Abstract
Introduction
Data are limited on the metabolic effects of resistance exercise (strength training) in adolescents.
Purpose
The objective of this study was to determine whether a controlled resistance exercise program without dietary intervention or weight loss, reduces body fat accumulation, increases lean body mass, and improves insulin sensitivity and glucose metabolism in sedentary obese Hispanic adolescents.
Methods
Twelve obese adolescents (15.5±0.5y; 35.3 ±0.8kg/m2;40.8±1.5% body fat), completed a 12 wk resistance exercise program (2×1h/wk, exercising all major muscle groups). At baseline and completion of the program, body composition was measured by DXA, abdominal fat distribution by Magnetic Resonance Imaging, hepatic and intramyocellular fat by Magnetic Resonance Spectroscopy, peripheral insulin sensitivity by the Stable Labeled IV Glucose Tolerance Test and hepatic insulin sensitivity by the Hepatic Insulin Sensitivity Index =1000/(GPR*fasting insulin). Glucose production rate (GPR), gluconeogenesis and glycogenolysis were quantified using Stable Isotope-Gas Chromatography/Mass Spectrometry techniques.
Results
All participants were normoglycemic. The exercise program resulted in significant strength gain in both upper and lower body muscle groups. Body weight increased from 97.0±3.8 to 99.6±4.2 kg (p<0.01). The major part (~80%) was accounted for by increased lean body mass (55.7±2.8 to 57.9±3.0 kg; p≤0.01).Total, visceral, hepatic and intramyocellular fat content remained unchanged. Hepatic insulin sensitivity increased by 24±9% (p<0.05), while peripheral insulin sensitivity did not change significantly. GPR decreased by 8±1% (p<0.01) due to a 12±5% decrease in glycogenolysis (p<0.05).
Conclusion
We conclude that a controlled resistance exercise program without weight loss increases strength and lean body mass, improves hepatic insulin sensitivity and decreases GPR without affecting total fat mass or visceral, hepatic and intramyocellular fat content.
Keywords: resistance, peripheral insulin sensitivity, visceral fat, hepatic fat, intramyocellular fat, adolescents
Introduction
Physical activity is a primary intervention in the combat against obesity and obesity related disorders in children and adolescents. According to the American Academy of Pediatrics, both aerobic (running, biking, swimming) and resistance exercise (strength training) should be part of a multifaceted approach to stimulate exercise and improve fitness in children and adolescents (23).
We and others have demonstrated positive effects of aerobic exercise programs on abdominal fat distribution, hepatic fat content and peripheral as well as hepatic insulin sensitivity in children and adolescents (12, 13, 27, 41, 42). In contrast, there are only limited data on the impact of programs containing resistance training alone or in combination with a diet intervention on body composition and insulin sensitivity in children and adolescents (4, 8, 24, 32, 39). Increased strength and lean body mass (muscle mass) were observed in three of these studies (24, 32, 39), while one (32) reported improved insulin sensitivity. Studies in adults have demonstrated increased strength (6, 15, 16), muscle mass (6, 15) and insulin sensitivity (6, 15, 16) in response to resistance exercise programs.
There is no published information on the impact of a resistance exercise program on hepatic and intramyocellular fat content, or glucose and lipid metabolism in adolescents. Neither has peripheral and hepatic insulin sensitivity (representing different mechanisms to maintain glucose homeostasis) been determined separately in response to resistance training.
Resistance exercise might be an attractive alternative to aerobic training for obese individuals because of its lower aerobic intensity and the positive feedback from the visible strength gain. Studies investigating the effects of resistance exercise programs on metabolic and body composition parameters are crucial in designing strategies that provide various intervention options to prevent obesity related disease.
The aim of the present study was to determine the effect of a controlled resistance exercise program alone, without additional dietary intervention or weight loss, on body composition, abdominal, hepatic and intramyocellular fat content, peripheral and hepatic insulin sensitivity, and glucose and lipid metabolism in obese adolescents. We focused on sedentary obese Hispanics because of their high risk of obesity related illnesses (25, 29, 31).
We hypothesized that in these adolescents, a 12 wk resistance exercise program would increase lean body mass, reduce visceral, hepatic and intramyocellular fat accumulation (IMCL) and improve peripheral and hepatic insulin sensitivity.
Methods and Procedures
Participants
After approval of the protocol by the Baylor College of Medicine Institutional Review Board for Human Subject Research, and the General Clinical Research Center Advisory Board, obese adolescents were recruited by local advertisement. Adolescents were screened and enrolled in the study after written assent from the adolescents and consent from the legal guardian were obtained.
Twelve post pubertal (2 Tanner IV; 10 Tanner V) obese Hispanic adolescents (6m; 6f; 15.5 ± 0.5 y) were studied (Table 1). All participants had BMI >95th percentile for age (20). Participants had been obese for ≥5 years and reported stable body weight for at least 6 months. Only sedentary adolescents were included, i.e. they did not participate in any school or after school organized athletic activities and performed <45 min light to moderate physical activity/week (by self report).
Table 1.
Body composition and fat distribution at baseline and post-exercise (mean ± SE)
| Baseline | Post-Exercise | |
|---|---|---|
| Weight (kg) | 97.0 ± 3.8 | 99.6 ± 4.2** |
| BMI (kg/m2) | 35.3 ± 0.7 | 36.1 ± 0.9** |
| Body fat % | 40.8 ± 1.5 | 40.2 ± 1.7 |
| Lean body mass (kg) | 55.7 ± 2.8 | 57.9 ± 3.0** |
| Fat mass (kg) | 39.7 ± 1.9 | 40.3 ± 2.3 |
| Bone mineral density (g/cm2) | 1.07 ± 0.03 | 1.08 ± 0.03** |
| Subcutaneous fat (cm2) | 531 ± 24 | 565 ± 27* |
| Visceral fat (cm2) | 58 ± 4 | 55 ± 4 |
| Hepatic fat % | 9.2 ± 2.9 | 9.4 ± 3.1 |
| Intramyocellular fat %$ | 3.4 ± 0.4 | 3.4 ± 0.5 |
Different from baseline:
p<0.05,
p<0.01.
