Abstract
MicroRNAs (miRNAs) are generated by a two-step processing pathway to yield RNA molecules of approximately 22 nucleotides that negatively regulate target gene expression at the post-transcriptional level1. Primary miRNAs are processed to precursor miRNAs (pre-miRNAs) by the Microprocessor complex2–4. These pre-miRNAs are cleaved by the RNase III Dicer5–8 to generate mature miRNAs that direct the RNA-induced silencing complex (RISC) to messenger RNAs with complementary sequence9. Here we show that TRBP (the human immunodeficiency virus transactivating response RNA-binding protein10), which contains three double-stranded, RNA-binding domains, is an integral component of a Dicer-containing complex. Biochemical analysis of TRBP-containing complexes revealed the association of Dicer–TRBP with Argonaute 2 (Ago2)11,12, the catalytic engine of RISC. The physical association of Dicer–TRBP and Ago2 was confirmed after the isolation of the ternary complex using Flag-tagged Ago2 cell lines. In vitro reconstitution assays demonstrated that TRBP is required for the recruitment of Ago2 to the small interfering RNA (siRNA) bound by Dicer. Knockdown of TRBP results in destabilization of Dicer and a consequent loss of miRNA biogenesis. Finally, depletion of the Dicer–TRBP complex via exogenously introduced siRNAs diminished RISC-mediated reporter gene silencing. These results support a role of the Dicer–TRBP complex not only in miRNA processing but also as a platform for RISC assembly.
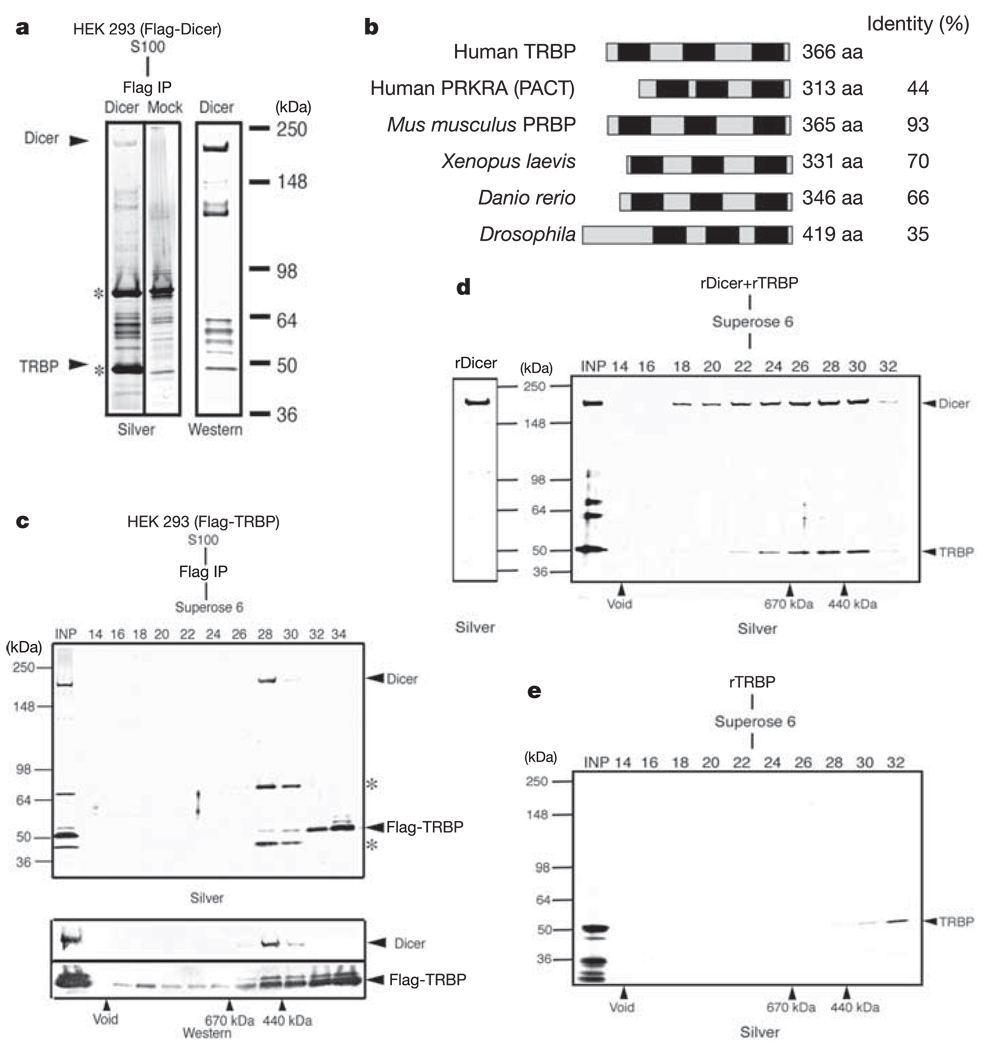
To gain an insight into the components of the miRNA/siRNA processing machinery, we isolated a Dicer-containing complex from human cells. This was accomplished by developing HEK293-derived stable cell lines expressing Dicer tagged with Flag (Flag-Dicer). Flag-Dicer was isolated using affinity chromatography, and the affinity eluate was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) followed by silver staining and western blot analysis.Western blot and mass spectrometric analyses indicated that most polypeptides in the Dicer affinity eluate were products of the proteolytic break down of Dicer (Fig. 1a). However, mass spectroscopy identified a 50-kDa band (six peptide sequences that migrated slightly above the contaminating MEP50 band) corresponding to the human immunodeficiency virus (HIV)-1 transactivating response (TAR) RNA-binding protein (TRBP)10. The TRBP gene encodes a protein with three double-stranded RNA-binding domains (dsRBDs). Analysis of the non-redundant protein database by Blast identified proteins with close homology to TRBP in both vertebrates and Drosophila (CG6866) (Fig. 1b).
Figure 1. Isolation of a Dicer–TRBP-containing complex.
