Abstract
Background and Aims
Animal pollination is typically an uncertain process that interacts with self-incompatibility status to determine reproductive success. Seed set is often pollen-limited, but species with late-acting self-incompatibility (SI) may be particularly vulnerable, if self-pollen deposition results in ovule discounting. Pollination is examined and the occurrence of late-acting SI and ovule discounting assessed in Cyrtanthus breviflorus.
Methods
The pollination system was characterized by observing floral visitors and assessing nectar production and spectral reflectance of flowers. To assess late-acting SI and ovule discounting, growth of self- and cross-pollen tubes, and seed set following open pollination or hand pollination with varying proportions of self- and cross-pollen, were examined.
Key Results
Native honeybees Apis mellifera scutellata pollinated flowers as they actively collected pollen. Most flowers (≥70 %) did not contain nectar, while the rest produced minute volumes of dilute nectar. The flowers which are yellow to humans are visually conspicuous to bees with a strong contrast between UV-reflecting tepals and UV-absorbing anthers and pollen. Plants were self-incompatible, but self-rejection was late-acting and both self- and cross-pollen tubes penetrated ovules. Seed set of open-pollinated flowers was pollen-limited, despite pollen deposition exceeding ovule number by 6-fold. Open-pollinated seed set was similar to that of the cross + self-pollen treatment, but was less than that of the cross-pollen-only treatment.
Conclusions
Flowers of C. breviflorus are pollinated primarily by pollen-collecting bees and possess a late-acting SI system, previously unknown in this clade of the Amaryllidaceae. Pollinators of C. breviflorus deposit mixtures of cross- and self-pollen and, because SI is late-acting, self-pollen disables ovules, reducing female fertility. This study thus contributes to growing evidence that seed production in plants with late-acting SI systems is frequently limited by pollen quality, even when pollinators are abundant.
Keywords: Amarydillaceae, Cyrtanthus breviflorus, honeybee pollination, late-acting self-incompatibility, ovule discounting, pollen limitation, pollen quantity and quality
INTRODUCTION
Animal pollination is typically an uncertain and inefficient process (Harder and Thomson, 1989). Uncertainty arises because pollinators vary in their spatial and temporal abundance, and differ in their ability to disperse and deposit pollen (Wilson and Thomson, 1991). Pollination uncertainty has both ecological and evolutionary consequences. A direct and common ecological effect is pollen limitation of seed production. Pollen limitation occurs when some ovules remain unfertilized because either too few pollen grains are deposited or pollen is of poor quality (Ashman et al., 2004; Knight et al., 2005). Owing to its potential effects on plant fitness, pollen limitation creates opportunities for selection on floral traits that influence pollen dispersal and receipt (Ashman and Morgan, 2004; Harder and Aizen, 2010). Further, selection in response to pollen limitation is expected to generate floral trait shifts that may ultimately be sufficient to cause speciation (Stebbins, 1970; Johnson, 2006; Harder and Johnson, 2009).
The vulnerability of plants to the uncertainties of pollination depends on their level of self-incompatibility. Comparative surveys reveal that pollen limitation is more likely in self-incompatible (SI) than self-compatible species (Larson and Barrett, 2000; Knight et al., 2005). SI entails the active rejection of male gametophytes that carry the same S-alleles as the female sporophyte (de Nettancourt, 2001). This rejection has been traditionally viewed as occurring in the stigma or style, but with late-acting SI (LSI) rejection occurs in the ovary (Seavey and Bawa, 1986). In one form of LSI, self-sterility involves a pre-zygotic reduction in the availability of fertile ovules, resulting from embryo sac degeneration following self-pollination (Sage et al., 1999, 2006). In other cases, rejection is post-zygotic and fertilization takes place, but embryos fail to develop (Gibbs et al., 1999; Sage and Samson, 2003; Bittencourt et al., 2003). However, LSI remains controversial because, for most species, genetic control of self-sterility has not been investigated, and, for this reason, some putative cases of LSI may be better explained by early-acting inbreeding depression. One notable exception, however, is the study by Lipow and Wyatt (2000) that demonstrates LSI in Asclepias exaltata is controlled by a single locus with multiple alleles.