Intramyocellular fat % measurements were not obtained in 2 participants
All participants were Hispanic (parents and grandparents of Hispanic descent by self report). They were in good health as determined by a medical history, a physical examination and a standard blood chemistry analysis including blood lipids, liver- and kidney function tests, hemoglobin, hematocrit, hemoglobin A1c and fasting and 2 h post-prandial glucose response. Participants were taking no medications including birth control pills and had no first-degree relatives with diabetes. Adolescents with morbid obesity (body fat % >50, sleep apnea, Pickwick syndrome or cor pulmonale) were excluded.
Study Design
Each participant was studied on two occasions: 1) The weekend before start of the exercise program (baseline), 2) Three days after the final exercise session of the 12 wk program (post-exercise). All procedures were identical on both study occasions.
To exclude effects of dietary intake on measurements obtained at baseline versus post-exercise, prior to both studies, each participant received an identical 7 d low-carbohydrate (CHO)/high-fat diet at home (30% CHO, 55% fat, and 15% protein; 20% of the total CHO content as fructose) (33, 34, 40, 41, 42). Total energy intake was calculated to correspond to each individual’s requirement according to the Institute of Medicine Dietary Reference Intakes (30). We have previously shown that estimating energy requirements based on these criteria results in accurate energy balance (33, 34, 40). A pack-out strategy with return and examination of non-consumed food was used (33, 34, 40). In order to determine the effect of exercise alone, participants were told not to make lifestyle changes and keep to their habitual diet except adhere to the controlled diet provided the week prior to both study occasions.
On both occasions, the participants were admitted to the General Clinical Research Center at Texas Children’s Hospital in the evening before the metabolic study. After dinner and a snack, participants were fasted overnight (except for water) i.e. from 2000 h until completion of the isotope infusion study at 1300 h the next day. Subsequently, the participants were transferred to the radiology department at Texas Children’s Hospital for Magnetic Resonance Imaging (MRI) and Magnetic Resonance Spectroscopy (MRS) of abdominal, hepatic and intramyocellular fat content.
Resistance Exercise Program
For the duration of 12 wks, participants came to the Physiotherapy Unit at Texas Children's Hospital twice a week for a 1h resistance exercise session. The program was based on the guidelines of the American College of Sport Medicine, Seventh Edition (1). Briefly, during wk 1 and 2, the program starts with resistance (weights) corresponding to ~50% of 3 repetitions max (3R max) with 2–3 sets of 8–12 repetitions. The weights and repetitions are then increased gradually according to each individual’s ability reaching ~80–85% of 3R max with 3 × 15–20 repetitions during wk 9 to 12.
The 1h session included: 10 min of warm up, 40 min of resistance training and 10 min of cool down. There was at least 1d of rest between sessions. All major muscle groups were trained during each exercise session using one of the alternative exercises for each muscle group outlined in Table 2. During the exercise program, first the number of repetitions and subsequently resistance (weight) were increased.
Table 2.
Resistance Exercises
| Muscle Group | Exercises |
|---|---|
| Chest | Hand-held weights Flys; Push-ups; Chest press |
| Back | Seated Row; Overhead Press |
| Triceps | Triceps Extension with hand-held weights |
| Biceps | Hand-held weights Curls; Cable Curls |
| Shoulders | Lateral Raises; Hand-held weights Press |
| Quadriceps | Leg Extension; Static Lunges; Squats with and without weights |
| Hamstrings | Dead Lifts with and without weights; Leg Curl with and without weights |
| Calves | Calf Raises |
| Gluts | Bridges on Physioball |
| Abs | Curl ups (straight and obliques) |
We present the result of the program as the dynamic strength gain by comparing sets × repetitions and (strength) weights for corresponding exercises at start and completion of the program. Specifically, we evaluated strength improvements in chest, triceps, biceps, quadriceps and hamstring muscles (Table 3) Quadriceps strength gain was evaluated using a Biodex isokinetic dynamometer (Shirley, NY) at velocity 180°/sec. as described by Wiggin et al. (44). All exercise sessions were supervised by trained exercise physiologists. Participants performed no exercise outside the program. Their weight was assessed twice a week in conjunction with the exercise sessions to assure that no weight loss occurred. The purpose of the study was to determine the “chronic” effects of the exercise program and not the “acute” effect of an exercise session. Therefore, the last exercise session took place three days prior to the metabolic and MRI/MRS measurements.
Table 3.
Strength improvements in response to the exercise program (mean ± SE).