a, Flag-Dicer isolated from a HEK293-derived cytoplasmic extract (S100) resolved by SDS–PAGE and visualized by silver staining (left panel), and western blot analysis using anti-Flag antibodies (right panel). Arrows point to the bands containing Dicer and TRBP proteins. The molecular masses of marker proteins (kDa) are indicated (right). Asterisks denote contaminating polypeptides that were also present in control immunoprecipitation (IP) from naive cells (Mock). b, Diagrammatic representation of the human TRBP protein and alignment with orthologous proteins. Conserved dsRBDs are depicted as black squares. Protein accession numbers (NCBI) are: AAB50581 (human TRBP), NP_003681 (human PRKRA), NP_03345 (mouse PRBP), AAH55390 (Danio rerio), MGC82499 (Xenopus), NP_609646 (Drosophila). aa, amino acids. c, The Dicer–TRBP complex isolated from S100 by Flag-TRBP, fractionated by Superose 6 gel filtration, and visualized by silver staining and western blot analysis. INP denotes the input to the column; asterisks denote the common contaminants (SKB1 and MEP50). Fractions of the column are shown along the top; molecular mass markers are indicated along the bottom. d, Silver stains of recombinant Dicer (rDicer) and recombinant Dicer–TRBP complex (rDicer + rTRBP) reconstituted and purified by gel filtration. e, Silver stain analysis of recombinant TRBP fractionated by gel filtration.
To confirm the association of TRBP and Dicer, we developed Flag-TRBP cell lines. Polypeptides associated with TRBP were isolated and subjected to chromatographic fractionation. Analysis of gel filtration fractions by silver staining and western blot analysis using antibodies against Dicer and Flag epitope demonstrated the co-elution of a fraction of TRBP and Dicer as a component of an approximately 500-kDa complex (Fig. 1c). The presence of Dicer was also confirmed by mass spectrometric sequencing. Moreover, additional bands (indicated with an asterisk in Fig. 1c) correspond to SKB1 and MEP50, common contaminants of Flag purification. Although most of TRBP eluted in smaller fractions (32 and beyond; perhaps as a consequence of overexpression), a minor portion of TRBP eluted as a large complex (fractions 16 and 18) not easily visualized by silver staining. To demonstrate the direct association of Dicer and TRBP, we generated recombinant Dicer and TRBP proteins from insect cells and bacteria, respectively. Recombinant Dicer protein was further purified to near homogeneity by gel filtration (Fig. 1d, left panel). Next, we mixed stoichiometric amounts of recombinant Dicer and TRBP and analysed the elution profile of the complex by gel filtration. Consistent with the behaviour of the endogenous complex, recombinant Dicer–TRBP co-elute as a complex, peaking in fractions 28–30 (Fig. 1d, right panel). To confirm the direct physical association of TRBP with Dicer, and to rule out the possibility that the observed co-elution was due to overlapping elution profiles of the individual proteins, we examined the elution profile of TRBP alone by gel filtration. Analysis of Superose 6 column fractions by silver staining revealed that recombinant TRBP eluted in a distinct fraction (fraction 32) compared with that of the Dicer–TRBP complex (compare Fig. 1e and d).
