Abstract
The development of nanotechnologies may lead to environmental release of nanomaterials that are potentially harmful to human health. Among the nanomaterials, multi walled carbon nanotubes (MWCNTs) are already commercialized in various products which can be in direct contact with populations. However, few studies address their potential toxicity. Although a few reports on the cytotoxicity of carbon nanotubes (CNTs) have been published, very little is known about their toxicity or genotoxicity in mammalian cells. We have for the first time compared the clastogenic/genotoxic potential of functionalized and non-functionalized MWCNTs in bone marrow cells of Swiss-Webster mice; using mitotic index (MI), chromosome aberrations (CA), micronuclei (MN) formation, and DNA damage in leukocytes as toxicologic endpoints. Six groups of five male mice, each weighing approximately 30 ± 2 g, were administered intraperitoneally, once a day for five days with doses of 0.25, 0.5, 0.75, mg/kg body weight (BW) of functionalized and non-functionalized MWCNTs. Four vehicle control groups (negative) and a positive control group (carbon black) were also made of 5 mice each. Chromosome and micronuclei from bone marrow cells and comet slides from leukocytes were examined following standard protocols. The results demonstrated that MWCNTs exposure significantly increased (p<0.05) the number of structural chromosomal aberrations, the frequency of micro-nucleated cells and the level of DNA damage, and decreased the mitotic index in treated groups compared to control groups. MWCNTs were shown to be toxic at sufficiently high concentrations, however purified functionalized MWCNTs had a higher clastogenic/genotoxic potential compared to non-functionalized form of MWCNT. The results of our study suggest that exposure to MWCNT has the potential to cause genetic damage. Hence, careful monitoring should be done with respect to designing/synthesing biocompatible carbon nanomaterials. Further characterization of their systemic toxicity, genotoxicity and carcinogenicity is also essential.
Keywords: multiwalled carbon nanotubes, bone marrow cells, leukocytes, Swiss-Webster mice, chromosomal aberrations, micronucleus formation, mitotic index, Comet assay, DNA damage
INTRODUCTION
Nanomaterials are part of an industrial revolution to develop lightweight but strong materials for a variety of purposes (Lam et al., 2004). Multi-walled carbon nanotubes (MWCNT's) are an important member of this class of materials because of their unique physicochemical properties and promises in technological applications (Iijima, 1991). They can be used in sensors, electronic devices, wastewater treatment and many other industrial applications. Importantly, broad biomedical uses of carbon nanotubes such as in drug delivery systems, bone cell growth and cancer treatment have been investigated (Yang et al., 2008). With the rapid advances in carbon nanotube-based new materials and technologies, there is a growing recognition that a fundamental understanding of the toxicological properties of carbon nanotubes is imperative (Warheit et al., 2007). However, the toxicity of carbon nanotubes is barely known when they are introduced into the blood circulation, as a result of their biomedical applications. The forecasted increase in manufacturing makes it likely that increasing human exposure will occur. As a result, carbon nanotubes (CNTs) are beginning to come under toxicological scrutiny, because the knowledge concerning their potential impacts on human health and the environment is still fragmentary (Lam et al., 2006; Donaldson et al., 2006).
With increasing interest in their potential toxicity, the adverse effects of engineered nanotubes are intensively being investigated. In recent years, many in vivo (Lam et al., 2004; Shvedova et al., 2003; Warheit et al., 2004; Takagi et al., 2008; Poland et al., 2008) and in vitro (Cui et al., 2005; Jia et al., 2005; Monteiro-Riviere et al., 2005; Tian et al., 2006; Sayes et al., 2006; Muller et al., 2008) studies have documented the potential adverse health effects associated with the exposure to carbonaceous nanomaterials. The bio-persistence, large aspect ratio and fibrogenic character of CNTs are important features that may cause adverse health effects. However, the available peer-reviewed toxicological data for CNT is rather divergent and sparse to assess their toxic effects to humans and laboratory animals. The reason for these discrepancies is not immediately evident, but may depend on experimental protocols and/or interferences with test system used (Colvin, 2003).
Many different forms of CNTs are found. They can be chemically modified and/or functionalized with either a hydroxyl or carboxyl or another nanomaterial. Non-functionalized or pristine single-walled CNTs can be visualized as a single sheet of graphite rolled-up in the form of a cylinder with seamless ends. Its diameter ranges from 0.4 nm to micrometer. Non-functionalized multi-walled CNTs consist of several single-walled CNTs stacked one inside another. Their diameter ranges up to 100 nm (Dresselhaus et al., 2001). These pristine CNTs are chemically inert and insoluble in aqueous solutions and therefore of little use in biological or medical applications. For many applications, CNTs are oxidized in strong acids to create hydroxyl groups and carboxyl groups (Liu et al., 1998) particularly in their ends, to which biomolecules or other nanomaterials can be, coupled (Bottini et al., 2005). These oxidized CNTs are much more readily dispersed in aqueous solutions and have been coupled to oligonucleotides, proteins or peptides (Bottini et al., 2006).
Interactions of unintentional anthropogenic nanomaterials with cells have been shown to modulate the expression of several cellular macromolecules. The most frequently affected macromolecules are those genes or proteins, which play a role in oxidative stress and DNA damage or injury to the immune system (Schins et al., 2002). Genotoxicity is expressed as varying types of DNA damage (DNA adduct, alkali labile sites, strand breaks) and mutations, ranging from gene to structural or numerical chromosome changes (aneuploidy and polyploidy) (Kirsch-Volders et al., 2002; Mateuca et al., 2006). The survival of the damaged cell will depend on the balance between the efficiency of the cellular protection and repair system (antioxidant defenses, base/nucleotide excision repair, mismatch or double strand break repair) and the processes leading to cell death (apoptosis or necrosis) (Muller et al., 2008).
Investigations of genotoxicity and cellular interactions of engineered nanomaterials and nanoparticles, manufactured on the low nanometer scale have been limited so far, and no experimental data have been published on the in vivo genotoxic/clastogenic potential of carbon nanotubes in bone-marrow cells especially using cytogenetic biomarkers and DNA damage in leukocytes. Studying cytogenetic biomarkers is very important as they represent the intermediate steps in the pathway from exposure to disease, in estimating the risk of cancer in populations. There is a strong association between cytogenetic changes induced by carcinogens/ mutagens and development of malignant and premalignant changes. Although the presence of cytogenetic markers themselves does not necessarily lead to adverse health outcomes, high levels of these markers apparently indicate that cells have been exposed to mutagens/carcinogens. However, such mutation events cause alterations in the activity or expression of growth control genes, which are the key steps in carcinogenesis (Patlolla et al., 2005; Bonassi et al., 2000).