Regardless of the mechanism involved, LSI could bear reproductive costs under natural conditions. If self- and cross-pollen tubes grow equally well into the ovary, then self-pollen could reduce female fertility by disabling ovules that would otherwise have participated in seed production (i.e. ovule discounting; Barrett et al., 1996). Several studies have used mixtures of self- and cross-pollen in hand-pollination experiments to demonstrate ovule discounting in species with LSI (Waser and Price, 1991; Sage et al., 1999; Gribel and Gibbs, 2002). However, whether ovule discounting contributes to pollen limitation in such species is still largely unknown. Further, ovule discounting has implications for the detection of pollen limitation, which is typically assessed by the addition of cross-pollen to flowers that are otherwise subject to natural pollination conditions (Aizen and Harder, 2007). Increased seed production in such experiments is usually interpreted as indicating an insufficiency in the quantity of pollen deposited on stigmas. But pollen quality may be the limiting factor if pollinators deposit self-pollen on stigmas causing some ovules to be discounted (Ashman et al., 2004). This problem raises questions about whether pollen quantity limits seed production as commonly as supplementation experiments suggest (Aizen and Harder, 2007).
LSI appears to be clustered in certain plant families or family alliances rather than being randomly distributed among angiosperm taxa (Gibbs and Bianchi, 1999). One such cluster is in the Liliales and Asparagales, the latter that includes the Amaryllidaceae (Chase, 2004). In this family, LSI has been reported in Narcissus tazetta (Dulberger, 1964) and N. triandrus (Sage et al., 1999). Interestingly, conventional SI has also been reported in the family. In Zephyanthes candida, self-pollen tubes are inhibited at the junction of the stigma and style (Ghosh and Shivanna, 1984). The Amaryllidaceae has a major centre of diversity in South Africa, but despite high levels of endemism and horticultural potential, pollination and self-incompatibility have been little studied. This is exemplified by Cyrtanthus, a genus of about 56 species that exhibit remarkable diversity in floral morphology and colour (Snijman and Meerow, 2010). Pollinator observations and inferred pollination systems based on the tubular floral form indicate that most species are pollinated by sunbirds or insects with long-mouth parts, such as butterflies, hawkmoths and long-proboscid flies (Johnson and Bond, 1997; Goldblatt and Manning, 2000; Manning and Snijman, 2002; Snijman and Meerow, 2010). One exception is C. breviflorus, which has campanulate flowers putatively suited for pollination by short-tongued generalist insects (Snijman and Meerow, 2010). This species therefore provides an ideal opportunity not only to understand better the pollination systems that characterize Cyrtanthus, but also to more fully assess the occurrence of LSI in the Amaryllidaceae.
Here, pollination and self-incompatibility are examined in Cyrtanthus breviflorus. The specific aims were: (a) to document floral visitors and their likely effectiveness as pollinators; (b) to examine nectar production and reflectance spectra of flowers; (c) to determine self-incompatibility status and whether LSI occurs; and (d) to assess pollen limitation and whether ovule discounting acts as a constraint on seed production.
MATERIALS AND METHODS
Study species and sites
Cyrtanthus breviflorus Harv. (Amaryllidaceae) is a bulbous perennial distributed from the Eastern Cape of South Africa to Kenya. Plants are either slender, short-stemmed and occur in coastal and inland grassland, or robust, tall-stemmed and occur in inland marshes. Intermediate forms occur and phenotypic differences disappear under cultivation. Inflorescences are umbellate. Flowers are campanulate, held upright and bright yellow in colour. Flowers are herkogamous and the style extends above the anthers, which occur at two heights. Fruits are capsules, containing flat, winged seeds. The slender form is one of the first grassland plants to flower after late winter and spring fires (Gordon-Grey and Wright, 1969; Reid and Dyer, 1984).
The study was conducted on plants of the slender growth form growing in grassland near Pietermaritzburg in KwaZulu-Natal, South Africa (29°37·713′S, 30°24·020'E; 700 m a.s.l.). Two discrete patches, separated by 600 m (Campus and Pelham), were studied during August and September 2009. Flowering occurred in response to two fires about 2 weeks apart. The Campus and Pelham patches had least 500 and 1000 plants, respectively. The study plants had one to four umbels, each with two to six flowers (Fig. 1A). Flowers opened mostly in the morning and anthers dehisced more or less synchronously a short time later. Anther dehiscence and stigma receptivity overlapped within flowers. The stigma-anther distance, calculated as the difference between the length of the style and the length of the tallest stamens, was 2·73 ± 0·23 mm (range, 0·98–4·18 mm; n = 20). Flowers last for 2–3 d and close their tepals at night.