| Muscle Group |
Exercise | Pre- Exercise |
Pre- Exercise |
Post- Exercise |
Post- Exercise |
% Diff. Pre vs. Post- Exercise |
% Diff. Pre vs. Post Exercise |
P-value ↑Weight |
P-value ↑ Sets x Reps |
|---|---|---|---|---|---|---|---|---|---|
| Weight lbs |
Sets x Reps |
Weight lbs |
Sets x Reps |
Weight | Sets x Reps | ||||
| Biceps | Cable curls n=2 Curls w. HW n=10 |
12 ± 4 n=12 |
26 ± 2 n=12 |
23 ± 8 n=12 |
54 ± 5 n=12 |
90 ± 11 n=12 |
144 ±39 n=12 |
0.02 n=12 |
0.0009 n=12 |
| Triceps | Extension w. HW n=12 |
12 ± 4 n=12 |
26 ± 2 n=12 |
23 ± 8 n=12 |
49 ± 3 n=12 |
108 ± 26 n=12 |
113 ± 25 n=12 |
0.02 n=12 |
0.0002 n=12 |
| Chest | Press w. HW n=7 HW Flys n=4 Push ups n=1 |
9 ± 2 n=11 |
30 ± 3 n=12 |
14 ± 3 n=11 |
50 ± 4 n=12 |
84 ± 20 n=11 |
86 ± 21 n=12 |
0.0005 n=11 |
0.0007 n=12 |
| Hamstrings | Leg Curls or Dead Lifts w. weight n=8 Leg Curls or Dead Lifts no weight N=4 |
27 ± 6 n=8 |
31 ± 4 n=12 |
37 ± 8 n=8 |
69 ± 8 n=12 |
33 ± 6 n=8 |
157 ± 53 n=12 |
0.008 n=8 |
0.0003 n=12 |
| Quadriceps | Leg ext w. weight n=2 |
50; 50 | 36 | 70; 70 | 36 | 40; 40 | 0 | ||
| Squats w. weight n=1 |
0 | 16 | 10 | 60 | (0 −18 lbs) | 275 | |||
| Biodex N/m at 180 deg. n=9 |
RL 88 ± 9 LL 101 ± 11 n=9 |
N/A | RL 114 ± 12 LL 115 ± 13 n=9 |
N/A |
RL 31 ± 7 LL 15 ± 6 n=9 |
N/A | RL 0.003 LL 0.02 n=9 |
N/A | |
Hand-held weights (HW); RL: Right leg; LL: Left leg.
Biodex data were not available in three subjects. Data on leg press and squats represent these three subjects.
Tracers
Deuterium oxide (99% 2H); [2H5]glycerol (99% [2H], 95% [2H5]); [1-13C]glucose (99% [13C]); and [6,6-2H2]glucose (99% [2H], 98% [2H2]) were purchased from Cambridge Isotope Laboratories (Andover, MA). The isotopes were tested for sterility and pyrogenicity by the investigation pharmacy at Texas Children’s Hospital (Houston, TX). The infusates were filtered through a Millex GP syringe filter (0.22 µm; Millipore Corporation, Bedford, MN) and stored at 4 °C for no more than 24–48 h before administration.
Administration of Tracers
On each study occasion, the participants received the following, stable isotopically labeled tracers as previously described (33–35).
During the overnight fast at 2100, 2300, 0100 and 0300 h, deuterium oxide (a total of 3 g/kg) was administered orally to measure total gluconeogenesis (7).
Between 0600 and 1300 h, a simultaneous, primed (60 × the minute infusion rate), constant rate i.v. infusion of [1-13C]glucose (0.33 ± 0 µmol/kg non-bone lean body mass (LBM) · min) and [2H5]glycerol (0.14 ± 0 µmol/kg LBM · min) was administered to measure glucose production and the plasma turnover of glycerol, an indicator of lipolysis (33–35).
The Stable Label Intravenous Glucose Tolerance Test (SLIVGTT) was started at 0900 h after the 0 min blood sample (see below). A bolus injection of glucose, 0.35 ± 0 g/kg LBM containing 10% [6,6-2H2]glucose, was administered over 90–120 sec to measure insulin sensitivity (33–35).
Blood Sampling
Blood samples were obtained just before start of the primed constant rate infusion of the [1-13C]glucose and [2H5]glycerol (designated as t = −180) (13 mL) and subsequently at t = −30, −20, −10, and 0 minutes (8 mL/sample). The injection of the SLIVGTT bolus (after the 0 min sample) was followed by blood sampling (3.6 mL per sample) at +2, 3, 4, 5, 8, 10, 18, 20, 23, 28, 32, 40, 60, 120, 180, and 240 min (33–35).
Analyses
Plasma concentrations of glucose, insulin, lipids, leptin, adiponectin and high sensitive C-reactive protein were measured as previously described (41). Non-bone lean body (LBM) and fat mass (FM) were measured by dual-energy x-ray absorptiometry (DXA) (QDR 11.2; Hologic Bedford, MA) (33, 34, 40).
Abdominal, hepatic and intramyocellular fat content were measured by Magnetic Resonance Imaging (MRI) and Magnetic Resonance Spectroscopy (MRS) using a Philips Achieva 1.5T whole body clinical scanner software release 1.5 (Philips Healthcare, Best, the Netherlands) as previously described in detail (33, 40, 42). The MR image of abdominal fat i.e. visceral (intra abdominal) and subcutaneous (peripheral) fat content was acquired in a single transversal slice at the level of the umbilicus (33, 40, 42). MRI data are expressed as cross-sectional area (cm2).
A PRESS single voxel technique was used to obtain the liver MR spectra as previously described in detail (42). Data were analyzed using the scanner software and results are expressed as total lipid/water peak area ratio (%). Hepatic fat was considered normal if the MRS lipid peak/water peak was <5.6 % and high if the MRS lipid peak/water peak was >5.6 % (37).
A PRESS chemical shift imaging technique was used for measuring IMCL in the soleus muscle (42). Data were analyzed using jMRUI v3.0 (26) with the AMARES algorithm to obtain the peak areas as described by Szczepaniak et al. (36). IMCL is expressed as the relationship between the areas of the IMCL and water peaks, respectively (%).
Calculations
Rates of glucose production and glycerol turnover (an indicator of lipolysis) were calculated under approximate steady-state conditions from the average isotopic enrichments obtained for [13C1]glucose and [2H5]glycerol, respectively, in the samples obtained at −30, −20, −10 and 0 min (33–35).
During the same period, the gluconeogenic contribution to glucose (GNG) was determined using 2H2O and the average 2H enrichments of carbons 1,3,4,5,and 6 of glucose (7).