The aim of the present study was to compare the genotoxic/clastogenic effects of functionalized and non-functionalized MWCNT in bone marrow cells of Swiss-Webster mice using mitotic index, structural chromosomal aberrations, micronuclei formation, and DNA damage in leukocytes as toxic endpoints. These endpoints were selected based on the fact that the bone-marrow assay for detecting chromosome aberrations, micronuclei formation and comet assay for assessing DNA damage are very sensitive and the technology/equipment needed to perform these assays is available in our laboratory.
MATERIAL AND METHODS
Multi-walled Carbon Nanotubes Characteristics
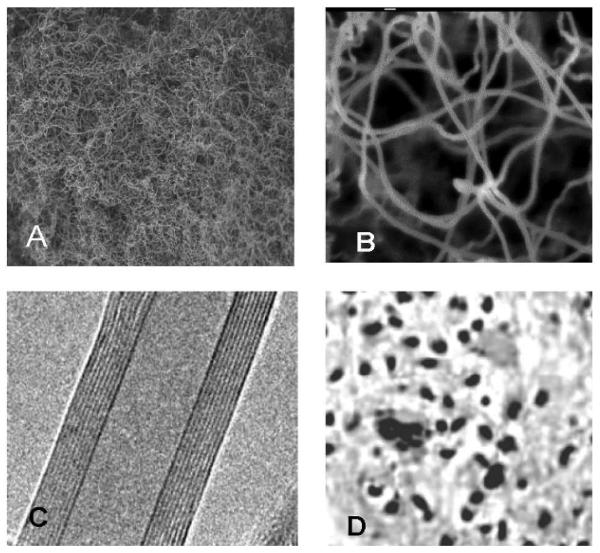
Multi-walled carbon nanotubes (MWCNTs) were synthesized by NanoLab Inc. (Newton MA, USA) by catalytic chemical vapor deposition (outer diameter of 15-30 nm, lengths of 15-20 μm, purity > 95%). After synthesis MWCNT were heated under argon (2L/min) at 2000 °C with 10 °C/min temperature up in order to extract catalyst (Fe-impurities). Purified MWCNTs (purity >95% by TGA) were submitted to a reflux process in sulfuric/nitric acid (8:1) to functionalize their surfaces. This process resulted in a large concentration of carboxyl (COOH) groups on the nanotube surface. After functionalization, these carboxylated nanotubes have 2-7% COOH by weight. Figures 1(A, B, C and D) represents the TEM structures of COOH functionalized nanotubes, and dispersed MWCNTs after sonication.
Fig. 1.
Transmission electron microscope (TEM) photographs of functionalized carbon nanotubes: A. low magnification, B: high magnification C: Inside multiwall nature of the carbon nanotube (10 nm inner diameter, 9 concentric walls, and a clear inner channel), D: Dispersed MWCNT after sonication.
Functionalized MWCNT morphology and size were determined by transmission electron microscopy (TEM). MWCNT were directly deposited on a TEM grid and allowed to dry. Samples were directly observed with a TEM. Surface areas were determined by the isothermal gas adsorption method BET (Brunauer et al., 1938) using a Micromeritics Flowsorb 2300 (Norcross, USA).
To characterize our system, we processed TEM observations of the carbon nanotubes (Fig. 1A - C). MWCNT suspension was correctly dispersed in 1% tween 80 + 0.9% sterile saline as surfactant during sonication. The length of the carbon nanotubes was up to 12 μm for the longer ones (60 minutes of sonication). The diameter was 11.5 nm after functionalization. Specific surface of carbon nanotube was measured by the classical BET method (Brunauer et al, 1938). The specific surfaces of long carbon nanotubes were 41 m2/g and 42m2/g for non-purified and purified form, respectively.
Chemicals
Amorphous carbon (carbon black: CB) was generously donated from a laboratory in India. Methanol, glacial acetic acid and superfrost microscope slides were purchased from Fischer-Scientific (Houston TX, USA). Potassium chloride solution (0.075M), Giemsa stain stock solution (0.4%), and May-Grunwald stain were obtained from Sigma-Aldrich (St. Louis MO, USA). Hanks Balanced Salt Solution was purchased from GIBCO (Grand Island NY, USA). Fetal Bovine Serum (FBS) was obtained from Hyclone (Logan UT, USA). Comet assay kit was purchased from Trevigen, Inc. (Gaithersburg MD, USA). 2′,7′-dichlorofluorescin diacetate (DCFH-DA) from Molecular Probes, Inc. USA.
Animal Maintenance
Healthy adult male Swiss-Webster mice (5-7 weeks of age, with average body weight (BW) of 30 ± 2 g) were used in this study. They were obtained from Charles River Laboratories (Wilmington MA, USA). The animals were randomly selected and housed in polycarbonate cages (five mice per cage) with steel wire tops and corn-cob bedding. They were maintained in a controlled atmosphere with a 12h:12h dark/light cycle, a temperature of 22 ± 2°C and 50-70% humidity with free access to pelleted feed and fresh tap water. The animals were supplied with dry food pellets commercially available from PMI Feeds Inc. (St. Louis MO, USA). They were allowed to acclimate for 10 days before treatment.
Dosing
MWCNTs were suspended and sonicated in a sterile 0.9% saline solution containing 1% Tween-80 (Muller et al., 2005) and were dispersed using an ultrasonic liquid processor (Misonix, Long Island, NY) at 4° C and 30% amplitude with pulse 1 sec on and 1 sec off during 30 min for long MWCNT. This suspension showed a majority of MWCNT aggregates with a hydrodynamic diameter of ~1 μm. The concentration of the suspension was 0.5 mg/ml. Fifty-five mice were randomly divided into eleven groups, five for each group. Six groups were experimental groups, three for functionalized and three for non-functionalized MWCNT. One group was chosen as positive control (carbon black, CB) and the other four groups were used as solvent controls (negative). Functionalized and non-functionalized MWCNT suspension was administered intraperitoneally to animals at the doses of 0.25, 0.5, and 0.75 mg/kg BW, one dose per 24 h given for 5 days. Intraperitoneal administration is not the natural route of exposure, however; it is the most commonly used method to study the toxicity of chemicals in bone marrow cells as it tends to maximize chemical exposure to target cells. Treatment by multiple injections was done for two reasons; firstly from pharmacological evidence it indicates the necessity for multiple injections to obtain required doses to the bone marrow (Preston et al., 1987), and secondly in order to induce toxic effects in rodents very high doses are required.