Fig. 1.
Flowers of Cyrtanthus breviflorus (A) approached by a honeybee and (B) with honeybee contacting anthers. Abbreviation: s, stigma. Scale bars = 5 mm.
Pollinator observations
Transects through patches were walked and the identity and number of insects visiting flowers noted. Transects were 60 m long, took 10 min to complete and were conducted five times per day over 4 d at each site, between 1000 h and 1400 h. It was noted whether visitors collected pollen or nectar, and whether they contacted stigmas as they foraged. Observations were reported for fine sunny days; no pollinators visited flowers on rainy overcast days.
An assessment to find out if floral age affected visits was made by monitoring either young (6 h old) or old flowers (24 h old) in the Campus patch. Young flowers had anthers with pollen whereas old flowers had little or no pollen in anthers. Eighteen plants that had one young and one old flower were used. Each plant was monitored once for 30 min between 0900 h and 1200 h on one day, and different plants were observed over 3 d.
It was also assessed whether the presence of pollen affected visits by monitoring young (6 h old) flowers that were either emasculated (– pollen) or left intact (+ pollen). Anthers on emasculated flowers were removed with forceps, leaving intact flowers untouched. Treatments were assigned to 18 pairs of adjacent plants and plant pairs were monitored once for 30 min as above. Prior to observations, pollinators were excluded from flowers with fine mesh bags.
The effects of floral age and pollen presence on visits were analysed with partially nested ANOVAs. Plant or plant pair was a random factor, nested within days, which was a fixed factor. Floral age and emasculation treatments were also fixed factors.
Floral rewards and attractants
Nectar volume and concentration (percentage sucrose equivalent by weight) were measured in both patches using 5-μL microcapillary tubes and a 0–50 % hand-held refractometer, respectively. Plants with large buds that were just about to open were bagged and measurements were taken about 24 h later (n = 20 flowers at each site). Destructive sampling was needed to ensure that all nectar was collected.
Spectral reflectance across the 300–700 nm range was assessed using an Ocean Optics S2000 spectrometer (Johnson and Andersson, 2002). Using flowers from the Campus patch, upper and lower tepals, dehisced anthers and pollen were assessed. For each, three or four replicates were taken from separate plants and the mean spectrum was calculated.
Experimental pollinations
Controlled pollinations were conducted in each patch to establish whether plants were self-incompatible. Plants were allocated to one of four treatments: (1) bagged and unmanipulated to test for auto-fertility; (2) bagged and self-pollinated; (3) bagged and cross-pollinated using two donors at least 5 m distant; and (4) left open to receive natural pollination. Bags were left on flowers until they wilted. Flowers were emasculated prior to self- and cross-pollinations. In all treatments, only the first flower to open on plants was used to reduce the likelihood of resources limiting seed production. Other flowers were removed only after they had wilted to ensure that floral display in the open-pollinated treatment was not negatively affected.
Five weeks later, fruit set (fruits/flower) and seed set (seeds/unfertilized ovules + aborted seeds + filled seeds) were scored. For the self- and cross-pollination treatments, both of these components were incorporated in an index of combined reproductive output (CRO) calculated as: (fruit set × mean seed set). Then an index of self-compatibility (ISI) was calculated as the ratio of self to cross CROs (Lloyd and Schoen, 1992). ISI values range from 0 (fully self-incompatible) to 1·0 (fully self-compatible).
Fruit set was compared with analysis of deviance (using generalized linear models), with site and pollination treatment as factors. For seed set, a two-way ANOVA, with both site and treatment as fixed factors, was used. CROs were not statistically analysed. The bagged, unpollinated treatment did not produce fruits and was excluded from analyses.
Pollen tubes
To determine the location of the self-sterility barrier, growth of self- and cross-pollen tubes were examined using fluorescence microscopy. Flowers in the Campus patch were self- or cross-pollinated as above (both n = 15). Thirty hours later, pistils were fixed in Carnoy's solution for 24 h and stored in 70 % alcohol. Pistils were softened in 1 n NaOH for 15 h, rinsed in distilled water for 1 h and stained in a 0·1 % solution of aniline blue in 0·1 n K2HPO4 for 10 h. Pistils were mounted in a drop of stain on a microscope slide and squashed under a coverslip. To assist in exposing ovules, the ovary wall was gently removed prior to squashing. Pollen tubes were examined at four levels in the pistil: stigma, mid-style, top of ovary and ovule. Quantifying ovule penetration, however, proved problematic because the micropyle of many ovules was obscured by pollen tubes. Accordingly, the percentage of ovules that were penetrated was scored using only those ovules in which the micropyle was clearly visible (approx. 10 % of ovules in each ovary for five flowers in each treatment).