Peripheral insulin sensitivity (the sensitivity of glucose disposition to insulin) was calculated by applying the minimal model to SLIVGTT data (2, 33–35).
Hepatic insulin sensitivity was calculated in the fasting state by the hepatic insulin sensitivity index (HISI): 1000/[GPR (µmol/kg LBM· min) × fasting plasma insulin (µU/mL)], where 1000 is a constant that results in numbers between 1 and 10, as described by Matsuda et al. (22).
Statistical Methods
Power calculations were based on data from our previous study on the effects of an aerobic exercise program (41, 42). Thus, 12 subjects would be sufficient to detect changes of the same magnitude as those found in response to the aerobic exercise program with a power of 0.8 and a type 2 error of 0.05. Data are presented as mean ± SE. Differences between values obtained on the two study occasions were tested by paired t-test. Generalized estimating equations (GEE) (SPSS 17.0) were used to assess the effects of gender and the interaction between the effect of the exercise program and gender. Correlation analysis was used to test potential relationships between measures of insulin sensitivity (hepatic and peripheral) and body fat distribution (visceral, hepatic and intramyocellular fat accumulation). A p<0.05 was considered statistically significant.
Results
Exercise Compliance and Strength Improvement
The participants completed 96 ± 1% of the total 24 exercise sessions (range: 22 – 24 exercises). Strength increased for upper body muscles (represented by biceps, triceps and chest) as well as lower body muscles (represented by quadriceps and hamstrings) (Table 3).
Peak torque measures (Nm) were performed in the leg extensor muscles at 180° using a Biodex instrument (data were available for 9 subjects). As shown in Table 3, peak torque increased for both legs. Muscle mass in the left and right leg were obtained by DXA and the peak torque per gram leg muscle was calculated pre and post exercise as a surrogate measure of muscle quality. In the right leg, peak torque/g leg muscle increased from 0.0093 to 0.0115 Nm/g (p=0.008) and in the left leg from 0.0108 to 0.0119 Nm/g (p=0.06) indicating that at least in the leg muscle not only strength and muscle mass but also muscle quality increased.
Energy Intake
Average daily energy intake over the 7-d pre-study diet period were not different at baseline (2911 ± 170 kcal/day) and post-exercise (2853 ± 158 kcal/day). The macronutrient distribution of the intake corresponded to the designed (31 ± 1% CHO, 54 ± 1% fat and 15 ± 1% protein) on both study occasions.
Effect of Resistance Exercise on Body Composition (Table 1)
Total Body Composition (DXA): Total body weight increased (p<0.01). This increase was primarily accounted for (~80%) by an increase in lean body mass (2.1 ± 0.5 kg; p<0.01), while total body fat mass did not change. Bone mineral density was slightly higher post exercise (0.015 g/cm2 i.e. 1.4%) (p<0.01) (Table 1). There was no difference between males and females. Our data for both boys and girls were all within the 97th to 3rd percentile (7/12 were close to the 50th percentile) reported in Mexican Males and Females of corresponding age (21).
Abdominal Fat Distribution (MRI): Visceral fat content did not change significantly, while a minor increase (5 ± 2%), (p<0.05) in subcutaneous fat was observed.
Hepatic Fat Content (MRS) did not change significantly. Seven of twelve adolescents (58%) had high liver fat content (lipid peak/water peak >5.6 %) (37). In these seven participants, hepatic fat content averaged 13.9 ± 4.3 at baseline and 14.2 ± 4.5% post-exercise (NS). In the five participants with a lipid/water peak <5.6%, hepatic fat content was 2.7 ± 0.7 at baseline and 2.7 ± 0.9% post exercise (NS).
Intramyocellular Fat Content (MRS) did not change significantly.
Effect of Resistance Exercise on Insulin Sensitivity, Glucose and Lipid Metabolism
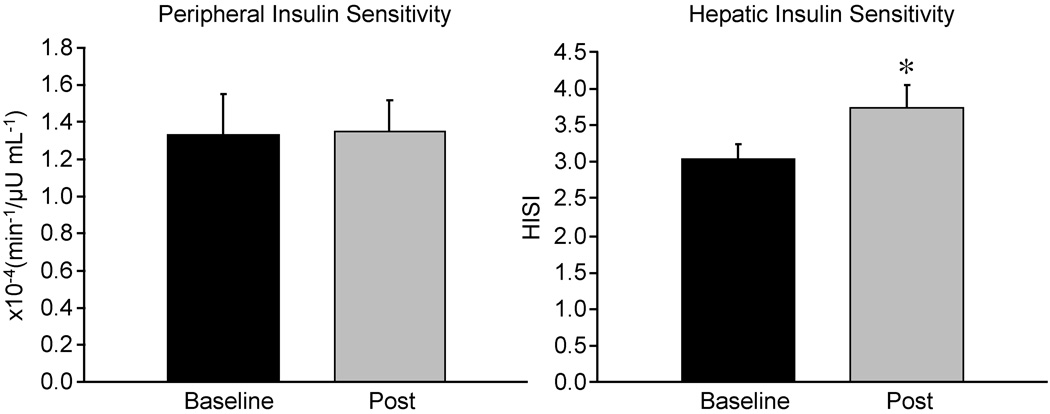
Fasting Glucose and Insulin concentrations did not change (Table 4). Peripheral Insulin Sensitivity: The average peripheral insulin sensitivity did not change significantly. In 8/12 adolescents, peripheral insulin sensitivity increased, while it decreased to the same extent in 4/12 participants (Figure 1).
Table 4.
