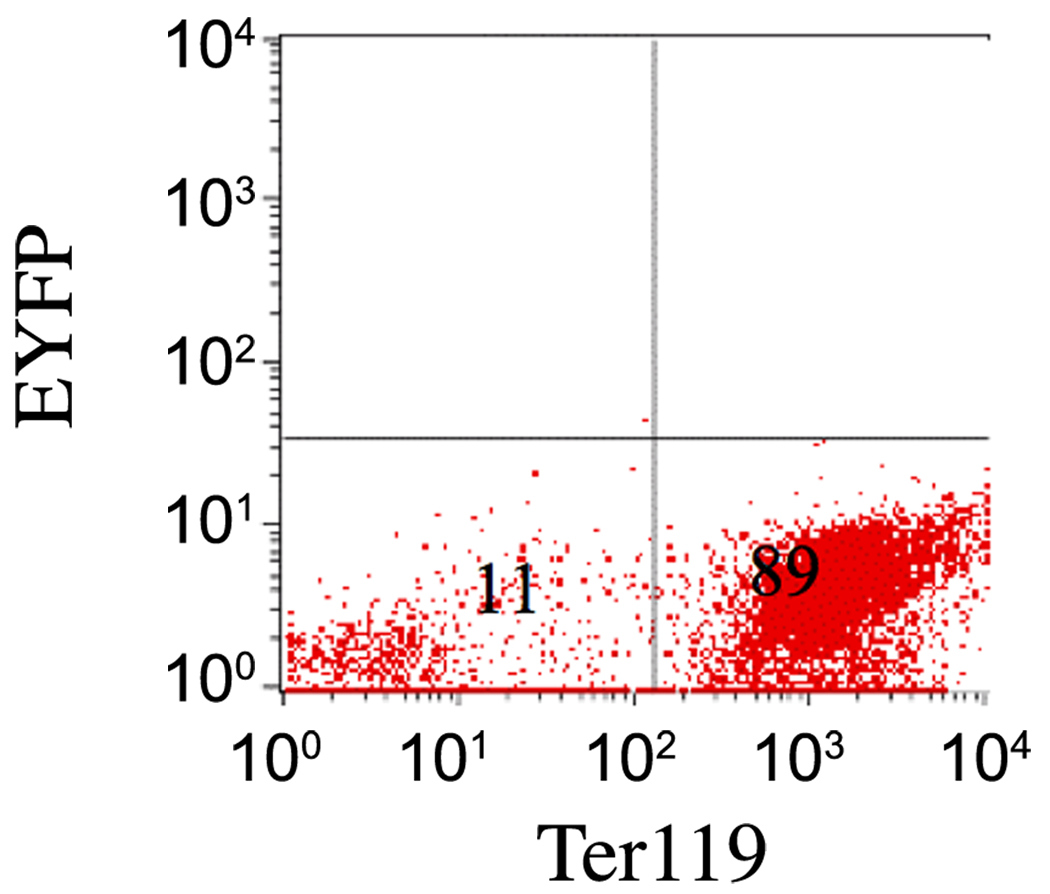
Tie2 and other endothelial markers have been reported to be expressed in mesodermal progenitors that contribute to primitive erythropoiesis (Ema et al., 2006). However, the activity of Tie2 promoter in these cells is unknown. To address this, circulating embryonic blood cells were isolated from Tie2-ER-Cre;Rosa26R-EYFP embryos, and then treated with 4OTH to induce Cre recombinase expression. No significant expression of EYFP was detected in this population (Fig. 3), suggesting that the Tie2 promoter is no longer active in circulating embryonic hematopoietic cells.
Figure 3. The Tie2-Cre transgene is not active in circulating embryonic blood cells.
Peripheral blood cells were collected from Tie2-CreER;Rosa26R-EYFP mice at E12.5 and induced in vitro with 4OTH for 24 hours in suspension. Cells were then analyzed for EYFP expression within the Ter119+ population. No significant expression of EYFP was observed.
Embryonic hematopoiesis is a dynamic process coordinated by multiple signals. Our study, using a highly utilized Tie2-Cre transgenic strain, demonstrates that the majority of adult blood cells derive from a Tie2+ origin. Therefore, our study shows that Tie2 promoter/enhancer elements are widely active in the hematopoietic lineage in addition to endothelial lineages during development.