Pollen limitation and ovule discounting
Ovule discounting in the Campus patch was assessed by allocating plants to one of the four following treatments: (1) cross-only: bagged and cross-pollinated; (2) cross + self: bagged and pollinated with equal amounts of cross-pollen and self-pollen; (3) cross + dead: bagged and pollinated with equal amounts of cross-pollen + dead cross-pollen; and (4) open: naturally pollinated. For all crossing treatments, one anther from each of two donors, located at least 5 m from recipient plants, was used. For cross + self and cross + dead treatments, two self anthers and two anthers with dead pollen, respectively, were added to the two cross anthers. The purpose of the treatment with dead pollen was to control for the effects of halving the amount of cross-pollen when mixtures of cross- and self-pollen were applied. Pollen was killed by microwaving at 800 W for 2 min. To verify the pollen was dead, flowers were pollinated with similarly treated pollen; no fruits were produced (n = 6). Pollinations were standardized to ensure more or less equal pollen loads from each donor by harvesting undehisced anthers into 1·5-mL Eppendorf tubes, and when anthers had dehisced, mixing the pollen thoroughly before saturating stigmas with a toothpick. Flowers were emasculated in the cross-only, cross + self and cross + dead treatments. As above, only the first flower on plants was used in all treatments. Fruit and seed set were calculated as above.
Fruit set was compared among treatments with analysis of deviance. For seed set, a one-way ANOVA was used and then four pair-wise comparisons were conducted using non-orthogonal contrasts corresponding to specific hypotheses, using α = 0·0125 to control for multiple comparisons: (1) the cross-only and open treatments were compared to test whether seed set of open-pollinated flowers was pollen-limited; (2) the cross-only and cross + dead treatments were compared to test whether halving the quantity of cross-pollen reduced seed set; (3) the cross + self and cross + dead treatments were compared to test whether self-pollen interferes with cross-pollen and causes ovule discounting; and (4) the cross + self and open treatments were compared. Provided pollen quantity is not limiting under natural conditions, similar seed set in these two treatments would indicate that pollinators deposit both cross- and self-pollen on stigmas and that self-pollen causes ovule discounting.
Pollen deposition
Stigmatic pollen loads of hand- and open-pollinated flowers were assessed in the Campus patch (both n = 10). Flowers were hand-pollinated as they opened with cross-pollen from two donors at least 5 m distant. Open-pollinated flowers were left untouched. Pistils were harvested 30 h later. The stigmas were placed on small cubes of glycerin jelly with basic fuchsin on a microscope slide and squashed under a coverslip. The number of pollen grains was counted at ×40 magnification. The number of ovules per ovary on 200 different plants was also counted, allowing ovule number to be compared with the number of pollen grains deposited on stigmas. By estimating pollen deposition following hand- and open-pollination and seed set of the cross-only, cross + dead and open treatments (see above), it was possible to assess whether pollen quantity limits seed set.
Statistical analyses
For analyses of deviance, GLMStat 6·0 (Beath, 2004), with binomial error structures and logit link functions, was used. Residual deviances were not significant (all P > 0·50). For ANOVAs, JMP 5·0·1 (SAS Institute Inc., Cary, NC, USA) was used. To meet assumptions of ANOVA, pollinator visits and percentage seed set were transformed (square-root and arcsin square-root, respectively). Means (± standard error) are given.
RESULTS
Pollinator observations
At both sites, about 85 % of floral visitors were pollen-collecting honeybees (Apis mellifera scutellata) (Campus, 86·2 %, n = 94 visitors; Pelham, 84·5 %, n = 71 visitors). Ninety-three percent of honeybees contacted the stigma as they foraged (n = 118 visits; Fig. 1B). All other visitors were small solitary bees, of which 92 % collected pollen and 8 % crawled into the floral tube (n = 24 visits). However, only 13 % of solitary bees contacted the stigma as they foraged, owing to their small size (n = 24 visits).