Biochemical measurements at baseline and post-exercise (mean ± SE). Baseline data from previously studied lean adolescents are included for comparison (41)
| Baseline Participants in Resistance Exercise (6m/6f) |
Post-Exercise Participants in Resistance Exercise (6m/6f) |
Baseline Previously Studied Lean Adolescents (41) (10m/4f) |
|
|---|---|---|---|
| Glucose (mmol/L) | 5.1 ± 0.1 | 5.1 ± 0.1 | 5.1 ± 0.1 |
| Insulin (µU/mL) | 23.0 ± 1.8 | 21.0 ± 1.9 | 7.3 ± 0.9 |
| Triglycerides (mg/dL) | 94 ± 8 | 94 ± 8 | 68 ± 8 |
| Free Fatty Acids (mmol/L) |
0.46 ± 0.02 | 0.46 ±0.04 | 0.46 ± 0.03 |
| LDL cholesterol (mg/dL) |
90 ± 7 | 95 ± 8 | 85 ± 8 |
| HDL cholesterol (mg/dL) |
41 ± 1 | 42 ± 1 | 52 ± 4 |
| Total cholesterol (mg/dL) |
150 ± 8 | 156 ± 9 | 152 ± 8 |
| Adioponectin (µg/mL) | 5.7 ± 0.6 | 5.4 ± 0.6 | 7.2 ± 0.9 |
| Leptin (ng/mL) | 46 ± 7 | 45 ± 7 | 6 ± 1 |
| Hs-CRP (mg/L) | 1.9 ± 0.4 | 2.1 ± 0.4 | 0.2 ± 0.0 |
Figure 1.
Peripheral Insulin Sensitivity, calculated by the minimal model applied to SLIVGTT data (SI) and Hepatic Insulin Sensitivity, measured by Hepatic Insulin Sensitivity Index (HISI) at baseline and post-exercise (mean ± SE). Baseline is depicted by black bars, post exercise by gray bars. Different from baseline: * p<0.05, ** p<0.01
Hepatic Insulin Sensitivity increased by 24 ± 9% (p<0.05) (Figure 1).
Neither peripheral nor hepatic insulin sensitivity was significantly correlated with visceral, hepatic or intramyocellular fat content.
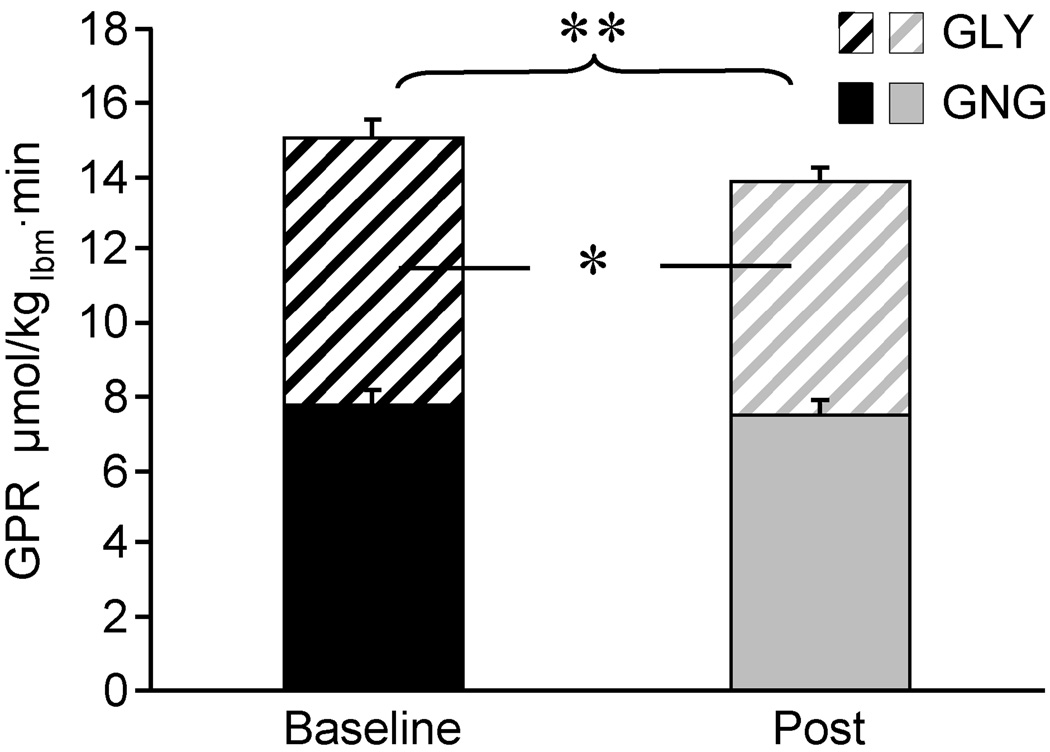
Glucose Production from Gluconeogenesis and Glycogenolysis: Glucose production rate decreased by 8 ± 1% (p<0.01) accounted for by a 12 ± 5% decrease in glycogenolysis (p<0.05). Gluconeogenesis remained unchanged (Figure 2).
Figure 2.
Glucose Production Rate (GPR), consisting of Gluconeogenesis (GNG) (solid part of the bar) and Glycogenolysis (GLY) (hatched part of the bar), at baseline (black bars) and post-exercise (gray bars) (mean ± SE).
Significant differences in GPR are depicted above the bars. Significant difference in GLY is depicted inside the bar. Different from baseline: * p<0.05, ** p<0.01
Lipolysis: The exercise program did not have any effect on total glycerol Ra (µmol/min) (Baseline: 225 ± 15 µmol/min; Post: 248 ± 24 µmol/min) or blood lipids (Table 4).
Effect of Resistance Exercise on Adiponectin, Leptin and Hs-CRP
Concentrations of leptin, adiponectin and hs-CRP did not change (Table 4). For comparison, baseline values previously published in lean adolescents of the same age and Tanner stage are included in Table 4 (41) .