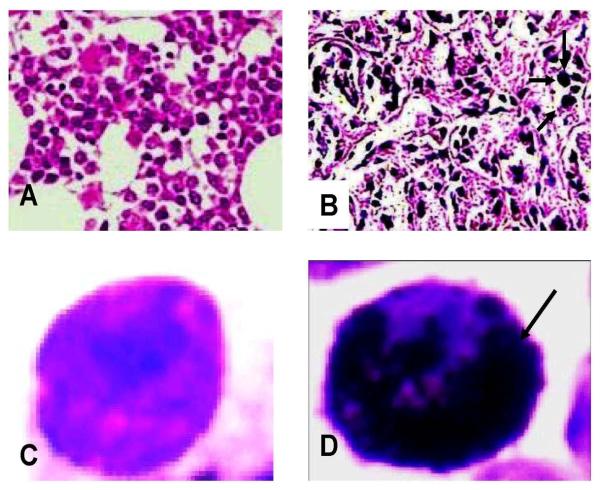
Each mouse received a total of five doses at 24 h intervals. Saline (0.9%), 1% tween-80, a mixture of 0.9% saline and 1% tween-80, carbon black (CB) and dimethyl formamide (dmf) were administered to the 5 animals each of control groups in the same manner as in the treatment groups. The same dose regimes were used in both chromosome aberration (CA) and micronuclei (MN), and comet assays. Figure 2 (A-D) shows the microphotographs of bone marrow cells.
Fig. 2.
A - Photograph (light compound microscope, magnification 400 x) of normal bone marrow cells; B - Bone marrow cells showing uptake of multi-walled carbon nanotube; CNormal individual bone marrow cell after hypotonic treatment; D- Individual bone-marrow cell after hypotonic treatment showing uptake of carbon nanotube.
The local Ethics committee for animal experiments [Institutional Animal Care and Use Committee] at Jackson State University, Jackson, MS, (USA) approved this study. Procedures involving the animals and their care conformed to the institutional guidelines, in compliance with national and international laws and guidelines for the use of animals in biomedical research (Giles et al., 1987).
Preparation of Homogenate
At the end of the 5-day exposure to functionalized and non-functionalized MWCNTs, one marrow was excised under anesthesia. The bone marrow was washed thoroughly in ice-cold physiological saline and weighed. A 10% homogenate of each tissue was prepared separately in 0.05 M phosphate buffer (pH 7.4) containing 0.1 mM EDTA using a motor driven Teflon-pestle homogenizer (Fischer), followed by sonication (Branson sonifer), and centrifugation at 500 x g for 10 min at 4° C. The supernatant was aspirated and centrifuged at 2000 x g for 60 min at 4° C. The cellular fraction obtained after centrifugation was called ‘homogenate’ and used for the assays.
Reactive Oxygen Species (ROS) Detection
ROS production was quantified by the 2′, 7′- dichlorofluorescin diacetate (DCFH-DA) method (Lawler et al., 2003). DCFH-DA passively enters the cell where it reacts with ROS to form highly fluorescent compound dichlorofluorescein (DCF). Aliquot of homogenates were centrifuged at 1000x g for 10 min (4° C). The supernatants were re-centrifuged at 1000x g for 20 min at 4° C, and then the pellets were re-suspended. The DCFH-DA solution with the final concentration of 50 μM and re-suspension were incubated for 30 min at 37° C. Fluorescence of the samples was monitored at an excitation wavelength of 485 nm and an emission wavelength of 538 nm using Fluorescence plate reader (Turner Biosystems, Sunnyvale, CA, USA).
Chromosome Aberration Assay
The mice were sacrificed by cervical dislocation 24h after administration of the last dose for chromosome aberration assay. Cytogenetic analysis was performed in bone marrow cells following the protocol of Preston et al (1987), with slight modifications. Experimental animals were injected (i.p.) with colchicine (2mg/kg) 1.5 h prior to sacrifice. Both femora were dissected out and cleaned of any adhering muscle. Bone-marrow cells were collected from both femora by flushing in KCL (0.075 M, at 37 ° C) and incubated at 37 ° C for 25 min. Collected cells were centrifuged at 2000 x g for 10 min, and fixed in aceto-methanol (acetic acid:methanol, 1:3, v/v). Centrifugation and fixation were repeated five times at an interval of 20 min. The cells were resuspended in a small volume of fixative, dropped onto chilled slides, flame-dried and stained the following day with freshly prepared 2% Giemsa stain for 3-5 min, and washed in distilled water to remove excess stain.
Mitotic Index Determination
The mitotic index (number of dividing cells/total number of cells x 100) was used to determine the rate of cell division. The slides prepared for the assessment of chromosomal aberrations were also used for calculating the mitotic index. Randomly selected views on the slides were monitored to determine the number of dividing cells (metaphase stage) and the total number of cells. At least 1000 cells were examined in each preparation.
Micronucleus Test
Mice were sacrificed by cervical dislocation 24 h after the last treatment. The frequency of micronucleated cells in femoral bone marrow was evaluated according to the procedure of Schmid (1976), with slight modifications as reported by Agarwal and Chauhan (1993). The bone marrow was flushed out from both femora using 2ml of Fetal Calf Serum and Hanks Balanced Salt Solution (3:1) and centrifuged at 2000x g for 10 min. The supernatant was discarded. Evenly spread bone marrow smears were stained using the May-Grunwald and Giemsa protocol.
Scoring of Slides
Bone marrow preparations for the analysis of chromosome aberrations in metaphase cells were obtained using the technique by Preston et al (1987). The slides were stained with 2% Giemsa. Well-spread metaphases presenting 40 ± 1 chromosomes were analyzed. One hundred metaphases per animal were screened to a total of 500 metaphases for each treatment and control to obtain the total number of chromosomal aberrations. The mitotic indices were obtained by counting the number of mitotic cells in 1000 cells per animal to a total of 5000 cells per treatment and control. The mitotic index was calculated as the ratio of the number of dividing cells to the total number of cells, multiplied by 100. A total of 5000 cells/treatment were scored, on coded slides to evaluate the frequency of micronucleated cells in bone marrow under an Olympus BX41 microscope.
Comet Assay
Single Cell gel electrophoresis (SCGE) or comet assay was performed using Singh et al. (1988) method with slight modifications. Mice lymphocytes were isolated and re-suspended in phosphate buffer saline. Following isolation the cells were mixed with 0.4% Trypan blue solution. After 15-20 min cells were counted and checked for viability. The remaining cells were immediately used for single-cell gel electrophoresis. In a 2 ml centrifuge tube, 50 μl of the lymphocyte suspension and 500 μl of low melting agarose were mixed and 75 μl of the suspension pipetted onto a pre-warmed comet-slide. The slides were placed flat in the dark at 4° C for 10 min for the mixture to solidify. The slides were then placed in pre-chilled lysing solution at 4° C for 1 hr. Slides were removed from lysing solution, tapped on a paper towel to remove any excess lysis solution and immersed in alkaline solution (pH =13) for 45 min at room temperature in the dark. The slides were washed twice for 5 min with Tris-Borate (TBE) buffer. Next the slides were electrophoresed at low voltage (300 mA, 25V) for 20 min. Slides were removed from the electrophoresis unit after the designated time, tapped to remove excess tris-borate buffer and immediately placed in 70% ethanol for 5 min and air-dried overnight at room-temperature. After overnight drying the slides were stained with SYBR-Green designed for comet assay and allowed to dry overnight. All the steps of the comet assay were conducted under yellow lamp in the dark to prevent additional DNA damage. The slides were read using an automated epifluorescence microscope and computer based DNA damage analysis software from Loats & Associates (Westminster, MD). The data were based on 100 randomly selected cells per sample, i.e., 50 cells were from each of the two replicate slides. Percent DNA in the tail was selected as an indicator of DNA damage.
