Summary
Aging is a major risk factor for tendon injury and impaired tendon healing, but the basis for these relationships remains poorly understood. Here we show that rat tendon-derived stem/progenitor cells (TSPCs) differ in both self-renewal and differentiation capability with age. The frequency of TSPCs in tendon tissues of aged animals is markedly reduced based on colony formation assays. Proliferation rate is decreased, cell cycle progression is delayed and cell fate patterns are also altered in aged TSPCs. In particular, expression of tendon lineage marker genes decreased while adipocytic differentiation increased. Cited2, a multi-stimuli responsive transactivator involved in cell growth and senescence, was also downregulated in aged TSPCs while CD44, a matrix assembling and organizing protein implicated in tendon healing, was upregulated, suggesting that these genes participate in the control of TSPC function.
Keywords: aging, tendon-derived stem/progenitor cells, self-renewal, differentiation, Cited2, CD44
Introduction
Age is a major risk factor for tendon disorders. Age-related changes in structural and mechanical properties may predispose tendons to injury; moreover, tendon healing is often impaired in the elderly (Vogel 1978; Birch et al. 1999; Magnusson et al. 2003; Couppe et al. 2009). Tenoblasts and tenocytes, the major tendon cellular elements that produce and organize tendon extracellular matrix (ECM) (Kannus 2000), also undergo age-dependent changes in number and activity (Ippolito et al. 1980; Nakagawa et al. 1994), but the mechanisms behind these changes remain unclear. Recently, tendons of several species (Zhang & Wang 2010; Bi et al. 2007; Rui et al. 2009) were shown to contain a small population of cells with stem cell properties, termed tendon-derived stem/progenitor cells (TSPCs). Since adult or tissue-resident stem/progenitor cells are considered essential for tissue maintenance and repair, we investigated whether the self-renewal capacity and differentiation potential of rat TSPCs are influenced by age.
Results and discussion
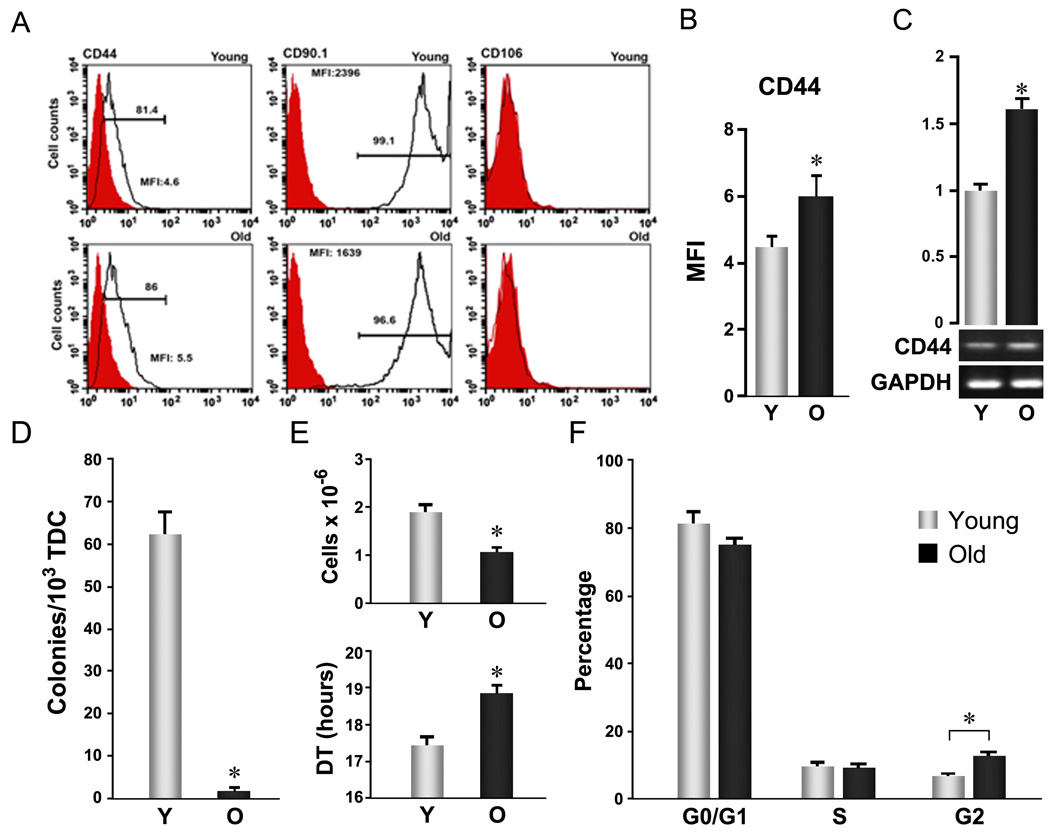
TSPCs were prepared from rat tendon tissues based on their preferential attachment and potent clonal expansion over the majority of resident tendon cells (Bi et al. 2007). We found that nearly 100% of both young and aged TSPCs stained positively for three stem cell markers: nucleostemin, Oct-4, and SSEA-4 (Suppl. Fig. 1), indicating that this population retains features reflecting their stemness regardless of age. Rat TSPCs also expressed surface antigens CD44 (Fig. 1 A, left panels) and CD90.1 (Fig. 1 A, middle panels), but not the endothelial cell marker CD106 (VCAM-1) (Fig. 1 A, right panels), as shown for murine and human TSPCs (Bi et al. 2007) and recently reported for rat TSPCs (Rui et al. 2009). Aged TSPCs expressed lower levels of CD90.1 than young cells, but