Effect of Gender
The females had lower weight; height; and lean body mass (all p<0.05) than the males, while their fat% and leptin concentrations were higher (both p<0.05). No other variable differed between genders at baseline. Except for a greater increase in lean body mass in the males, effects of the exercise program did not differ between genders.
Discussion
The results of the present study demonstrate that this 12 wk controlled resistance exercise program increased strength, lean body mass and hepatic insulin sensitivity and slightly reduced glucose production in obese Hispanic adolescents. In contrast, the program had no effect on total body, visceral, hepatic and intramyocellular fat or peripheral insulin sensitivity.
Although increased muscle mass and hepatic insulin sensitivity are important results of the resistance exercise program, aerobic exercise seems to have more extensive effects. We demonstrated in a similar group of obese adolescents that the same volume of aerobic exercise significantly reduced total, visceral and hepatic fat content and increased both peripheral and hepatic insulin sensitivity (41, 42). These findings are in agreement with other studies in adults and children (12, 13, 28, 45). In contrast, the impact of resistance exercise is less clear (4, 6, 8, 15, 24, 32, 39). While most studies reported increased strength and lean body mass (muscle mass) in response to resistance exercise (6, 15, 24, 32, 39), only a few studies found effects on body fat (4, 24, 32). Similarly, its influence on insulin sensitivity was inconsistent. Some studies reported improved insulin sensitivity (6, 15, 16, 32), while others found no metabolic effects of resistance exercise (4, 8, 39). The methods used in the referenced studies (unlabeled FSIVGTT, unlabeled clamp, oral glucose tolerance test and HOMA-IR) provide a measure of whole body (i.e. peripheral + hepatic) insulin sensitivity. Using the stable label IVGTT and the Hepatic Insulin Sensitivity Index enabled us to determine peripheral and hepatic insulin sensitivity separately.
The increase in body weight resulting from the exercise program was almost completely explained by the increase in lean body mass. Theoretically, one would expect that increased lean body mass (muscle mass) i.e. increased insulin sensitive tissue mass would result in increased insulin sensitivity, primarily peripherally. The increase in lean body mass resulting from our program might have been insufficient to achieve this effect. Neither lean body mass at baseline and post-exercise nor exercise induced change in lean body mass correlated with peripheral or hepatic insulin sensitivity (data not shown). This is in agreement with the referenced studies, where changes in whole body insulin sensitivity (or lack thereof) were independent of changes in lean body mass (6, 15, 16, 32, 39). In addition, muscular strength alone has been shown to be a positive predictor for insulin sensitivity (5). It has been suggested that resistance training might increase insulin sensitivity as a result of qualitative changes within the muscle (6, 15). Brooks et al. (6) and Holten et al. (15) conducted muscle biopsies in adults with type 2 diabetes participating in a whole body (6) or one leg resistance exercise program (15). Brooks et al. (6) demonstrated increased muscle quality (strength/unit of muscle mass) and increased areas of type I and type II fibers. The increase in type I fiber area correlated significantly with the decrease in insulin resistance. Holten et al. (15) showed increased insulin activity and Glut 4 protein but no effect on markers of oxidative capacity in skeletal muscle. Since muscle biopsies cannot be performed in healthy children and adolescents for ethical reasons, we were not able to pursue potential cellular mechanisms. We did calculate peak torque/g leg muscle from the DXA and Biodex analyses as a surrogate measure of muscle quality. We found an increase in this value for the right leg and a value that approached significance (p=0.06) for the left. These values did not correlate with insulin sensitivity.
A failure to increase lipid oxidation during fasting might lead to intramyocellular fat deposition in obese individuals, subsequently contributing to patterns of insulin resistance (17). Exercise could potentially improve fat oxidation, which might lead to reduced intramyocellular fat content. Indeed, Koopman et. al. (19) demonstrated a decrease in intramyocellular fat directly after a resistance exercise session. However, 120 min after the exercise session the intramyocellular fat content had returned to pre-exercise levels indicating only a short term effect (19). To our knowledge there are no published data on the effects of a resistance exercise program on intramyocellular fat content. We measured intramyocellular fat at baseline and post the exercise program using magnetic resonance spectroscopy but found no changes.
Resistance exercise significantly increased fasting hepatic insulin sensitivity. The mechanism for this effect is not clear. Hepatic fat content measured by magnetic resonance spectroscopy did not change in response to the program and did not correlate with hepatic insulin sensitivity. Thus, the improvements in hepatic insulin sensitivity could not be explained by any reduction in hepatic fat accumulation. This finding indicates that liver lipid content is not a reliable predictor of hepatic insulin resistance. Heled et al. (14) reported that aerobic exercise training (treadmill) increased hepatic insulin sensitivity due to ameliorated insulin signaling response and inhibited PEPCK activity in the hepatocyte of diabetes prone fat sand rats. Since we could not perform liver biopsies in our healthy adolescents, hepatocellular mechanisms could not be investigated. While none of the more metabolically active fat deposits (visceral, hepatic and intramyocellular) changed in response to our resistance exercise program, we observed a small increase in subcutaneous abdominal fat. This finding might be of importance. It has been speculated that defective differentiation of subcutaneous fat might lead to defective fat storage in this compartment with subsequent overflow of fat into visceral, hepatic and intramyocellular fat deposits (9, 38). Increased subcutaneous abdominal fat without changes in visceral fat was also reported by Treuth et al. (39) in response to a 5 month resistance exercise program in pre-pubertal girls.