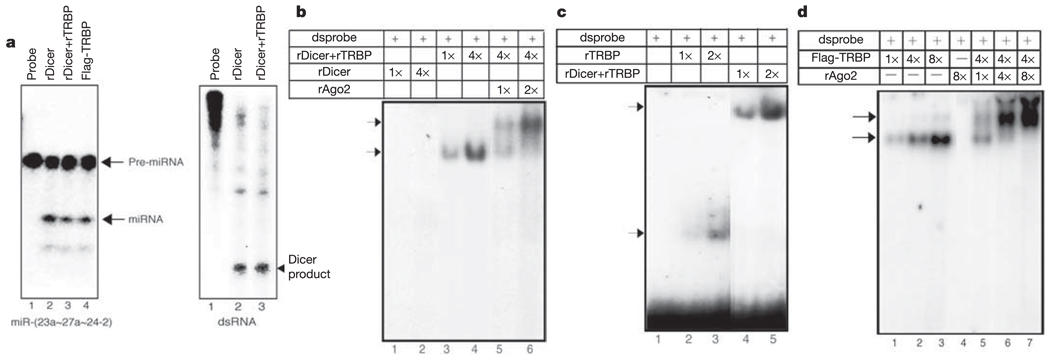
We have shown previously that recombinant Drosha requires its binding partner, the dsRBD protein DGCR8, for processing primary miRNAs to pre-miRNAs2. Unlike Drosha, recombinant Dicer was reported to process long double-stranded RNA to yield products of 21–23 nucleotides13. To assess the processing of pre-miRNA by the Dicer–TRBP complex, an approximately 60-nucleotide pre-miRNA corresponding to miR-(23a ~ 27a ~ 24-2) was prepared through the action of the Microprocessor complex. Addition of equal amounts of either Dicer alone or Dicer–TRBP to the reaction generated the final 22-nucleotide mature miRNA (Fig. 2a, left panel). Analysis of the processing of a number of miRNAs by different concentrations of Dicer or the Dicer–TRBP complex revealed a specific activity that was comparable for the two enzyme preparations (data not shown).We next monitored the processing of long double-stranded RNA by Dicer and Dicer–TRBP. Similar to results obtained with pre-miRNA, the addition of equal amounts of recombinant Dicer or recombinant Dicer–TRBP complex to the reaction yielded comparable amounts of cleavage products of approximately 22 nucleotides (Fig. 2a, right panel). These results are reminiscent of the association of the double-stranded RNA-binding protein R2D2 with Drosophila Dicer2. Indeed, in the absence of R2D2, Dicer2 can still process double-stranded RNA efficiently, but the resulting siRNAs are not effectively channelled into RISC14. Moreover, a recent report suggests that the R2D2 protein is the sensor for choosing the guide strand of the siRNA duplex to be loaded into the RISC15.