higher levels of CD44 as determined by both the percentage of positive cells (Fig. 1 A, left panels) and mean fluorescence intensity (Fig. 1B). These differences in CD44 expression were also seen at mRNA levels by RT-PCR (Fig. 1C). CD44, implicated in healing of many tissues, is downregulated during scarless fetal tendon healing (Favata et al. 2006); moreover, mice genetically deficient in CD44 showed improved patellar tendon healing (Ansorge et al. 2009). These findings suggest that the increased CD44 expression in aged TSPCs may contribute to reduced TSPC repair capacity with age.
Figure 1. Aging-related cellular changes in TSPC self-renewal.
Young and aged TSPCs were prepared, as described by Bi et al (Bi et al. 2007), from 3–4 and 24–26 month Sprague-Dawley male rats, respectively. Patellar tendons were digested with collagenase A/dispase (2 h, 37 °C) and cultured in DMEM plus 10% FBS for 7–9 days. Adherent cells (passage 0, P0) at the end of culture were used as TSPCs for all assays unless otherwise specified. (A–C) Age-related surface antigen expression changes in TSPCs. A: Representative histogram plots. TSPCs stained with fluorescein-conjugated anti-rat antibodies (open) or isotype control antibodies (filled) were analyzed by FACS. B: Mean fluorescence intensity of CD44 based on FACS analysis of surface expression as shown in A (n=3, independent experiments, *P<0.05). C: CD44 mRNA levels determined by RT-PCR. Upper Panels represent the quantification of band intensity from the gel shown in lower panels. (D) Colony formation. Total tendon-derived cells (TDC) were plated at 1×103 cells/well (6-well plate) and grown for 9 days. Colony forming units (CFUs) were scored after methylene blue staining. Error bars represent SD (n=3, *P<0.05 versus aged TSPC). (E) Proliferation. TSPCs were seeded at 1×103 cells/well (6 well plate) and cultured for 8 days. Cell numbers were counted and cell population doubling times were estimated from start (0) and end (1) points. Error bars represent SD (n=3, *P<0.05 versus aged TSPC). (F) Cell cycle distribution. TSPCs were fixed with 70% ethanol, stained with propidium iodide (PI), and analyzed by FACS. Error bars represent SD (n=3 independent experiments; * P<0.05)
TSPCs accounted for a dramatically smaller fraction of total tendon cells in old rats (0.171±0.06%) than young rats (6.26±0.55%, P<0.001) (Fig. 1D) based on the number of colonies formed by whole tendon-derived cells (TDC) (Bi et al. 2007; Delorme & Charbord 2007). This corresponded to a 70% reduction in the total number of cells recovered from old versus young tendons. However, colony formation assays using P0 TSPCs from aged and young rats showed only small differences (data not shown). These results indicate that diminished colony formation by aged TSPCs primarily were due to low TSPC numbers likely reflecting a depleted TSPC pool in aged tendon tissues.