Our study population consists of Hispanic adolescents, thus, the studies by Goran’s group (8, 32, 43) in a similar population are of particular interest. In a first study, overweight adolescent Latino boys performed a resistance exercise program, 2 × 1 h/wk for 16 wks (32). Insulin sensitivity increased by 45%. Lean body mass also increased and body fat% (but not fat mass) decreased. In a later study, overweight adolescent Latino boys and girls were subjected to the same resistance exercise program with an additional diet component aiming at reducing sugar and increasing fiber intake (8). Surprisingly, in this study insulin sensitivity and body composition were completely unaffected. The investigators speculated that the diet intervention might have cancelled out the effect of the exercise. However, a post hoc analysis of the response to the diet intervention showed no difference between a control group, a diet alone group and the diet + strength training group (43).
A weakness of our study might be that we did not include a non-exercising control group. However, it would be ethically questionable to subject healthy adolescents to comparatively invasive metabolic studies without any intervention. Muscle mass and strength increases until adulthood (3). Since our participants were post pubertal and, thus, had passed their growth peak, it is unlikely that growth and maturation explain the increase in strength and muscle mass that was observed during the relatively short 12 wk exercise program. For the same reason, pubertal effects on insulin sensitivity are very unlikely.
Resistance exercise resulted in a small decrease (8%) in glucose production due to a decrease (12%) in glycogenolysis while gluconeogenesis remained unchanged. Most likely, the decrease in glucose production has limited clinical relevance in our normoglycemic adolescents with normal glucose tolerance. However, in diabetic adolescents, increased glucose production might result in hyperglycemia (10). Thus, one might speculate that in these individuals, resistance exercise could be a tool to reduce glucose production and subsequently hyperglycemia. The association between decreased glycogenolysis and increased hepatic insulin sensitivity confirms that glycogenolysis is more sensitive to insulin than gluconeogenesis, as suggested by Gastaldelli et al. (11).
Baseline leptin and hs-CRP concentrations were significantly higher compared to a previously studied group of lean adolescents of the same age (41). The higher CRP concentrations indicate a low grade whole body inflammation in obese adolescents. Data regarding the effect of resistance exercise on adiponectin, leptin and hs-CRP concentrations are limited (6, 18). Brooks et al. (6) found that adiponectin increased and CRP decreased in response to resistance exercise, while Klimcakova et. al. (18) reported unchanged adiponectin and hs-CRP but decreased leptin concentration. We observed no exercise induced changes in these parameters.
In conclusion: Resistance exercise might be an attractive alternative to aerobic exercise for obese adolescents. Increased strength, lean body mass and hepatic insulin sensitivity are important findings. However, the more comprehensive effects of aerobic exercise involving metabolic parameters, body composition and body fat distribution might have a greater potential to prevent obesity related illnesses (41, 42). Thus, a program combining resistance and aerobic exercise might be a viable strategy to achieve the positive effects of both types of exercise.
Acknowledgments
This work is a publication of the U.S. Department of Agriculture/Agricultural Research Service, Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, Texas. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. government.
We would like to thank all research participants for their time and commitment; Dr. Morey Haymond, Dr. Luisa Rodriguez, research nurses Amy Pontius, Cindy Bryant, Linda Peasant and Shawn Asphall, research coordinator Janette Gonzalez, dietitian Ann McMeans, exercise physiologists Tomas Green, Cynthia Newberry, Ashley Albright, Veronica Victorian and Jennifer Watts, the General Clinical Research Center staff at Texas Children’s Hospital for their invaluable help in conducting these studies. We thank Susan Sharma, Marcia Ekworomadu, Shaji Chacko and Dan Donaldson for excellent technical assistance.
Grant information:The study was supported by NICHD RO1 HD044609; Baylor General Clinical Research Center Grant MO1-RR-00188-34 and USDA Cooperative Agreement 6250-51000-046.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
The results of the present study do not constitute endorsement by ACSM
References
- 1.ACSM’s guidelines for exercise testing and prescription. 7th ed. Philadephia (Penn): 2006. American College of Sports Medicine; pp. 154–158. [DOI] [PubMed] [Google Scholar]
- 2.Avogaro A, Bristow JD, Bier DM, Cobelli C, Toffolo G. Stable-label intravenous glucose tolerance test minimal model. Diabetes. 1989;38(8):1048–1055. doi: 10.2337/diab.38.8.1048. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Or O, Rowland T. Pediatric Exercise Medicine From Physiologic Principles to Health Care Application. 1st ed. Champaign (IL): Human Kinetics; 2004. pp. 35–37. [Google Scholar]
- 4.Benson AC, Torode ME, Fiatarone Singh MA. The effect of high-intensity progressive resistance training on adiposity in children: a randomized controlled trial. Int J Obes (Lond) 2008;32(6):1016–1027. doi: 10.1038/ijo.2008.5. [DOI] [PubMed] [Google Scholar]
- 5.Benson AC, Torode ME, Singh MA. Muscular strength and cardiorespiratory fitness is associated with higher insulin sensitivity in children and adolescents. Int J Pediatr Obes. 2006;1(4):222–231. doi: 10.1080/17477160600962864. [DOI] [PubMed] [Google Scholar]
- 6.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci. 2007;4(1):19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chacko SK, Sunehag AL, Sharma S, Sauer PJ, Haymond MW. Measurement of gluconeogenesis using glucose fragments and mass spectrometry after ingestion of deuterium oxide. J Appl Physiol. 2008;104(4):944–951. doi: 10.1152/japplphysiol.00752.2007. [DOI] [PubMed] [Google Scholar]