Figure 2. The Dicer–TRBP complex processes miRNAs and associates with siRNA to form a ternary complex with Ago2.
a, Pre-miRNA processing assay (left panel). The pre-miRNAs generated by the Microprocessor complex were subjected to a second step of processing using recombinant Dicer, recombinant Dicer–TRBP, or fraction 28 of the Superose 6 column of immunopurified TRBP complex (Flag-TRBP) to yield a 22-nucleotide mature miRNA. The right panel shows the long double-stranded RNA processing assay. Double-stranded RNA was incubated with recombinant Dicer or recombinant Dicer–TRBP to yield a 22-nucleotide mature miRNA. b, EMSA performed using radiolabelled double-stranded siRNA. Recombinant Dicer and recombinant Dicer–TRBP (fraction 28 of the Superose 6 column) was analysed for complex formation with siRNA. 1× corresponds to 20 ng of Dicer and 10 ng of recombinant Ago2. c, EMSA performed using radiolabelled siRNA. Recombinant Dicer–TRBP (fraction 28 of the Superose 6 column) and recombinant TRBP (fraction 32 of the Superose 6 column) were analysed for complex formation with siRNA. 1× corresponds to 20 ng for each protein. d, EMSA performed with Dicer–TRBP (fraction 28 of the Superose 6 column corresponding to Flag-TRBP) and addition of recombinant Ago2. 1 × corresponds to 5 ng of native complex and 2.5 ng of recombinant Ago2. Arrows indicate the two ribonucleoprotein complexes.
To determine whether TRBP may function by bridging the association of Dicer and Ago2 with the siRNA, we assessed the physical association of Ago2 with Dicer–TRBP by mobility shift gel electrophoresis using a radiolabelled siRNA. Although recombinant Dicer did not associate with the siRNA, addition of recombinant Dicer–TRBP (fraction 28 of the Superosoe 6 gel filtration shown in Fig. 1d) resulted in the formation of a complex between Dicer–TRBP and the siRNA (Fig. 2b). Notably, TRBP alone (fraction 32 of the Superose 6 gel filtration; Fig. 1e) could form a stable, rapidly migrating complex with double-stranded siRNA, which was distinct from that of the Dicer–TRBP complex (Fig. 2c). Although Ago2 cannot associate with the siRNA on its own (Fig. 2d, lane 4), addition of recombinant Ago2 to the Dicer–TRBP–siRNA complex resulted in the appearance of a slower migrating Dicer–TRBP–Ago2 complex (Fig. 2b). These results indicate that TRBP may have a role in mediating Dicer association with siRNA and the recruitment of Ago2.