Statistical Analysis
Data were evaluated by ANOVA (one-way) with statistical significance assessed at p ≤ 0.05 using SAS for Windows 2003 package program and all the values were reported as means ± SDs.
RESULTS
ROS Detection in Bone Marrow Cells
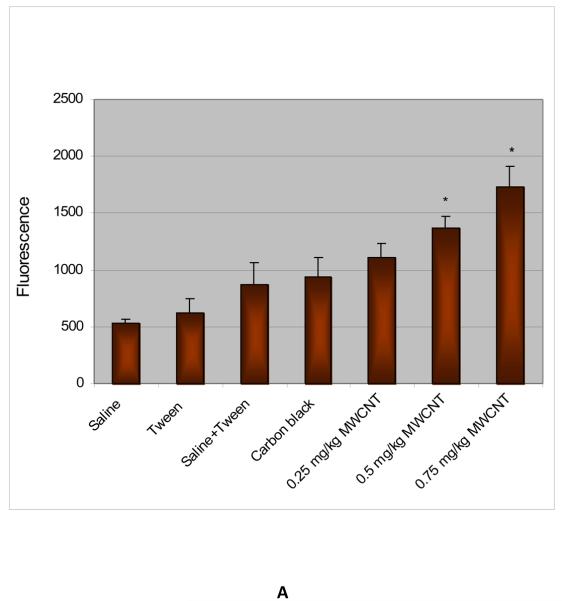
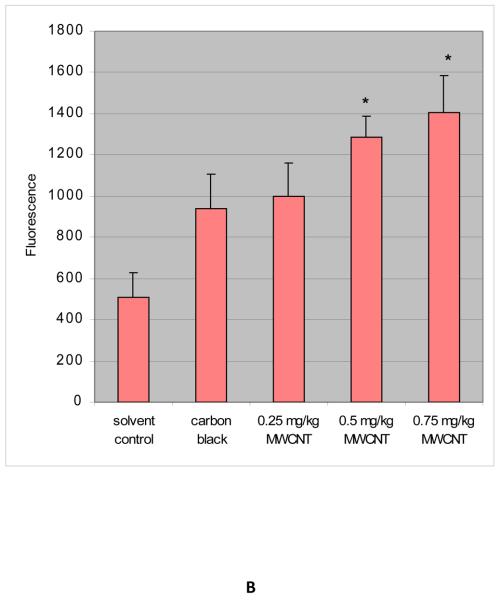
ROS were determined in the bone marrow homogenates in control and treatment groups after administration of functionalized and non-functionalized MWCNT to mice for 5 days. The administration of MWCNT to mice significantly enhanced the ROS level at three tested doses as compared to the control animals and increases were dose-dependent. The mean values of fluorescence were 535.6 ± 33.0, 618.9 ± 124.8, 870.8 ± 193.4, 938.4 ± 169.9, 1,104.6 ± 125.0, 1,365.3 ± 102.3, and 1,734.6 ± 180.2 for saline (0.9%), 1% tween - 80, saline (0.9%) + tween-80 (0.25%), positive control carbon black (CB), and functionalized MWCNT doses of 0.25, 0.50 and 0.75 mg/kg BW, respectively. Non-functionalized MWCNT exposure also induced ROS in a statistically significant manner compared to controls. The mean values of fluorescence induced were 505.4 ± 124.8, 938.0 ± 169.9, 1,000.5 ± 158.8, 1,285.0 ± 102.3, and 1,406.2 ± 180.2 for solvent control (dimethyl formamide), positive control carbon black (CB), and non-functionalized MWCNT doses of 0.25, 0.5 and 0.75 mg/kg BW, respectively. However, the level of ROS in non-functionalized exposed mice was lower compared to functionalized at all three dose levels. Figure 3 (A & B) represents the results of ROS analysis.
Fig. 3.
A- ROS induction in bone marrow cells exposed to functionalized MWCNT; B- ROS induction in bone marrow cells exposed to non-functionalized MWCNT. Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
Chromosome Aberrations
The metaphase analysis of bone marrow cells revealed various types of chromosomal aberrations, which consisted of chromatid and isochromatid types of gaps, breaks, unions, and fragments. Chromatid gaps and breaks were noted to be more frequent than others (Table 1 & 2). Relatively higher frequencies of gaps were observed for all the doses of functionalized and non-functionalized MWCNTs tested. However functionalized MWCNT was found to show more clastogencity/genotoxicity than the non-functionalized form. A quantitative assessment of the distribution of breaks and gaps revealed that the distal regions of the chromosomes were more vulnerable to the effects of MWCNT.
Table 1.
Mitotic Index and different types of chromosome aberrations, in bone-marrow cells of Swiss-Webster mice exposed to functionalized MWCNT.
| Dose (mg/Kg) | Total metaphases Examined |
MI% | Chromatid |
Chromosome |
Fragment | Union | Total SCA | Cells with SCAa (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Gap | Break | Gap | Break | |||||||
| Saline (0.9%) | 500 | 11.98 | 3 | 2 | 2 | 1 | 1 | 1 | 10 | 2.0 ± 0.9a |
| 1% Tween-20 | 500 | 11.5 | 2 | 2 | 3 | 2 | 2 | 1 | 12 | 2.4 ± 0.6a |
| Saline + Tween | 500 | 10.06 | 3 | 2 | 5 | 3 | 3 | 2 | 18 | 3.6 + 0.9a |
| Carbon Black (CB) | 500 | 7.12 | 6 | 3 | 7 | 2 | 2 | 2 | 22 | 4.4 ± 1.73a |
| 0.25 | 500 | 4.5 | 7 | 3 | 8 | 4 | 5 | 3 | 30 | 6.0 ± 3.7a |
| 0.5 | 500 | 2.88 | 9 | 6 | 8 | 3 | 15 | 5 | 46 | 9.2 ± 1.5a |
| 0.75 | 500 | 2.12 | 16 | 6 | 13 | 10 | 21 | 4 | 70 | 14.0 ± 4.2a |
One hundred cells were analyzed per animal, to a total of 500 metaphase cells/treatment, CB: carbon black, SCA: structural chromosomal aberrations; MI: mitotic index
Standard Deviation (S.D)
Table 2.