Honeybees visited young flowers with pollen more often than old flowers without pollen [young, 9·7 ± 1·0 visits h−1; old, 0·4 ± 0·2 visits h−1 (F1,24 = 149·86, P < 0·0001)]. The age × day interaction was not significant (F2,24 = 0·94, P = 0·40). Neither days nor plants affected visitation (day, F2,6 = 2·54, P = 0·16; plants, F6,24 = 1·10, P = 0·39).
Honeybees visited young intact flowers more often than young emasculated flowers [intact, 13·6 ± 1·3 visits h−1; emasculated, 5·2 ± 0·7 visits h−1 (F1,24 = 28·32, P < 0·0001)]. The treatment × day interaction was not significant (F2,24 = 0·40, P = 0·68). Neither days nor plant pairs affected visitation (day, F2,6 = 0·89, P = 0·46; plant pair, F6,24 = 1·22, P = 0·33).
Floral rewards and attractants
Most flowers produced no detectable nectar during the first 24 h of floral life (Campus, 70 %; Pelham, 90 %; both n = 20 flowers). For remaining flowers, the volume and concentration of nectar was 0·24 ± 0·04 µL and 14·1 ± 2·4 %, respectively (sites pooled, n = 8).
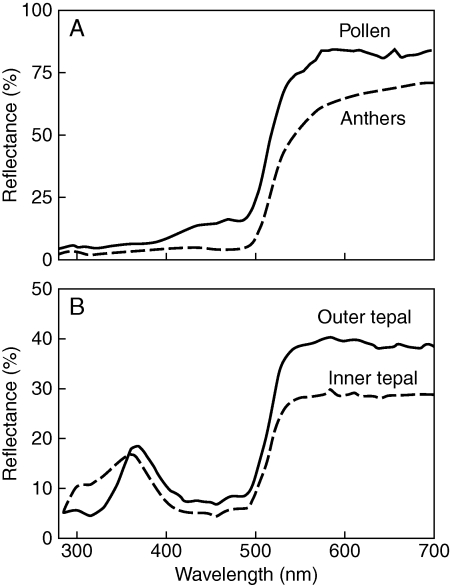
Flowers reflected maximally in the 500–600 nm range, which is perceived as yellow by humans (Fig. 2). Tepals exhibited UV reflectance with a peak in the 300–400 nm range, while anthers and pollen were contrastingly UV-absorptive (Fig. 2).
Fig. 2.
Reflectance spectra for (A) pollen and anthers and (B) upper and lower tepals in Cyrtanthus breviflorus. Each curve represents the mean spectrum calculated from individual replicates.
Experimental pollinations
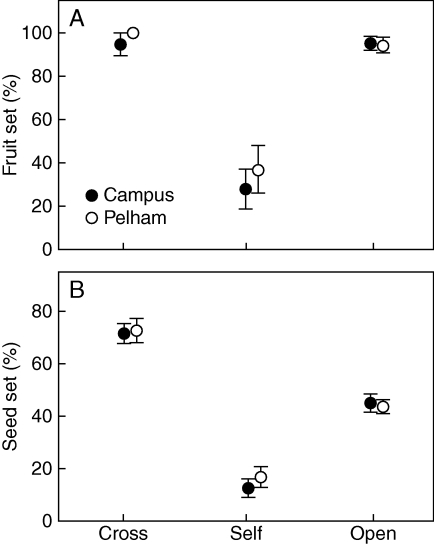
None of the bagged, unpollinated flowers set fruits, indicating that pollinators are essential for seed production. Forty per cent of self-pollinated flowers produced fruits, which was significantly less than cross- and open-pollinated flowers (>90 %, Fig. 3A, χ2 = 69·8, d.f. = 2, P < 0·0001). Seed set differed significantly among the three pollination treatments (Fig. 3B; F2,155 = 56·6, P < 0·001). Selfed seed set was reduced by 80 % and 67 % compared with crossed and open seed set, respectively. Seed set of open-pollinated plants was pollen-limited and was reduced by 38 % compared with crossed seed set (Fig. 3B). Sites did not differ in either analysis (fruit set, χ2 = 0·32, P = 0·572; seed set F1,155 = 0·21, P = 0·647), and for seed set, the site × treatment interaction was not significant (F2,155 = 0·32, P = 0·726).
Fig. 3.
Mean (± s.e.) percentage of (A) fruit set and (B) seed set following cross-, self- and open-pollination in the Campus and Pelham patches of Cyrtanthus breviflorus, as indicated.