Analysis of the interaction between the endogenous Dicer–TRBP complex and the siRNA resulted in the appearance of two complexes: a minor, slower migrating complex, and a major, faster migrating complex (Fig. 2d). Addition of ATP to the reaction did not affect either complex (data not shown). Whereas the faster migrating complex corresponds to that observed with recombinant Dicer–TRBP, the slower migrating complex resembled that of Dicer–TRBP–Ago2. Indeed, addition of increasing concentrations of recombinant Ago2 to native Dicer–TRBP complex resulted in the formation of a slower migrating complex indistinguishable from the minor band observed with the native Dicer–TRBP complex (Fig. 2d). These results suggested the possibility that the Ago2 protein may form a stable complex with Dicer–TRBP in vivo.
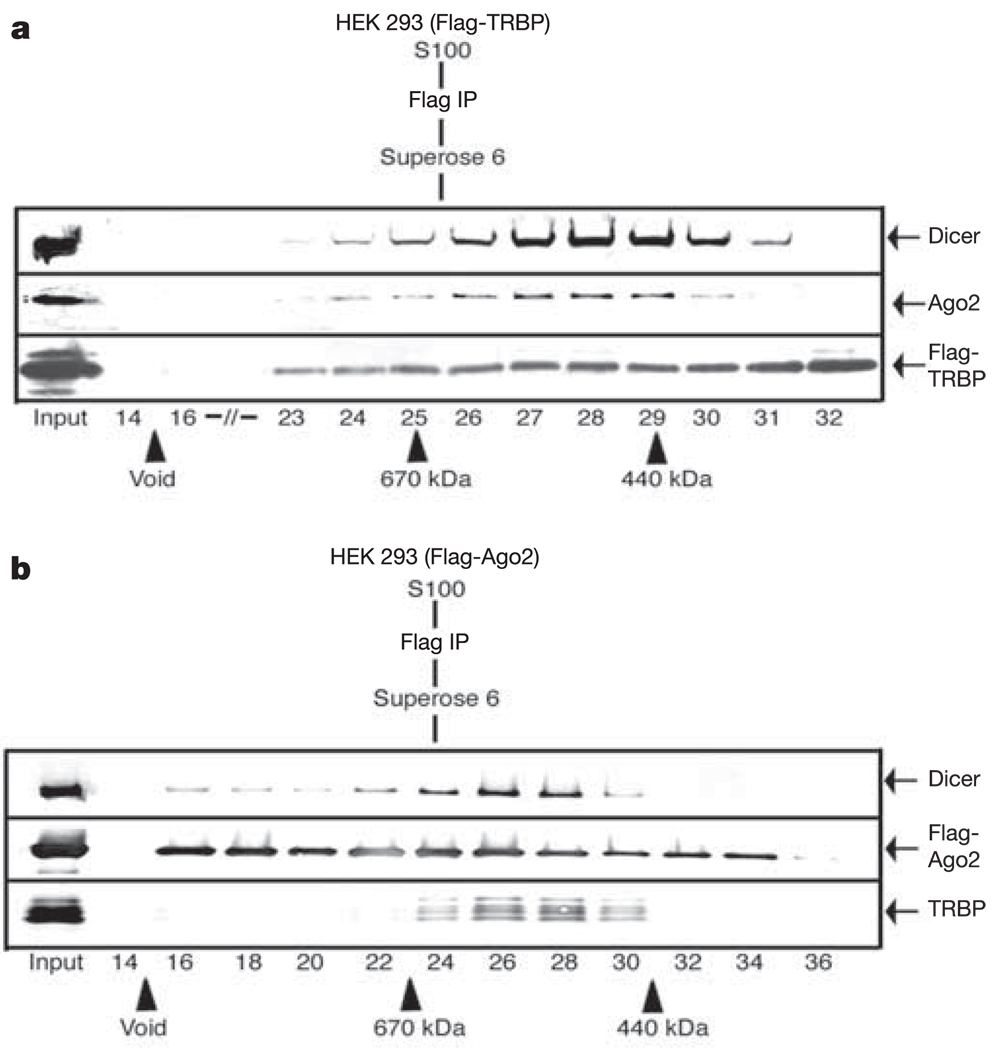
To test this possibility, we analysed the Dicer–TRBP complex purified through Flag-TRBP, and fractionated by gel filtration for the presence of Ago2. This revealed the precise co-elution of Ago2 with TRBP and Dicer (Fig. 3a). To demonstrate this association rigorously, we generated HEK 293-derived stable lines expressing Flag-Ago2. Flag-Ago2 was purified and subjected to gel filtration. Western blot analysis of Superose 6 gel filtration column fractions revealed the presence of Ago2 in multiple complexes of variable size (Fig. 3b). However, a distinct complex containing TRBP and Dicer was detected peaking in fractions 26–28, consistent with the presence of a Dicer–TRBP–Ago2 complex displaying a similar molecular mass as that observed after analysis of Flag-TRBP by gel filtration (Fig. 3a). Consistent with the presence of multiple isoforms of TRBP, we detected multiple TRBP bands associating with Dicer–Ago2 (Fig. 3b). Taken together, these data reveal the stable association of a trimeric protein complex containing Dicer–TRBP and Ago2 that can assemble onto siRNA duplexes.
Figure 3. Stable association of Dicer–TRBP with Ago2.
a, Western blot analysis of Flag-TRBP affinity eluate fractionated by Superose 6 gel filtration. Column fractions were analysed using the antibodies indicated. b, Western blot analysis of Flag-Ago2 affinity eluate fractionated by Superose 6 gel filtration. Column fractions were analysed using the antibodies indicated.