Mitotic Index and different types of chromosome aberrations, in bone-marrow cells of Swiss-Webster mice exposed to non-functionalized MWCNT.
| Dose (mg/Kg) | Total metaphases Examined |
MI% | Chromatid |
Chromosome |
Fragment | Union | Total SCA | Cells with SCAa (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Gap | Break | Gap | Break | |||||||
| Solvent controlb | 500 | 10.58 | 3 | 2 | 2 | 1 | 1 | 1 | 10 | 2.0 ± 0.9a |
| Carbon Black (CB) | 500 | 7.12 | 6 | 3 | 7 | 2 | 2 | 2 | 22 | 4.4 + 1.73a |
| 0.25 | 500 | 5.8 | 5 | 3 | 4 | 3 | 7 | 4 | 26 | 5.2 ± 1.5a |
| 0.5 | 500 | 5.4 | 6 | 6 | 8 | 3 | 8 | 3 | 34 | 6.8 ± 3.1a |
| 0.75 | 500 | 3.2 | 10 | 6 | 12 | 10 | 14 | 4 | 56 | 11.2 ± 4.7a |
One hundred cells were analyzed per animal, to a total of 500 metaphase cells/treatment, SCA: structural chromosomal aberrations; MI: mitotic index.
Standard Deviation (S.D).
dimethylforamide :solvent control
The results of the chromosomal aberration assay in bone marrow cells after intraperitoneal treatment with functionalized and non-functionalized MWCNT are summarized in Table 1 & 2. The frequency of chromosomal aberrations (CA) also increased with increasing doses of functionalized MWCNT, and statistically significant differences (p<0.05) from the control were observed. The mean percentages of the induced CAs were 2.0 ± 0.9 %, 2.4 ± 0.6, 3.6 ± 0.9, 4.4 ±1.73, 6.0 ± 3.7, 9.2 ± 1.5%, and 14.0 ± 4.2% for saline (0.9%), 1% tween - 80, saline (0.9%) + tween-80 (0.25%) positive control carbon black (CB), and functionalized MWCNT doses of 0.25, 0.5 and 0.75 mg/kg BW, respectively. Non-functionalized MWCNT exposure also induced CA in statistically significant manner compared to controls, however functionalized MWCNT were found to be slightly more toxic than non-functionalized form. The mean percentages of the induced CAs were 2.0 ± 0.9%, 3.5 ± 1.5%, 5.2 ± 1.5%, 6.8 ± 3.1% and 11.2 ± 4.7% for solvent control (dimethyl formamide), positive control carbon black (CB), and non-functionalized MWCNT doses of 0.25, 0.5 and 0.75 mg/kg BW, respectively.
Mitotic Index
The mitotic index was used to determine the rate of cell division. The slides prepared for the assessment of chromosomal aberrations were used for calculating the mitotic index. It was found that the mitotic index significantly decreased as the MWCNT doses increased. Mitotic indices of 11.98 ± 1.5%, 11.5 ± 0.56%, 10.06 ± 1.65%, 7.12 ± 0.79%, 4.55 ± 0.83, 2.88 ± 0.24 and 2.12 ± 0.26% were recorded for 0.9% saline, 1% tween-80, 0.9% saline + 1% tween-80, positive control carbon black (CB) and functionalized MWCNT doses of 0.25, 0.5 and 0.75 mg/kg BW, respectively (Table 1). Non-functionalized MWCNT exposure was shown to decrease the mitotic index in a dose-dependent manner. Mitotic indices of non-functionalized MWCNT are summarized in Table 2. Our study demonstrated that the decrease in MI was higher in functionalized MWCNT than the non-functionalized form.
Micronuclei Induction
The micronuclei frequencies in bone marrow cells after intraperitoneal treatment with MWCNT are summarized in Table 3 and 4. MWCNT induced a dose-related increase in micronuclei frequency, and significant differences (p<0.05) from the control were observed. The mean percentages of micro-nucleated cells were 2.33 ± 0.58%, 2.66 ± 2.08%, 4.0 ± 2.0%, 4.33 ± 1.15%, 6.33 ± 2.51, 10.6 ± 3.05%, and 13.0 ± 3.61% for 0.9% saline, 1% tween-80, 0.9% saline + 1% tween-80 (0.25%), positive control carbon black (CB), and 0.25, 0.5, 0.75 mg/kg BW of functionalized MWCNT, respectively. Non-functionalized MWCNTs were also found to induce a dose-related increase in the frequency of micronuclei induction which were statistically significant (p<0.05) compared to control. The mean percentages of micro-nucleated cells were 2.0 ± 1.0, 3.0 ± 1.73, 6.3 ± 2.52, and 9.0 ± 2.0 for solvent control (dmf) and non-functionalized MWCNT doses of 0.25, 0.5 and 0.75 mg/kg BW respectively. However, functionalized MWCNT were found to induce more micronuclei than the non-functionalized form.
Table 3.
Functionalized MWCNT induced micronuclei formation in the bone marrow cells of Swiss-Webster mice.
| Dose | Mice Number | Fixation time (h) | MN/1000 cellsa |
|---|---|---|---|
| Saline (0.9%) | 1 | 3 | |
| 2 | 2 | ||
| 3 | 30 | 2 | |
| 2.33 ± 0.58b | |||
| 1%tween-80 | 4 | 2 | |
| 5 | 5 | ||
| 6 | 30 | 1 | |
| 2.66 ± 2.08b | |||
| [S + T]c | 7 | 4 | |
| 8 | 2 | ||
| 9 | 30 | 6 | |
| 4.0 ± 2.0b | |||
| Carbon black | 10 | 5 | |
| 11 | 3 | ||
| 12 | 30 | 5 | |
| 4.33 ± 1.15b | |||
| 0.25 | 13 | 6 | |
| 14 | 4 | ||
| 15 | 30 | 9 | |
| 6.33 ± 2.51b | |||
| 0.5 | 16 | 10 | |
| 17 | 8 | ||
| 18 | 30 | 14 | |
| 10.6 ± 3.05b | |||
| 0.75 | 19 | 12 | |
| 20 | 17 | ||
| 21 | 30 | 10 | |
| 13.0 ± 3.61b |
Micronuclei
Mean ± S.D.
Suspension of 0.9% saline + 1% tween-80
Table 4.
