Abstract
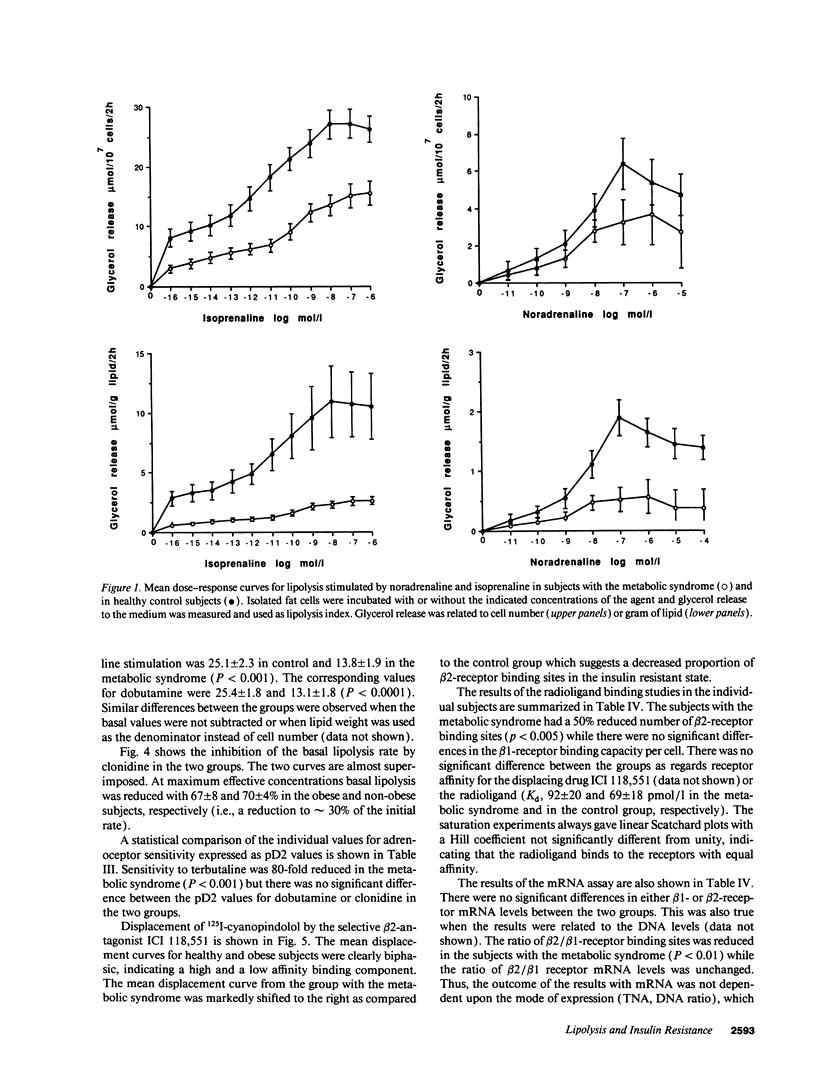
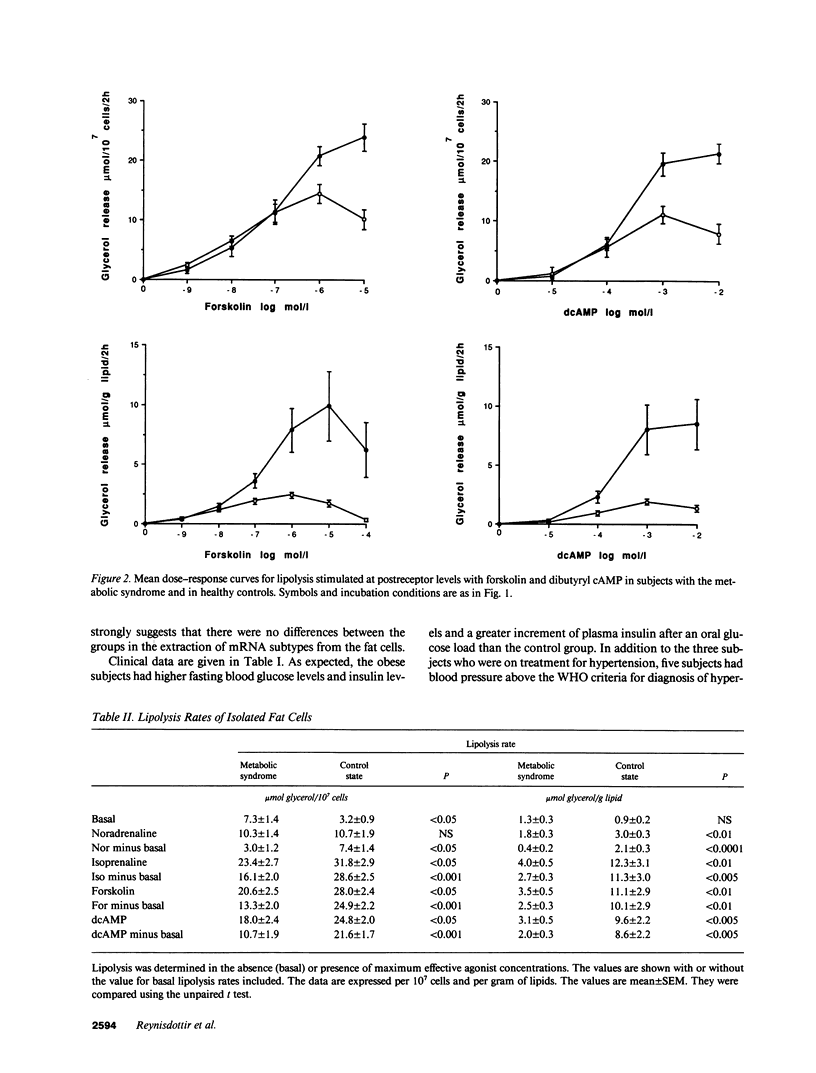
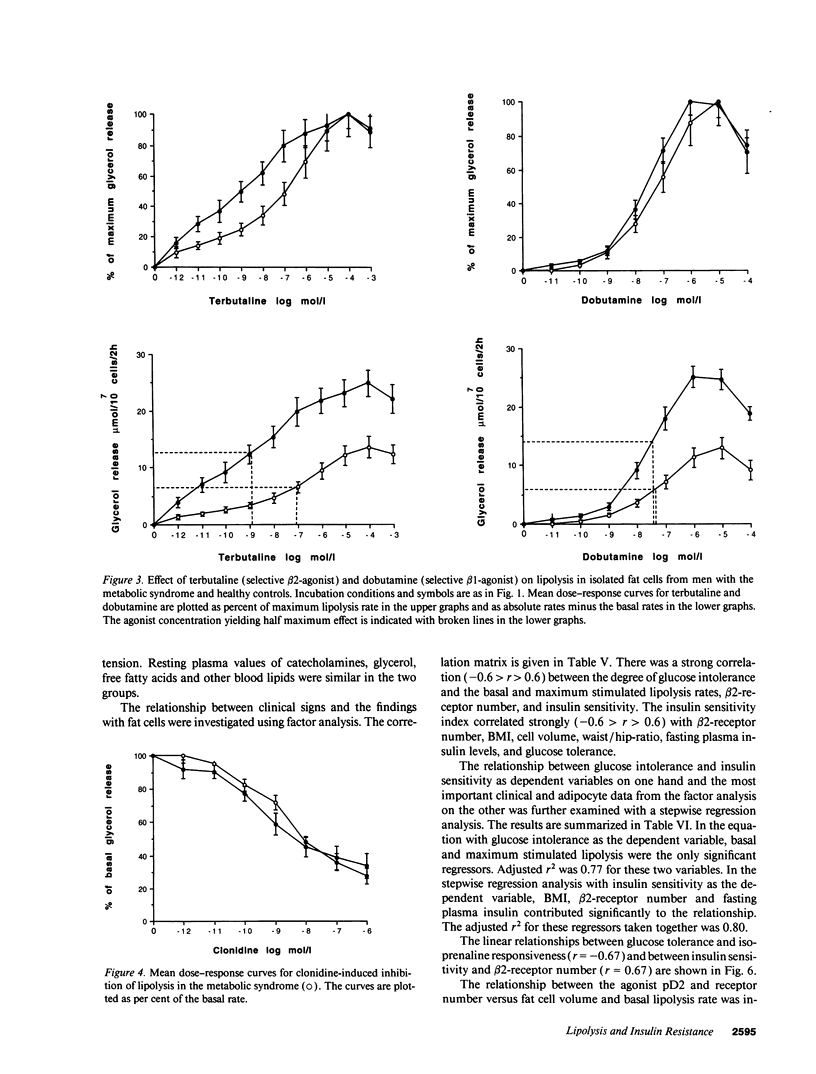
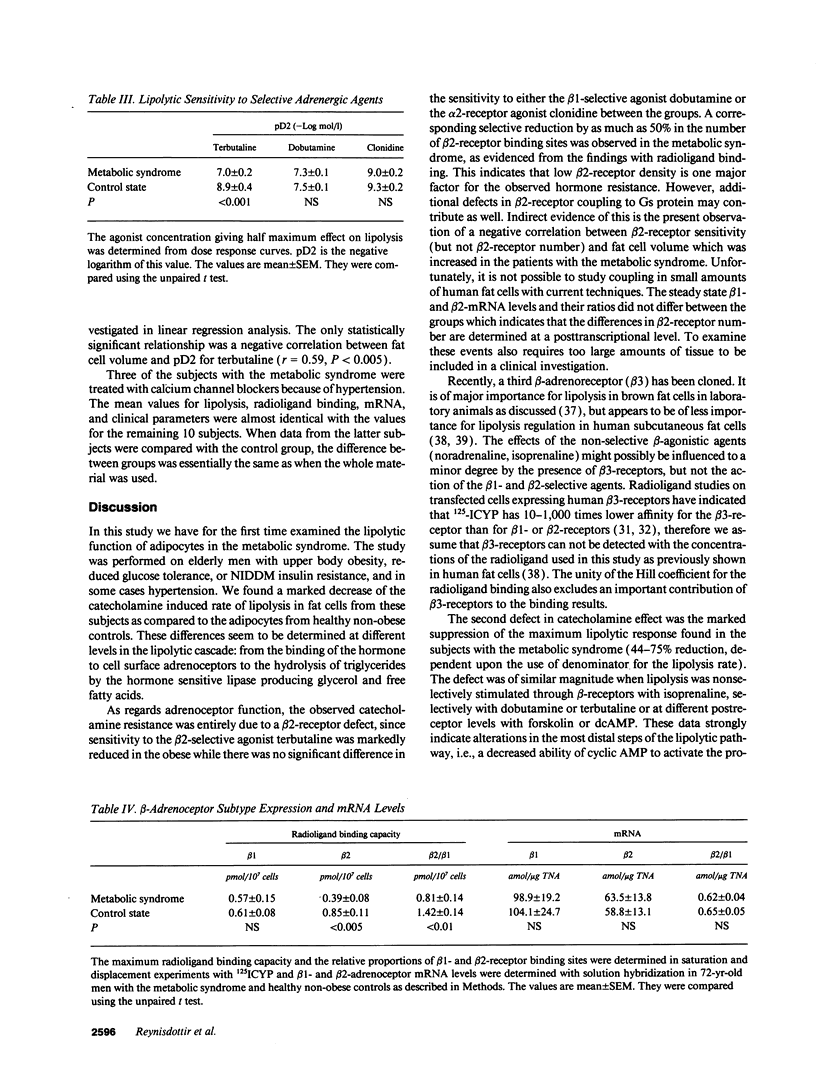
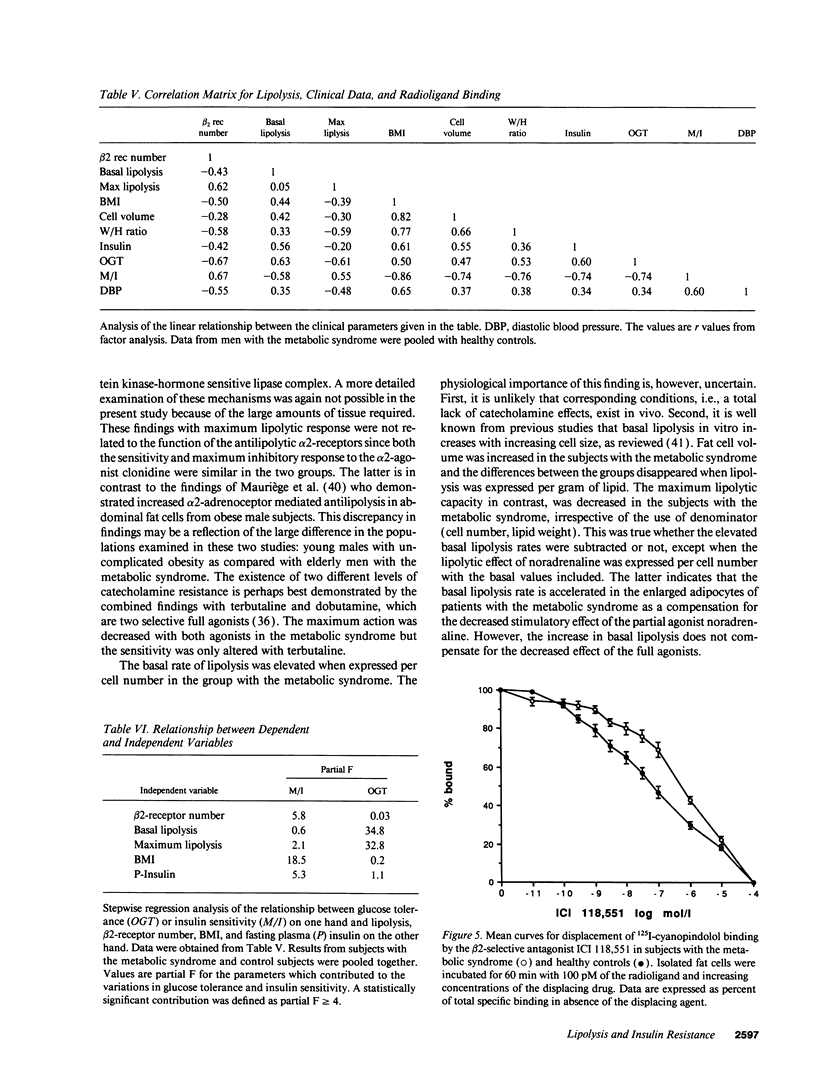
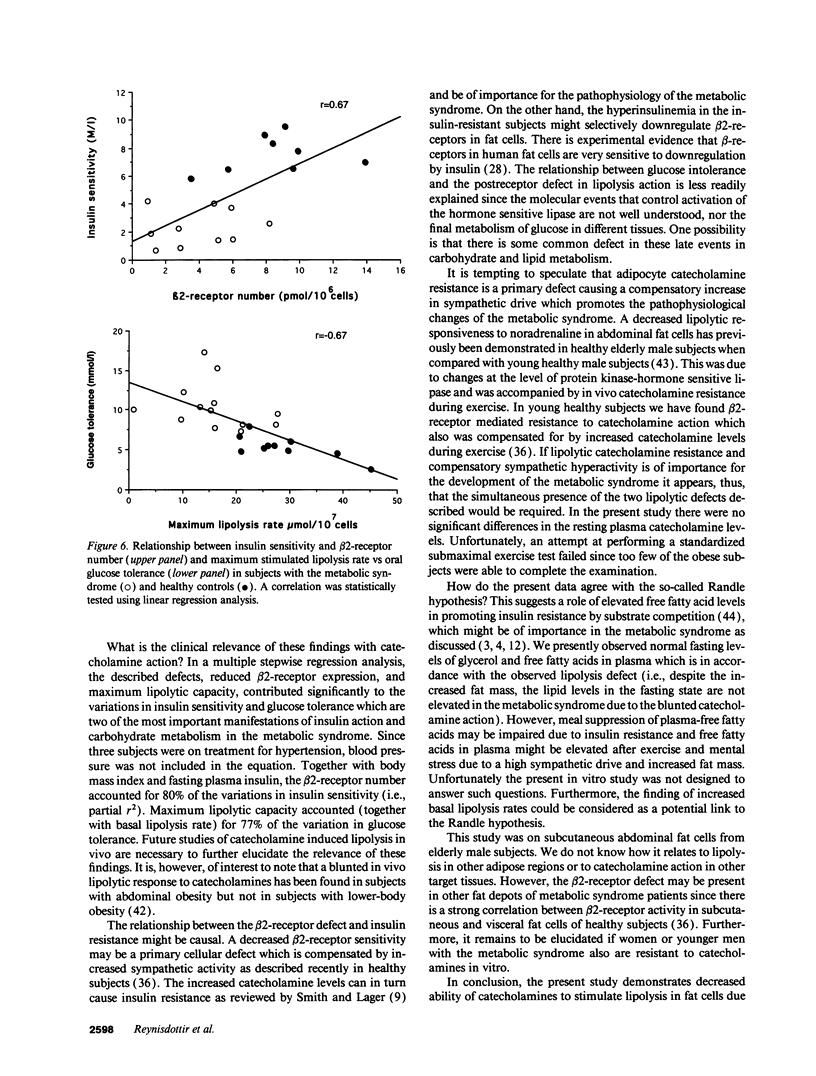
Bearing in mind the importance of upper-body obesity for the insulin resistance (or metabolic) syndrome and the abnormalities in free fatty acid metabolism associated with this disorder, the regulation of lipolysis in isolated subcutaneous adipocytes was investigated in 13 72-yr old upper-body obese men with insulin resistance and glucose intolerance and in 10 healthy 72-yr-old men. There was a marked resistance to the lipolytic effect of noradrenaline in the metabolic syndrome due to defects at two different levels in the lipolytic cascade. First, an 80-fold decrease in sensitivity to the beta 2-selective agonist terbutaline (P < 0.001) which could be ascribed to a 50% reduced number of beta 2-receptors (P < 0.005) as determined with radioligand binding. The groups did not differ as regards dobutamine (beta 1) or clonidine (alpha-2) sensitivity, nor beta 1-receptor number. The mRNA levels for beta 1- and beta 2-receptors were similar in the two groups. Second, the maximum stimulated lipolytic rate was markedly reduced in the metabolic syndrome. This was true for isoprenaline (nonselective beta-agonist), forskolin (activating adenylyl cyclase), and dibutyryl