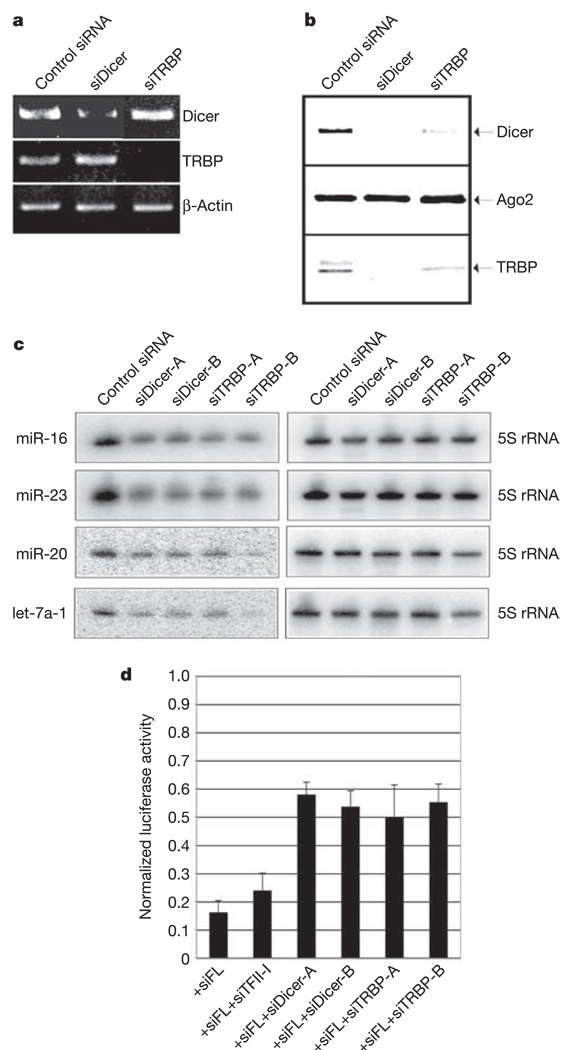
To address the role of the Dicer–TRBP complex in the generation of mature miRNAs in vivo, we used RNA interference (RNAi) to deplete Dicer and TRBP. Two different siRNAs targeting Dicer and TRBP were used to knockdown the levels of each protein, whereas a siRNA targeting TFII-I was used as a control. Polymerase chain reactionwith reverse transcription (RT–PCR) demonstrated the gene specificity of the siRNAs in reducing mRNA levels (Fig. 4a); however, depletion of either Dicer or TRBP resulted in the destabilization of both proteins, reflecting a role for their association in maintaining protein stability (Fig. 4b). Predictably, analysis of miRNA levels after abrogation of Dicer–TRBP resulted in a substantial decrease in the level of mature miRNA (Fig. 4c). The low level of miRNAs detected after Dicer depletion could be due to either residual Dicer protein after siRNA treatment, or may represent persistence of mature miRNAs over the relatively short time period of these experiments.
Figure 4. The Dicer–TRBP complex is required for miRNA biogenesis and post-transcriptional gene silencing.
a, Analysis of the transcript levels of TRBP, Dicer and β-actin by RT–PCR, after treatment of HeLa cells with a representative siRNA to TFII-I (control), TRBP and Dicer. b, Western blot analysis of Flag-Ago2 immunoprecipitate after siRNA-mediated knockdown of TFII-I (control), TRBP and Dicer. c, Northern blot analysis of miR-16, miR-23, let-7a-1 and miR-20 after treatment of HeLa cells with the indicated siRNA. d, HeLa cells co-transfected with firefly and Renilla luciferase plasmids and a combination of siRNA targeting firefly luciferase and one of the siRNAs shown in the bottom of the figure. Firefly luciferase activity was normalized relative to that of Renilla luciferase. Error bars represent standard error of the mean for three independent experiments.
The association of the Dicer–TRBP complex with Ago2 is suggestive of a coupling of the initiation step of RNAi and its effector role in RNA-mediated gene silencing. We depleted Dicer or TRBP using siRNA and then assessed the extent of post-transcriptional gene silencing by measuring the effectiveness of siRNA targeting firefly luciferase to decrease firefly luciferase activity. A Renilla luciferase that was not a target of the siRNA was used as a control to normalize the luciferase activity. Treatment of HeLa cells with siRNA against firefly luciferase resulted in 80% inhibition of the firefly luciferase activity (Fig. 4d). Depletion of Dicer or TRBP (approximately 80% depletion of Dicer and TRBP levels in three independent experiments) using siRNA relieved this inhibition to about 40% compared with cells that were treated with control siRNA against TFII-I. These results implicate a role for the Dicer–TRBP complex in linking the initiation step of siRNA production to the execution step of post-transcriptional gene silencing.