Non-functionalized MWCNT induced micronuclei formation in the bone marrow cells of Swiss-Webster mice.
| Dose | Mice Number | Fixation time (h) | MN/1000 cellsa |
|---|---|---|---|
| 0 | 1 | 2 | |
| 2 | 1 | ||
| 3 | 30 | 3 | |
| 2.0 ± 1.0b | |||
| 0.25 | 4 | 4 | |
| 5 | 4 | ||
| 6 | 30 | 1 | |
| 3.0 ± 1.73b | |||
| 0.5 | 7 | 9 | |
| 8 | 6 | ||
| 9 | 30 | 4 | |
| 6.3 ± 2.52b | |||
| 0.75 | 10 | 11 | |
| 11 | 9 | ||
| 12 | 30 | 7 | |
| 9 ± 2.0b |
Micronuclei
Mean ± S.D.
DNA Damage
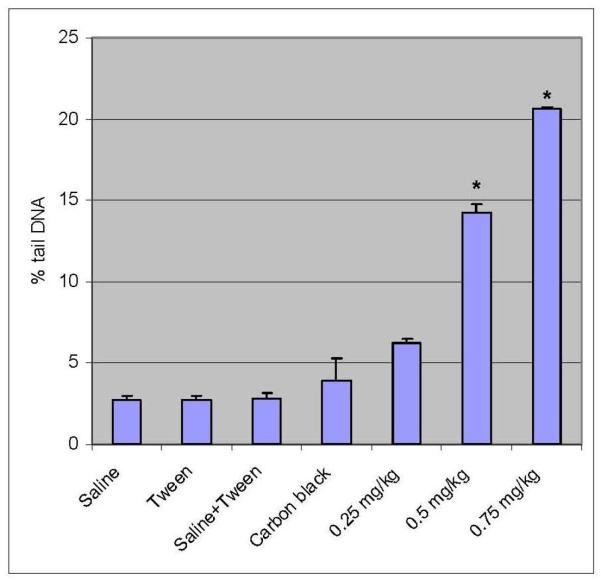
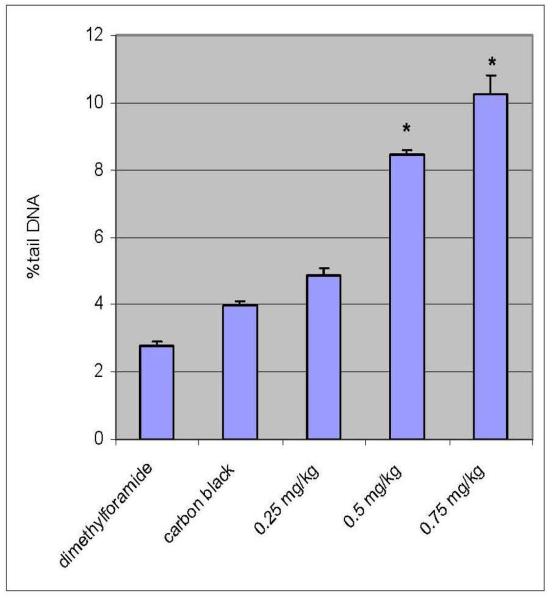
Percent tail DNA is an important parameter in evaluating the DNA damage. All the doses of functionalized and non functionalized MWCNT induced statistically significant increase in percent tail DNA [6.25 ± 0.251 – 20.65 ± 0.05% - Functionalized]; [4.85 ± 0.23 – 10.24 ± 0.57 % – non-functionalized] indicating DNA damage when compared with controls [2.69 ± 0.3; 2.78 ± 0.14%]. Maximum increase in mean comet tail-length was observed at 0.75 mg/kg (BW) of functionalized MWCNT after 5 days post-treatment [20.65%]. Both functionalized and non-functionalized MWCNT showed a clear dose-dependent increase in the mean comet tail length from 0.25 to 0.75 mg/kg BW. However, the DNA damage in mice leukocytes exposed to functionalized MWCNT was found to be 50% more compared to non-functionalized MWCNT. The results of DNA damage are illustrated in Figures 4, 5, 6, and 7, respectively.
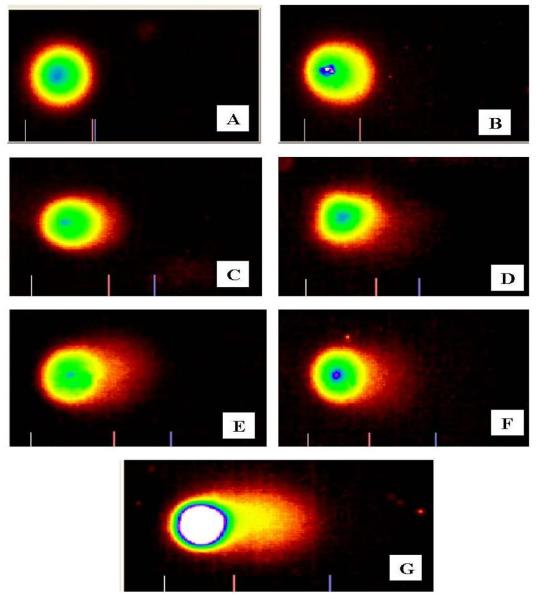
Fig. 4.
Comet Assay images of peripheral blood leukocytes of Swiss Webster mice exposed to functionalized MWCNTs. Representative photographs of comets from A- Saline; B-Tween; C-Saline + Tween; D − Carbon black (0.75 mg/kg); E-0.25 mg/kg; F-0.5 mg/kg and G- 0.75 mg/kg.
Fig. 5.
Percent tail DNA in peripheral blood leucocytes exposed to functionalized MWCNT of 0.25mg/kg, 0.5 mg/kg, and 0.75 mg/kg. Each experiment was done in triplicate. Data represents mean ± SD. Statistical significance (p<0.05) is depicted as (*).
Fig. 6.
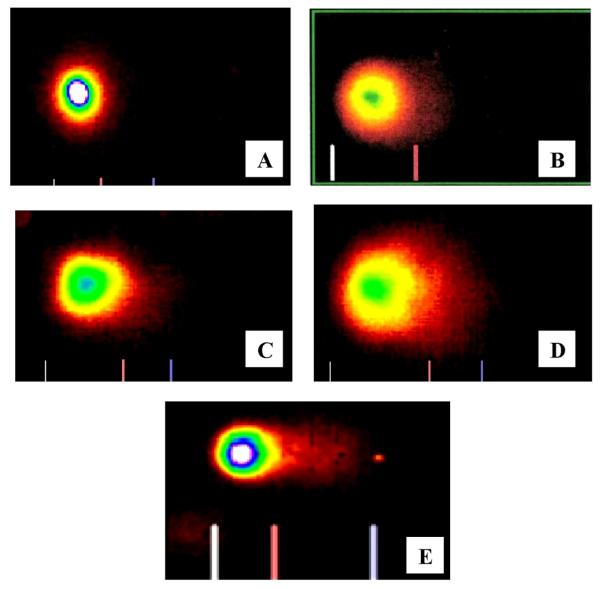
Representative Comet assay images of peripheral blood leukocytes of Swiss Webster mice exposed to non-functionalized MWCNTs. A- dimethylforamide; B- Carbon black (CB); C-0.25 mg/kg; D-0.5 mg/kg; and E-0.75 mg/kg.