cAMP (activating protein kinase). In regression analysis, the observed abnormalities in lipolysis regulation correlated in an independent way with the degree of glucose intolerance (r = -0.67) and beta 2-receptor number with insulin resistance (r = 0.67). In conclusion, the results of this study indicate the existence of lipolytic resistance to catecholamines in the adipose tissue of elderly men with the metabolic syndrome, which may be of importance for impaired insulin action and glucose intolerance. The resistance is located at a posttranscriptional level of beta 2-receptor expression and at the protein kinase-hormone sensitive lipase level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R. The brown adipocyte beta-adrenoceptor. Proc Nutr Soc. 1989 Jul;48(2):215–223. doi: 10.1079/pns19890032. [DOI] [PubMed] [Google Scholar]
- Arner P. Adrenergic receptor function in fat cells. Am J Clin Nutr. 1992 Jan;55(1 Suppl):228S–236S. doi: 10.1093/ajcn/55.1.228s. [DOI] [PubMed] [Google Scholar]
- Arner P., Arner O., Ostman J. The effect of local anaesthetic agents on lipolysis by human adipose tissue. Life Sci. 1973 Jul 16;13(2):161–169. doi: 10.1016/0024-3205(73)90191-4. [DOI] [PubMed] [Google Scholar]
- Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988 Aug;4(5):507–515. [PubMed] [Google Scholar]