We have isolated a trimeric protein complex of approximately 500 kDa that contains TRBP, Dicer and Ago2. We demonstrate that this complex is required for miRNA biogenesis and links miRNA processing with the assembly of an active RISC. This contention is based on the following. First, affinity purification and gel filtration of Flag-TRBP and Flag-Ago2 revealed a stable association of Dicer–TRBP–Ago2 in a discrete complex of about 500 kDa that displayed pre-miRNA processing activity in vitro. Second, the close association of Dicer and TRBP was further demonstrated by the fact that depletion of Dicer or TRBP resulted in the loss of stability of the reciprocal binding partner in vivo and concomitant loss of miRNA processing. Third, TRBP was required for the recruitment of Ago2 to the siRNA duplexes. Finally, knockdown of Dicer–TRBP resulted in impaired post-transcriptional gene silencing in vivo. Previous studies have pointed to a mechanistic coupling between the initiation phase of RNAi and the execution phase9. Specifically, these results point to a role for Dicer in facilitating siRNA-directed mRNA cleavage16,17. Our results extend these contentions and delineate a role for the double-stranded RNA-binding protein TRBP in coupling the initiation and execution steps of RNAi. These results are also consistent with a stepwise assembly of RISC in which the Dicer–TRBP complex, by virtue of its affinity for siRNA, recruits Ago2 to siRNA, leading to the formation of the trimeric complex. This trimeric complex provides the foundation for the assembly of an active holo-RISC complex through the recruitment of additional proteins9.
TRBP binds the HIV TAR, a stem-loop structure required for the transcriptional activation of HIV10. Furthermore, TAR has secondary structure that resembles miRNA precursors, raising the possibility that TAR is a viral pre-miRNA. Moreover, deletion of PRBP, the mouse homologue of TRBP, yields viable mice that often die at weaning. Surviving homozygous mutant males show defects in spermatogenesis18. In contrast, knocking out Dicer results in embryonic lethality19. Given that in humans Dicer requires TRBP for protein stability and RISC assembly, it seems likely that in mice there is a redundant factor, possibly PRKRA (also known as PACT in human), that compensates for PRBP during development but not spermatogenesis.
METHODS
Affinity purification of Flag-Dicer, Flag-TRBP and Flag-Ago2
Expression plasmids for Flag-Dicer (carboxy-terminal tag), Flag-TRBP (amino-terminal tag) and Flag-Ago2 (amino-terminal tag) were individually co-transfected with a puromycin resistance plasmid into HEK 293 cells. Complexes were purified (50–150 mg) from cytoplasmic extract (S100) with anti-Flag M2 affinity gel (Sigma). After washing with buffer A twice (20mM Tris-HCl (pH 7.9), 0.5M KCl, 10% glycerol, 1mM EDTA, 5mM dithiothreitol (DTT), 0.2mM phenylmethylsulphonyl fluoride (PMSF) 0.5% NP40), and once with buffer B (20mM Tris-HCl (pH 7.9), 0.1M KCl, 10% glycerol, 1mM EDTA, 5mM DTT, 0.2mM PMSF), the affinity column was eluted with Flag peptide. Analysis of Flag-TRBP and Flag-Ago2 by gel filtration was as described previously2. Protein identification using liquid chromatography tandem mass spectrometry (LC-MS/MS) was performed as described previously2. Anti-Dicer, anti-TRBP and anti-Ago2 polyclonal antibodies were generated to the N and C terminus of each protein (Open Biosystems). Recombinant Dicer, TRBP and Ago2 were purified according to methods previously described for purification of recombinant proteins from insect cells and bacteria2.
Pre-miRNA and long double-stranded RNA processing
Processing of in vitro-transcribed primary miRNA by the Microprocessor complex (Drosha–DGCR8) was carried out as described previously2. Long double-stranded RNA was prepared by in vitro transcription of a 150-nucleotide RNA from both strands of a DNA plasmid. The two complementary RNAs were mixed together in equimolar amounts in RNase-free water with 10U RNasin and incubated overnight at 37 °C. After gel purification, long double-stranded RNA was used in processing assays. Processing of pre-miRNA or long double-stranded RNA was performed by incubating the Dicer–TRBP complex, recombinant Dicer, or recombinant Dicer–TRBP complex with gel-purified pre-miRNA in a buffer containing 3.2mM MgCl2, 1mM ATP, 20mM creatine phosphate, 1U µl−1 HPRI (Takara), 20mMTris-HCl (pH 7.9), 0.1MKCl, 10% glycerol, 5mMDTT, 0.2mMPMSF, for 1 h at 37 °C. Subsequent to phenol:chloroform extraction and RNA precipitation, samples were resolved by 15% denaturing polyacrylamide gel.