Fig. 7.
Percent tail DNA in peripheral blood leucocytes exposed to non-functionalized MWCNTs of 0.25mg/kg, 0.5 mg/kg, and 0.75 mg/kg. Each experiment was done in triplicate. Data represents as mean ± SD. Statistical significance (p<0.05) is depicted as (*).
DISCUSSION
In the present study, we have compared the clastogenic/genotoxic effects of functionalized and non-functionalized MWCNTs in mice using CA, MN and comet assays. The results clearly indicated a significant increase of cytogenetic damage in the bone-marrow cells, due to exposure to MWCNTs through intraperitoneal administration. The percentages of aberrant cells in the bone-marrow of Swiss-Webster mice exposed to MWCNT showed statistically significant increases as compared to the controls. However, purified functionalized MWCNT was found to be more toxic than the non-functionalized MWCNT. Out of all types of aberrations, chromatid breaks isochromatid breaks, acentric fragments, minutes, translocations and gaps were the predominant forms of CA observed. Chromosome type aberrations such as dicentrics were also observed. We have noted a decrease in the mean mitotic index values in MWCNT-exposed groups as compared to the controls. This could be due to a slower progression of cells from S (DNA synthesis) phase to M (mitosis) phase of the cell cycle as a result of MWCNT exposure. Although it is most likely that this impairment in cell cycle progression is associated with MWCNT toxicity, further experiments are needed to elucidate the biochemical mechanisms involved. At present, there are no published studies assessing the effect of MWCNTs on mitotic index in biological systems. The results from our study showed that there was a statistically significant difference in the frequencies of MN induction and percent tail DNA between functionalized and non-functionalized MWCNT. However, the frequency of chromosomal aberrations did not show a statistically significant difference between functionalized and non-functionalized MWCNT.
While investigating the mechanisms of nanomaterials-induced genotoxicity, several reviews [Schins et al., 2002; Knaapen et al., 2004) have reported the theory of primary versus secondary genotoxicity. When it is directly related to the exposure of the substance, genotoxicity is referred to as primary. However, secondary genotoxicity is the result of the substance interacting with cells or tissues and releasing factors, which cause the adverse effects, such as inflammation and oxidative stress. It is generally accepted that some fibres (e.g. asbestos) and isometric particles (e.g silica) can cause cancer in humans and/or experimental animals through the induction of genotoxic events in cells (Muller et al., 2008). Above all, for any adverse effect to occur, the number of such fibres must reach a sufficient level to cause activation of inflammatory cells, genotoxicity, fibrosis and cancer in the target tissue. A superficial resemblance between nanomaterials such as CNTs and asbestos has challenged scientists to assess whether fibre-shaped nanoparticles present a unique health risk (Poland et al., 2008). Ultrafine- and nano-particles represent a new cause of concern because they generally appear more toxic than their micrometric counterparts (Oberdorster et al., 2005) and there is a lack of information concerning their potential genotoxic activity. The genotoxic activity of ultrafine titanium dioxide or ultrafine crystalline silica was explored in a limited number of in vitro studies. Rahman et al (2002) reported an induction of MN and apoptosis in hamster fibroblasts exposed to ultrafine titanium dioxide. They suggested that clastogenic events are involved in the formation of these MN. Consistent with this mechanism, significant genotoxicity was shown with comet, MN and hypoxanthine phosphoribosyl transferase assays in human lymphoblastoid cells exposed to ultrafine titanium dioxide (Wang et al., 2007). Recently, it was also demonstrated that crystalline silica exerted a genotoxic effect in human lymphoblastoid cells as reflected by the induction of MN and gene mutations (Wang et al., 2007).
The present study provides the first comparative evidence of the clastogenic/genotoxic potential of functionalized and non-functionalized MWCNTs in bone-marrow cells of Swiss-Webster mice. In vivo, we observed a dose-dependent increase of CA and MN in bone marrow cells, which could be ascribed to primary or secondary genotoxicity. We have demonstrated in our present study that both types of MWCNT induced intracellular reactive oxygen species (ROS) in the bone marrow cells of mice. DCF fluorescence intensity statistically increased after 5 days exposure to all examined MWCNT doses compared to the controls. Since we had demonstrated in this study that MWCNT induced ROS in the bone marrow cells, we could not exclude the implication of secondary genotoxicity mechanisms. Several in vitro and in vivo studies have shown that CNTs exhibit greater genotoxicity than any other nanoparticles which elicited more oxidative stress (Nel et al., 2006; Lindberg et al., 2009; Yang et al., 2009). Due to its fibrogenic nature, CNTs might penetrate into cell nucleus through nucleopores, and then destruct the DNA double helix (Pantarotto et al., 2004). Several hypotheses can be suggested to account for the clastogenic/genotoxic effects of MWCNT, including the formation of adduct and/or damage at the level of DNA or chromosomes.
In the present study MWCNT exposure caused a significant increase in tail DNA indicative of DNA damage at 5 days post-treatment when compared to controls. Our results are in accordance with those of Muller et al (2008); Yang et al., (2009), and Zhu et al., (2007) reporting a dose-dependent DNA damage exposed to MWCNTs in epithelial cells, mouse embryonic stem cells and mouse embryo fibroblasts cells. A direct interaction between the particles and the genetic material is also possible. Li et al (2005) has reported such possibilities suggesting that CNTs are efficient in interacting with biomolecules with similar dimensions such as DNA. Particles may also activate cells to enhance their intracellular production of ROS, of which the stable and diffusible forms such as hydrogen peroxide or lipid peroxidation intermediates could hit nuclear DNA (Schins et al., 2007; Grabinski et al., 2007). Several potential mechanisms can contribute to explain the aneugenic effect of MWCNTs, including a physical interaction with components of the mitotic spindle during cell division or the interaction with proteins directly or indirectly involved in chromosome segregation (e.g. tubulin, actin) (Muller et al., 2008).