Proliferation of aged and young TSPCs also differed. Cell numbers after 8 days of culture were lower in aged TSPCs (1.08±0.09×106 vs. 1.91±0.16×106, P<0.05, Fig. 1E, upper panel), with a corresponding increase in estimated mean population doubling time (DT) (18.8±0.22 hrs vs. 17.4±0.20 hrs, P<0.05, Fig. 1E, lower panel). Similar differences were observed in CFSE-dilution assays (Lyons 2000) assessed by FACS (data not shown). Analysis of cell cycle phase distribution using propidium iodide further showed that aged TSPCs contained a higher fraction in G2/M (Fig. 1F), suggesting that aged TSPCs were preferentially subject to late cell cycle arrest. This could result from accumulated genetic and/or epigenetic damage, as has been reported in other stem/progenitor cell populations (Rossi et al. 2007). Additionally, differences in apoptotic rates between young and old TSPCs could also contribute to the observed disparities in population size. However, to our knowledge, neither issue has yet been examined in TSPCs. Collectively, these data indicate that TSPCs undergo age-related declines in self-renewal capacity (Nishimura et al. 2005; Levi & Morrison 2008) that could account for reduced stem cell numbers with aging.
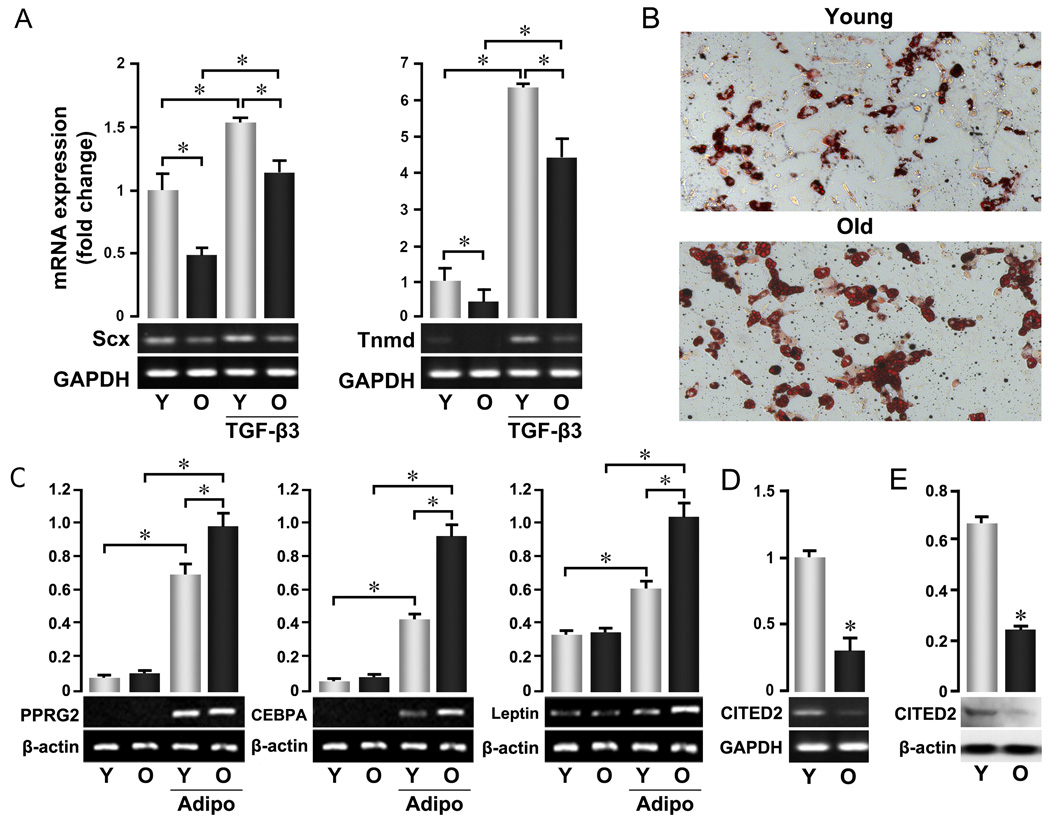
The ability of TSPCs to differentiate into tenocytes was also diminished by age. Expression of two tendon lineage-specific genes (Perez et al. 2003; Shukunami et al. 2006; Murchison et al. 2007), Scleraxis (Scx) and Tenomodulin (Tnmd), was lower in aged TSPCs than in young cells (Fig. 2A). Aged TSPCs also showed diminished induction of these markers by TGF-β3 (Kovacevic & Rodeo 2008). Whether these differences also extend to other tenocyte lineage-inductive stimuli like mechanical loading (Juncosa-Melvin et al. 2006; Kuo & Tuan 2008), and whether they reflect differentiation capacity in vivo remain to be determined. Interestingly, however, aged TSPCs formed adipocytes more readily than younger cells (Fig. 2B) and expressed higher levels of adipogenic markers PPARγ2 (PPARGC1A), C/EBPa (Cebpa/CEBPA), and leptin following induction (Fig. 2C). Young and old TSPCs showed no apparent difference in the ability to form osteoblasts or chondrocytes (Suppl. Fig. 2). These data may help explain the higher levels of adipose tissue normally associated with older tendons (Kannus & Jozsa 1991), a pattern similar to that observed in bone marrow, where adiposity was found to correlate inversely with the functionality of hematopoietic stem/progenitor cells (Naveiras et al. 2009).