Electrophoretic mobility shift analysis
Electrophoretic mobility shift analysis (EMSA) was performed using [γ32P]ATP end-labelled, double-stranded siRNA (luciferase) as a probe. Protein complexes/recombinant proteins were added to the reaction containing 15nM siRNA in the same buffer used for pre-miRNA processing, and incubated at 30 °C for 20 min. Complexes were resolved by 4% native PAGE at 4 °C.
siRNAs and northern blot analysis
The sequences of the siRNAs used in this study are: Dicer-A, 5′-UUUGUUGCGAGGCUGAUUCdTdT-3′ ; Dicer-B, 5′ - UCUAUUAGCACCUUGAUGUdTdT-3′ ; TRBP-A, 5′ -GCUGCCUAGUAUAGAGCAAdTdT-3′ ; TRBP-B, 5′-UCUACGAAAUUCAGUAGGAdTdT-3′ ; luciferase, 5′-UCGAAGUAUUCCGCGUACGdTdT-3′ ; TFII-I, 5′-UGUGGGGAAGCUCUUGGCCdTdT-3′. Transfection of HeLa 293 cells was performed with Lipofectamine 2000 (Invitrogen), as described. To examine the effect of siRNA on target gene expression, total RNA and complementary DNA synthesis was prepared 48–72 h after transfection, as described2. Primer sequences for RT–PCRs in Fig. 4a are: Dicer, 5′ -CATGGATAGTGGGATGTCAC-3′ and 5′ -CTACTTCCACAGTGACTCTG-3′; TRBP, 5′ -GGGCTGCCTAGTATAGAGC-3′ and 5′ - CCCTGACCAGTGCAGCTGGTG-3′; β-actin, 5′ -AAAGACCTGTACGCCAACAC-3′ and 5′ -GTCATACTCCTGCTTGCTGAT-3′. For northern blot analysis, total RNA (5–10 µg of each sample, isolated using TRIzol reagent) was resolved on 15% denaturing polyacrylamide gel and electrotransferred onto Hybond N + nylon membranes (Amersham). Hybridization with specific [γ32P]ATP end-labelled DNA oligonucleotides was carried out as described previously2.
Luciferase assay
Luciferase assays were performed as described17 with the following modification: HeLa cells were first co-transfected with firefly and Renilla luciferase reporter gene expression plasmids. A second transfection of siRNA against TRBP, Dicer or TFII-I together with siRNA against firefly luciferase was performed.
Acknowledgements
We thank T. Beer for assistance with the microcapillary HPLC/mass spectrometry. R.S. was supported by a grant from NIH and American Cancer Society. R.I.G. is a fellow of the Jane Coffin Childs Fund for Medical Research.
Footnotes
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Gregory RI, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 3.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 4.Han J, et al. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 6.Grishok A, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 7.Hutvagner G, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 8.Ketting RF, et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sontheimer EJ. Assembly and function of RNA silencing complexes. Nature Rev. Mol. Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- 10.Gatignol A, Buckler-White A, Berkhout B, Jeang KT. Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 1991;251:1597–1600. doi: 10.1126/science.2011739. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 12.Meister G, et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, et al. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science. 2003;301:1921–1925. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 15.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 17.Doi N, et al. Short-interfering-RNA-mediated gene silencing in mammalian cells requires Dicer and eIF2C translation initiation factors. Curr. Biol. 2003;13:41–46. doi: 10.1016/s0960-9822(02)01394-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhong J, Peters AH, Lee K, Braun RE. A double-stranded RNA binding protein required for activation of repressed messages in mammalian germ cells. Nature Genet. 1999;22:171–174. doi: 10.1038/9684. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein E, et al. Dicer is essential for mouse development. Nature Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]