- Arner P., Hellström L., Wahrenberg H., Brönnegård M. Beta-adrenoceptor expression in human fat cells from different regions. J Clin Invest. 1990 Nov;86(5):1595–1600. doi: 10.1172/JCI114880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Arner P., Pollare T., Lithell H. Different aetiologies of type 2 (non-insulin-dependent) diabetes mellitus in obese and non-obese subjects. Diabetologia. 1991 Jul;34(7):483–487. doi: 10.1007/BF00403284. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991 Dec;14(12):1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- Black H. R. The coronary artery disease paradox: the role of hyperinsulinemia and insulin resistance and implications for therapy. J Cardiovasc Pharmacol. 1990;15 (Suppl 5):S26–S38. [PubMed] [Google Scholar]
- Campbell P. J., Carlson M. G. Impact of obesity on insulin action in NIDDM. Diabetes. 1993 Mar;42(3):405–410. [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991 Mar;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Engfeldt P., Hellmér J., Wahrenberg H., Arner P. Effects of insulin on adrenoceptor binding and the rate of catecholamine-induced lipolysis in isolated human fat cells. J Biol Chem. 1988 Oct 25;263(30):15553–15560. [PubMed] [Google Scholar]
- Frayn K. N., Coppack S. W. Insulin resistance, adipose tissue and coronary heart disease. Clin Sci (Lond) 1992 Jan;82(1):1–8. doi: 10.1042/cs0820001. [DOI] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Groop L. C., Kankuri M., Schalin-Jäntti C., Ekstrand A., Nikula-Ijäs P., Widén