Figure 2. Altered cell fate of aged TSPCs.
P0 TSPCs were prepared as described in Figure 1 and used for all assays. (A) Expression of tendon lineage-specific genes. TSPCs were cultured with or without TGF-β3 for 3 days, and total RNA was extracted after culture. (B, C) Adipocyte-skewed differentiation of aged TSPCs. Cells were cultured in specific induction medium for 16 days. B: Oil Red O staining per (Gimble et al. 1995); C: mRNA expression of adipogenic marker genes in untreated and adipogenic-induced TSPCs. (D, E) Expression of Cited2 at mRNA (D) and protein (E) levels in young and old TSPCs. mRNA expression of indicated genes in A, C, D was assessed by RT-PCR. Upper Panels represent the quantification of band intensity from the gel shown in lower panels. Data are representative of 3 experiments and confirmed by real-time PCR.
Finally, we explored age-dependent changes in a potential regulator of TSPC function. Cited2 is a transcription factor implicated in the control of growth and senescence in several cell types (Sun et al. 1998; Kranc et al. 2003; Yokota et al. 2003; Sun 2009). Recent studies further revealed that Cited2 is required to maintain adult hematopoietic stem cells (Chen et al. 2007; Kranc et al. 2009). We found that Cited2 expression in aged TSPCs was reduced at both the mRNA (Fig. 2D) and protein levels (Fig. 2E). These data are consistent with positive roles for Cited2 in TSPC self-renewal. Furthermore, the coordinated expression of Cited2 and Scx suggests that it may also regulate TSPC differentiation.
The present study has demonstrated remarkable changes in number and function of tendon stem/progenitor cells with advancing age. It remains to be determined whether these age-related changes are also influenced by factors such as activity, which could affect tendon loading history. Second, while the functional characterizations of rat TSPCs in vitro may reflect properties of these cells, including the adaptation to an in vitro environment, further studies are also needed to determine whether TSPCs exhibit age-dependent differences in the ability to repopulate functional tenocyte pools in vivo.
Supplementary Material
P0 TSPCs were stained with anti-nucleostemin (RD system, CA#: af1638), or anti-Oct-4 (Abcam, CA#: ab18976), or -SSEA-4 (Abcam, CA#: ab16287; monoclonal Abs) antibodies and fluorescent-conjugated secondary antibodies. The nuclei of the cells (for Nst and SSEA-4 staining) were counterstained with 4′, 6-diaminodino-2-phenylindole (DAPI).
P0 Cells were incubated in osteogenic medium containing 50 µM ascorbate 2-phosphate, 10 mM glycerol phosphate, and 100 nM dexamethasone for 2 weeks or in chondrogenic induction medium consisting of high glucose (4.5 g/ml) DMEM supplemented with 6.25 µg/mL insulin, 6.25 µg/mL transferrin, 6.25 µg/mL selenous acid, 5.33 µg/mL linoleic acid, 1.25 mg/mL bovine serum albumin, 0.1 µM dexamethasone, 10 ng/mL TGF-β3, 50µg/mL ascorbate 2-phosphate for 3 weeks. Osteogenic and chondrogenic differentiation were visualized by Alizarian Red S staining and Safranin-O staining, respectively.