- 8.Davis JN, Kelly LA, Lane CJ, Ventura EE, Byrd-Williams CE, Alexandar KA, et al. Randomized Control Trial to Improve Adiposity and Insulin Resistance in Overweight Latino Adolescents. Obesity (Silver Spring). (Epub ahead of print) 2009 doi: 10.1038/oby.2009.19. PMID 19247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 10.Gastaldelli A, Miyazaki Y, Pettiti M, Buzzigoli E, Mahankali S, Ferrannini E, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89(8):3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 11.Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quinones-Galvan A, Sironi AM, et al. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50(8):1807–1812. doi: 10.2337/diabetes.50.8.1807. [DOI] [PubMed] [Google Scholar]
- 12.Gutin B, Barbeau P, Owens S, Lemmon CR, Bauman M, Allison J, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75(5):818–826. doi: 10.1093/ajcn/75.5.818. [DOI] [PubMed] [Google Scholar]
- 13.Gutin B, Owens S. Role of exercise intervention in improving body fat distribution and risk profile in children. Am J Hum Biol. 1999;11(2):237–247. doi: 10.1002/(SICI)1520-6300(1999)11:2<237::AID-AJHB11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Heled Y, Shapiro Y, Shani Y, Moran DS, Langzam L, Barash V, et al. Physical exercise enhances hepatic insulin signaling and inhibits phosphoenolpyruvate carboxykinase activity in diabetes-prone Psammomys obesus. Metabolism. 2004;53(7):836–841. doi: 10.1016/j.metabol.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53(2):294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21(8):1353–1355. doi: 10.2337/diacare.21.8.1353. [DOI] [PubMed] [Google Scholar]
- 17.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49(5):677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 18.Klimcakova E, Polak J, Moro C, Hejnova J, Majercik M, Viguerie N, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91(12):5107–5112. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 19.Koopman R, Manders RJ, Jonkers RA, Hul GB, Kuipers H, van Loon LJ. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol. 2006;96(5):525–534. doi: 10.1007/s00421-005-0118-0. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 21.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents and young adults. J Clin Endocrinol Metab. 2010 February 15; doi: 10.1210/jc.2009-1913. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 23.McCambridge TM, Stricker PR. Strength training by children and adolescents. Pediatrics. 2008;121(4):835–840. doi: 10.1542/peds.2007-3790. [DOI] [PubMed] [Google Scholar]
- 24.McGuigan MR, Tatasciore M, Newton RU, Pettigrew S. Eight weeks of resistance training can significantly alter body composition in children who are overweight or obese. J Strength Cond Res. 2009;23(1):80–85. doi: 10.1519/jsc.0b013e3181876a56. [DOI] [PubMed] [Google Scholar]
- 25.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 26.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2–3):141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 27.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54(11):1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary VB, Marchett CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100(5):1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 30.Otten J, Pitzi Hellwig J, Meyers L. Dietary (DRI) Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006. pp. 82–84. [Google Scholar]
- 31.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 32.Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Salem GJ, Crespo NC, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 33.Sunehag AL, Toffolo G, Campioni M, Bier DM, Haymond MW. Effects of dietary macronutrient intake on insulin sensitivity and secretion and glucose and lipid metabolism in healthy, obese adolescents. J Clin Endocrinol Metab. 2005;90(8):4496–4502. doi: 10.1210/jc.2005-0626. [DOI] [PubMed] [Google Scholar]
- 34.Sunehag AL, Toffolo G, Treuth MS, Butte NF, Cobelli C, Bier DM, et al. Effects of dietary macronutrient content on glucose metabolism in children. J Clin Endocrinol Metab. 2002;87(11):5168–5178. doi: 10.1210/jc.2002-020674. [DOI] [PubMed] [Google Scholar]
- 35.Sunehag AL, Treuth MS, Toffolo G, Butte NF, Cobelli C, Bier DM, et al. Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: an evaluation of their reproducibility. Pediatr Res. 2001;50(1):115–123. doi: 10.1203/00006450-200107000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 Pt 1):E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 37.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 38.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57(2):367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 39.Treuth MS, Hunter GR, Figueroa-Colon R, Goran MI. Effects of strength training on intra-abdominal adipose tissue in obese prepubertal girls. Med Sci Sports Exerc. 1998;30(12):1738–1743. doi: 10.1097/00005768-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF. Metabolic adaptation to high-fat and high-carbohydrate diets in children and adolescents. Am J Clin Nutr. 2003;77(2):479–489. doi: 10.1093/ajcn/77.2.479. [DOI] [PubMed] [Google Scholar]
- 41.van der Heijden G, Toffolo G, Manesso E, Sauer PJJ, Sunehag AL. Aerobic Exercise Increases Peripheral and Hepatic Insulin Sensitivity in Sedentary Adolescents. J Clin Endocrinol Metab. 2009;94(11):4292–4299. doi: 10.1210/jc.2009-1379. E-pub Oct. 6, 2009 PMID 19808855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Heijden GJ, Wang ZJ, Sauer PJJ, Haymond MW, Rodriguez LM, Sunehag AL. A 12 Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity (Silver Spring) 2010;18:384–390. doi: 10.1038/oby.2009.274. E-pub Aug. 20, 2009 PMID 19696755. [DOI] [PubMed] [Google Scholar]
- 43.Ventura E, Davis J, Byrd-Williams C, Alexander K, McClain A, Lane CJ, et al. Reduction in risk factors for type 2 diabetes mellitus in response to a low-sugar, high-fiber dietary intervention in overweight Latino adolescents. Arch Pediatr Adolesc Med. 2009;163(4):320–327. doi: 10.1001/archpediatrics.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiggin M, Wilkinson K, Habetz S, Chorley J, Watson M. Percentile values of isokinetic peak torque in children six through thirteen years old. Pediatr Phys Ther. 2006;18(1):3–18. doi: 10.1097/01.pep.0000202097.76939.0e. [DOI] [PubMed] [Google Scholar]
- 45.Wilmore JH, Despres JP, Stanforth PR, Mandel S, Rice T, Gagnon J, et al. Alterations in body weight and composition consequent to 20 wk of endurance training: The HERITAGE family study. Am J Clin Nutr. 1999;70(3):346–352. doi: 10.1093/ajcn/70.3.346. [DOI] [PubMed] [Google Scholar]