Methods
Mouse Strains
Tie2-Cre mice (Jackson Laboratory, B6.Cg-Tg(Tek-Cre)12Flv, stock 004128), Tie2-CreER mice (Forde et al., 2002) (provided by Bernd Arnold, German Cancer Research Center, Heidelberg, Germany) and VE-cadherin-Cre mice (Jackson Laboratory, B6.Cg-Tg(Cdh5-Cre)7Mlia/J, stock 006137) were crossed with Rosa26R-EYFP mice (Jackson Laboratory, B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J, stock 006148) to generate Tie2-Cre;Rosa26R-EYFP mice, Tie2-CreER;Rosa26R-EYFP mice, or VE-cadherin-Cre;Rosa26R-EYFP mice. The utilization of the mouse model in these experiments was approval by the Institutional Animal Care and Use Committee of Maine Medical Center.
The following primers were used for genotyping of the Tie2-Cre, VE-cadherin-Cre, and Tie2-CreER strains, and yields a ~200bp product:
5’-GCATTTCTGGGGATTGCTTA-3’ and 5’-CCCGGCAAAACAGGTAGTTA-3’
The Rosa26R-EYFP strain was genotyped using the following primers, which will amplify a 320bp transgenic product:
5’-AAGACCGCGAAGACTTTGTC-3’, 5’-AAAGTCGCTCTGAGTTGTTAT-3’, and 5’-GGAGCGGGAGGAATGGATATG-3’
Cell Staining and Sorting
The bone marrow and spleen were isolated from Tie2-Cre;Rosa26R-EYFP mice at two months of age and mechanically dissociated into a single-cell suspension, and treated with red blood cell lysis buffer. Cells were analyzed on a FACSCalibur with the following monoclonal antibodies: CD45-APC, CD4-PE-Cy7, CD8-APC, CD41-PE-Cy7, B220-APC, Gr1-APC, Mac1-PE-Cy7, and Ter119-APC. For Tie2 staining, the purified Tie2 antibody was used, and then stained with APC-conjugated secondary antibodies before performing FACS.
E9.5 yolk sacs of Tie2-Cre;Rosa26R-EYFP mice were visualized for EYFP+ cells under fluorescence microscopy, and then confirmed by genotyping. The yolk sacs were dissected and digested at 37°C for 60 minutes in 0.2% collagenase with 20% fetal bovine serum in phosphate-buffered saline. After digestion, yolk sacs were separated into single-cell suspension by passing through a 20-gauge needle. Single cell suspensions were stained with CD41 and Ter119 antibodies (eBioscience, San Diego, CA), and analyzed by FACS.
Embryos fromTie2-CreER; Rosa26R-EYFP crosses at E12.5 were bled in warm PBS after removal from their yolk sacs and placentas. The peripheral blood cells were centrifuged and resuspended in myelocult media (Stem Cell Technologies), supplemented with 10−6 M hydrocortisone (Stem Cell Technologies), and 5 µM 4OTH (Sigma) for 24 hours in suspension. Flow cytometry was then performed to detect the expression of EYFP and Ter119. All monoclonal antibodies and their appropriate controls were purchased from eBioscience. The double positive cell populations were normalized to the total single positive EYFP or respective antibody to get percentage of double positive cells.
Acknowledgements
This work was supported by NIH grants R01HL070865 (to L.L.), HL65301 (to R.E. Friesel) and P20RR1555 (PI: R.E. Friesel). The Flow Cytometry Core Facility is supported by P20RR181789 (PI: D. Wojchowski), and the Mouse Transgenic Facility is supported by NIH grant P20RR1555 (PI: R.E. Friesel), both from the National Center for Research Resources.
References
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108:4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- Forde A, Constien R, Grone HJ, Hammerling G, Arnold B. Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis. 2002;33:191–197. doi: 10.1002/gene.10117. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakhovitskaia A, Gribi R, Stamateris E, Villain G, Jaffredo T, Wilkie R, Gilchrist D, Yang J, Ure J, Medvinsky A. Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth. Stem Cells. 2009;27:1616–1624. doi: 10.1002/stem.71. [DOI] [PubMed] [Google Scholar]
- Lugus JJ, Park C, Ma YD, Choi K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood. 2009;113:563–566. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–1028. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Palis J, Chan RJ, Koniski A, Patel R, Starr M, Yoder MC. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci U S A. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci U S A. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Takakura N, Huang XL, Naruse T, Hamaguchi I, Dumont DJ, Yancopoulos GD, Suda T. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9:677–686. doi: 10.1016/s1074-7613(00)80665-2. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]