E., Kuismanen E., Eriksson J., Franssila-Kallunki A., Saloranta C. Association between polymorphism of the glycogen synthase gene and non-insulin-dependent diabetes mellitus. N Engl J Med. 1993 Jan 7;328(1):10–14. doi: 10.1056/NEJM199301073280102. [DOI] [PubMed] [Google Scholar]
- Hellmér J., Arner P., Lundin A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989 Feb 15;177(1):132–137. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Hellmér J., Wahrenberg H., Arner P. Stability over time of adrenergic sensitivity in isolated human fat cells. Int J Obes Relat Metab Disord. 1992 Jan;16(1):23–28. [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hollenga C., Brouwer F., Zaagsma J. Differences in functional cyclic AMP compartments mediating lipolysis by isoprenaline and BRL 37344 in four adipocyte types. Eur J Pharmacol. 1991 Aug 6;200(2-3):325–330. doi: 10.1016/0014-2999(91)90590-m. [DOI] [PubMed] [Google Scholar]
- Jensen M. D., Haymond M. W., Rizza R. A., Cryer P. E., Miles J. M. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989 Apr;83(4):1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Wieland E., Scheurer A., Vogel G., Wildenberg U., Joost C. Influences of variation in total energy intake and dietary composition on regulation of fat cell lipolysis in ideal-weight subjects. J Clin Invest. 1987 Aug;80(2):566–572. doi: 10.1172/JCI113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H. Insulin resistance in visceral obesity. Int J Obes. 1991 Sep;15 (Suppl 2):109–115. [PubMed] [Google Scholar]