Acknowledgments
The authors thank Drs. Zhengzhe Li, Li Sun, and Damien M. Laudier for scientific and technical support. Supported by NIH grants AR050968, AR 047628 (Sun H.B.), AR52743 (Flatow E.L.).
References
- Ansorge HL, Beredjiklian PK, Soslowsky LJ. CD44 deficiency improves healing tendon mechanics and increases matrix and cytokine expression in a mouse patellar tendon injury model. J Orthop Res. 2009;27:1386–1391. doi: 10.1002/jor.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J. 1999;31:391–396. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Haviernik P, Bunting KD, Yang YC. Cited2 is required for normal hematopoiesis in the murine fetal liver. Blood. 2007;110:2889–2898. doi: 10.1182/blood-2007-01-066316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, Soslowsky LJ. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–2132. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Morgan C, Kelly K, Wu X, Dandapani V, Wang CS, Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393–402. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- Ippolito E, Natali PG, Postacchini F, Accinni L, De Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62:583–598. [PubMed] [Google Scholar]
- Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, Nirmalanandhan VS, Bradica G, Butler DL. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranc KR, Bamforth SD, Braganca J, Norbury C, van Lohuizen M, Bhattacharya S. Transcriptional coactivator Cited2 induces Bmi1 and Mel18 and controls fibroblast proliferation via Ink4a/ARF. Mol Cell Biol. 2003;23:7658–7666. doi: 10.1128/MCB.23.21.7658-7666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranc KR, Schepers H, Rodrigues NP, Bamforth S, Villadsen E, Ferry H, Bouriez-Jones T, Sigvardsson M, Bhattacharya S, Jacobsen SE, Enver T. Cited2 is an essential regulator of adult hematopoietic stem cells. Cell Stem Cell. 2009;5:659–665. doi: 10.1016/j.stem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Levi BP, Morrison SJ. Stem cells use distinct self-renewal programs at different ages. Cold Spring Harb Symp Quant Biol. 2008;73:539–553. doi: 10.1101/sqb.2008.73.049. [DOI] [PubMed] [Google Scholar]
- Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–154. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci. 2003;58:123–127. doi: 10.1093/gerona/58.2.b123. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T, Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand. 1994;152:307–313. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Perez AV, Perrine M, Brainard N, Vogel KG. Scleraxis (Scx) directs lacZ expression in tendon of transgenic mice. Mech Dev. 2003;120:1153–1163. doi: 10.1016/j.mod.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and Characterization of Multi-potent Rat Tendon-derived Stem Cells. Tissue Eng Part A. 2009 doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Sun HB. CITED2 Mechanoregulation of Matrix Metalloproteinases. Annals of the New York Academy of Sciences. 2009 doi: 10.1111/j.1749-6632.2009.05305.x. (accepted) [DOI] [PubMed] [Google Scholar]
- Sun HB, Zhu YX, Yin T, Sledge G, Yang YC. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc Natl Acad Sci U S A. 1998;95:13555–13560. doi: 10.1073/pnas.95.23.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel HG. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res. 1978;6:161–166. doi: 10.3109/03008207809152626. [DOI] [PubMed] [Google Scholar]
- Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–47280. doi: 10.1074/jbc.M304652200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
P0 TSPCs were stained with anti-nucleostemin (RD system, CA#: af1638), or anti-Oct-4 (Abcam, CA#: ab18976), or -SSEA-4 (Abcam, CA#: ab16287; monoclonal Abs) antibodies and fluorescent-conjugated secondary antibodies. The nuclei of the cells (for Nst and SSEA-4 staining) were counterstained with 4′, 6-diaminodino-2-phenylindole (DAPI).
P0 Cells were incubated in osteogenic medium containing 50 µM ascorbate 2-phosphate, 10 mM glycerol phosphate, and 100 nM dexamethasone for 2 weeks or in chondrogenic induction medium consisting of high glucose (4.5 g/ml) DMEM supplemented with 6.25 µg/mL insulin, 6.25 µg/mL transferrin, 6.25 µg/mL selenous acid, 5.33 µg/mL linoleic acid, 1.25 mg/mL bovine serum albumin, 0.1 µM dexamethasone, 10 ng/mL TGF-β3, 50µg/mL ascorbate 2-phosphate for 3 weeks. Osteogenic and chondrogenic differentiation were visualized by Alizarian Red S staining and Safranin-O staining, respectively.