Furthermore, our results demonstrated that more hydrophobic non-functionalized or pristine CNTs appear to be less toxic than the functionalized/oxidized CNTs in inducing genotoxic/clastogenic effects in bone-marrow cells. The increased genotoxicity/clastogenicity of functionalized CNTs which are considered better suited for biological applications, may well be because they are better dispersed in aqueous solution and therefore reach higher concentrations of free CNTs at similar weight per volume values. Similar findings were reported in in vitro studies using human T-lymphocytes cells where the non-functionalized or pristine form was found to be less toxic than functionalized or oxidized CNTs (Bottini et al., 2006). Significantly, we find that the physical form of carbon has a major impact on toxicity. CNTs are more toxic than similar chemical amounts of carbon in amorphous carbon black, which is quite non-toxic even at the highest concentration (0.75mg/kg). Thus, the molecular structure and topology is essential for the clastogenicity/genotoxicity of carbonaceous nanomaterials. Our results suggest that MWCNTs indeed can cause genetic damage in a dose-dependent manner. Our results are also in support of findings by Tian et al (2006) and Bottini et al (2006) that besides other factors of surface chemistry, functionalization and refinement of carbon-based nanomaterial play a significant role in the induction of clastogenicity in the target system. Our studies do not imply that CNTs should be abandoned for biological or medical purposes. However, our proposed mechanism of toxicity of these nanomaterials based on their surface properties can assist materials scientists to design/synthesize biocompatible materials. It may also assist toxicologists to further characterize clastogenicity/genotoxicity of dispersed carbon nanomaterials in in vitro and in vivo studies. Since nanotechnology is entering into large-scale use, health and safety issues of CNTs should be promptly addressed (Warheit et al., 2007; Poland et al, 2008). Therefore, further toxicological studies in vivo have to be developed for evaluating hazards of occupational or environmental exposure to nanomaterials
Acknowledgement
This research was supported in part by a grant from the Air Forces Research Laboratory/Wright Patterson AFB (Grant No. FA8650-07-1-6851) and in part by a grant from National Institutes of Health-RCMI Center for Environmental Health (Grant No. 5G12RR01349-12) at Jackson State University.
References
- Agarwal DK, Chauhan LKS. An improved chemical substitute for fetal calf serum for the micronucleus test. Biotechnol Histochem. 1993;68:187–188. doi: 10.3109/10520299309104695. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Hagmar L, Stromberg U, Montagud AH, Tinnerberg H, Forni A, Heikkila P, Wanders S, Wilhardt P, Hansteen IL, Knudsen LE, Norppa H. Chromosomal aberrations in lymphocytes predict human cancer independently of exposure to carcinogens. Cancer Res. 2000;60:1619–1625. [PubMed] [Google Scholar]
- Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, Bergamaschi A, Mustelin T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol.Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Bottini M, Tautz L, Huynh H, Monosov E, Bottini N, Bellucci S, Mustelin T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005:758–760. doi: 10.1039/b412876a. [DOI] [PubMed] [Google Scholar]
- Brunauer SP, Emmett H, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60(2):309–319. [Google Scholar]
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single-wall carbon nanotubes on human HEK293 cells. Toxicol. Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon Nanotubes: A Review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006;92(1):5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- Dresselhans MS, Dresselhaus G, Avouris P, editors. Carbon nanotubes: Synthesis, structure, properties and applications. Springer; Berlin: 2001. [Google Scholar]
- Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemost. 1987;58(4):1078–1084. [PubMed] [Google Scholar]
- Grabinski C, Hussain S, Lafdi K, Braydich-Stolle L, Schlager J. Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon. 2007;45:2828–2835. [Google Scholar]
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–59. [Google Scholar]
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multiwall nantube, and fullerene. Environ. Sci. Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A Review of Carbon Nanotube Toxicity and Assessment of Potential Occupational and Environmental Health Risks. Critical Reviews in Toxicol. 2006;36(3):189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupt antioxidant capacity in skeletal muscle. Free Radical Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Li S, He P, Dong J, Guo Z, Dai L. DNA-directed self-assembling of carbon nanotubes. J. Am. Chem. Soc. 2005;127(1):14–15. doi: 10.1021/ja0446045. [DOI] [PubMed] [Google Scholar]
- Lindberg HK, Falck GC, Suhonen S, Vippola M, Vanhala E, Catalán J, Savolainen K, Norppa H. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol Lett. 2009 May 8;186(3):166–173. doi: 10.1016/j.toxlet.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Liu J, Rinzler AG, Dai HJ, Hafner JH, Bradley RK, Boul PJ, Lu A, Iversion T, Shelimov K, Huffman CB. Fullerene pipes. Science. 1998;280:1253–1256. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M, Vanhauwaert A, De Boeck M, Decordier I. Importance of detecting numerical versus structural chromosome aberrations. Mutat. Res. 2002;504:137–148. doi: 10.1016/s0027-5107(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJA, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie. 2006;88:1515–1531. doi: 10.1016/j.biochi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol. Lett. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl. Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, Lison D, Kirsch-Volders M. Clastogenic and aneugenic effects of multiwalled carbon nanotube in epithelial cells. Carcinogenesis. 2008;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nano level. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H, ILSI Research Foundation/Risk Science Institute Nanomaterial Toxicity Screening Working Group Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;6:2–8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004 Jan 7;(1):16–7. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- Patlolla A, Tchounwou P. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutat. Res. 2005;587(1-2):126–133. doi: 10.1016/j.mrgentox.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Dean BJ, Galloway S, Holden H, McFee AF, Shelby M. Mammalian in vivo cytogenetic assays: Analysis of chromosome aberrations in bone marrow cells. Mutat. Res. 1987;189:157–165. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110(8):797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes CM, Liang F, Hudson JL, Mendez J, Guo W, Beach JM, Moore VC, Doyle CD, West JL, Billups WE, Ausman KD, Colvin VL. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161(2):135–142. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhal. Toxicol. 2002;14:57–78. doi: 10.1080/089583701753338631. [DOI] [PubMed] [Google Scholar]
- Schins RP, Knaapen AM. Genotoxic effects of particles. In: Donaldson K, Borm P, editors. Particle Toxicology. Taylor and Francis Group; Boca Raton, FL: 2007. pp. 285–298. [Google Scholar]
- Schmid W. The micronucleus test for cytogenetic analysis. In: Hollaender A, editor. Chemical Mutagens, Principles and Methods for their Detection. Vol. 4. Plenum, Press; New York: 1976. pp. 31–53. [Google Scholar]
- Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, Maynard A, Baron P. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Tian F, Cui D, Sehwarz H, Estrada GG, Kobayashi Cytotoxicity of single-wall carbon nanotube on human fibrpblasts. Toxicology In Vitro. 2006;20:1202–1212. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Sanderson Barbara JS, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Sanderson BJ, Wang H. Cytotoxicity and genotoxicity of ultrafine crystalline SiO2 particulate in cultured human lymphoblastoid cells. Environ. Mol. Mutagen. 2007;48:151–157. doi: 10.1002/em.20287. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Borm PJ, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19(8):631–643. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77(1):117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Yang C, Liu D, Yang H, Zhang Z, Xi Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29(1):69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- Yang ST, Wang X, Jia G, Gu Y, Wang T, Nie H, Ge C, Wang H, Liu Y. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 2008;181:182–189. doi: 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryogenic stem cells. Nano. Lett. 2007 Dec;7(12):3592–3597 S. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]