- Kohrt W. M., Kirwan J. P., Staten M. A., Bourey R. E., King D. S., Holloszy J. O. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993 Feb;42(2):273–281. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Landsberg L. Obesity, metabolism, and hypertension. Yale J Biol Med. 1989 Sep-Oct;62(5):511–519. [PMC free article] [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Nyberg B., Wahrenberg H., Arner P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest. 1990 May;85(5):1614–1621. doi: 10.1172/JCI114612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist F., Wahrenberg H., Hellström L., Reynisdottir S., Arner P. Lipolytic catecholamine resistance due to decreased beta 2-adrenoceptor expression in fat cells. J Clin Invest. 1992 Dec;90(6):2175–2186. doi: 10.1172/JCI116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriège P., Després J. P., Prud'homme D., Pouliot M. C., Marcotte M., Tremblay A., Bouchard C. Regional variation in adipose tissue lipolysis in lean and obese men. J Lipid Res. 1991 Oct;32(10):1625–1633. [PubMed] [Google Scholar]
- Modan M., Halkin H. Hyperinsulinemia or increased sympathetic drive as links for obesity and hypertension. Diabetes Care. 1991 Jun;14(6):470–487. doi: 10.2337/diacare.14.6.470. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Pollare T., Lithell H., Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism. 1990 Feb;39(2):167–174. doi: 10.1016/0026-0495(90)90071-j. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Smith U., Lager I. Insulin-antagonistic effects of counterregulatory hormones: clinical and mechanistic aspects. Diabetes Metab Rev. 1989 Sep;5(6):511–525. doi: 10.1002/dmr.5610050604. [DOI] [PubMed] [Google Scholar]
- Smith U. Life style and genes--the key factors for diabetes and the metabolic syndrome. J Intern Med. 1992 Aug;232(2):99–101. doi: 10.1111/j.1365-2796.1992.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Stern M. P., Haffner S. M. Body fat distribution and hyperinsulinemia as risk factors for diabetes and cardiovascular disease. Arteriosclerosis. 1986 Mar-Apr;6(2):123–130. doi: 10.1161/01.atv.6.2.123. [DOI] [PubMed] [Google Scholar]
- Tate K. M., Briend-Sutren M. M., Emorine L. J., Delavier-Klutchko C., Marullo S., Strosberg A. D. Expression of three human beta-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur J Biochem. 1991 Mar 14;196(2):357–361. doi: 10.1111/j.1432-1033.1991.tb15824.x. [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Brechtel G., Henry R. R. Multiple defects in muscle glycogen synthase activity contribute to reduced glycogen synthesis in non-insulin dependent diabetes mellitus. J Clin Invest. 1991 Feb;87(2):489–495. doi: 10.1172/JCI115022. [DOI] [PMC free article] [PubMed] [Google Scholar]