Combined reproductive output of selfed and crossed flowers was 0·04 ± 0·01 and 0·68 ± 0·04 at Campus, and 0·06 ± 0·02 and 0·73 ± 0·04 at Pelham, respectively. The ISI at both sites was <0·1 (Campus, 0·05 ± 0·02; Pelham, 0·08 ± 0·02).
Pollen tubes
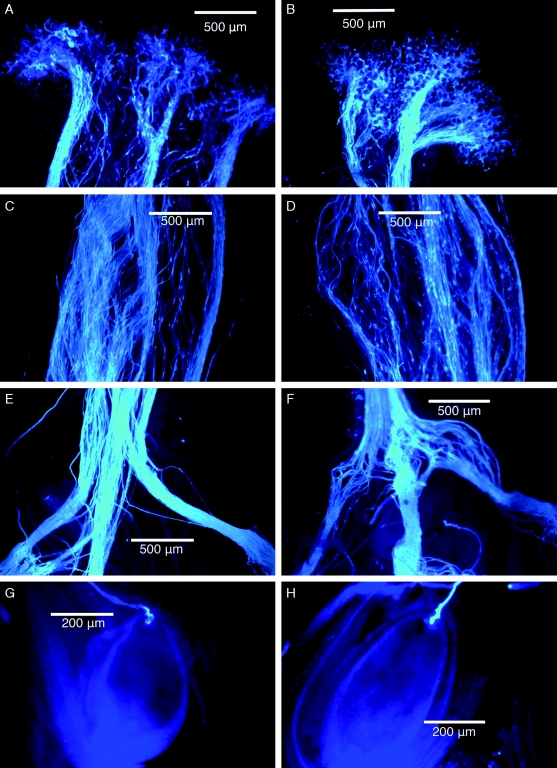
No differences in the growth of self- and cross-pollen tubes were observed (Fig. 4). In both treatments, all 15 flowers had ovules that were penetrated (Fig. 4G and H). For flowers in which individual ovules could be examined (n = 5 flowers), self- and cross-pollen tubes penetrated similar numbers of ovules (self, 78 %; cross, 79 %; n = 37 and 42 ovules, respectively).
Fig. 4.
Pollen tubes in cross-pollinated (on the left) and self-pollinated (on the right) pistils of Cyrtanthus breviflorus. Fluorescence micrographs show pollen tubes on stigmas (A, B), in mid-styles (C, D), at the top of ovaries (E, F) and associated with individual ovules (G, H).
Pollen limitation and ovule discounting
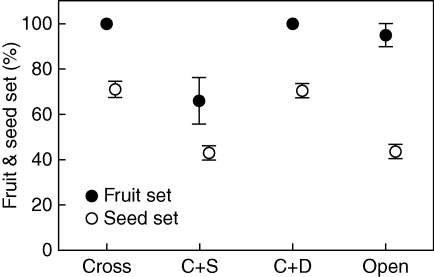
Fruit set in the cross + self treatment was about 66 % that of the other three treatments (Fig. 5; χ2 = 22·8, d.f. = 3, P < 0·0001). Seed set also differed among treatments (F3,80 = 30·91, P < 0·0001). For the four pair-wise comparisons: (1) seed set in the open treatment was only 61 % that of the cross-only treatment (F1,80 = 45·39, P < 0·0001); (2) seed set did not differ between the cross-only and cross + dead treatments (F1,80 = 0·10, P = 0·75); (3) seed set in the cross + self treatment was only 61 % that of the cross + dead treatment (F1,80 = 47·18, P < 0·0001); and (4) seed set did not differ between the cross + self and the open treatments (Fig. 5; F1,80 < 0·10, P = 0·97).
Fig. 5.
Mean (± s.e.) percentage of fruit set and seed set, as indicated, of Cyrtanthus breviflorus flowers pollinated with cross-pollen, cross + self-pollen and cross + dead pollen, and open-pollinated.
Pollen deposition
Open-pollinated flowers had 72 % as many pollen grains on their stigmas as did hand cross-pollinated flowers (open-pollinated, 205·5 ± 15·5; hand-pollinated, 284·6 ± 18·6 grains; F1,18 = 10·74, P < 0·005). Despite having fewer grains deposited, pollen loads of open-pollinated flowers exceeded the number of ovules per flower by almost 6-fold (ovules, 35·9 ± 0·5).
DISCUSSION
Pollinators and floral traits
The results indicate that the pollination system of C. breviflorus is specialized for active pollen collection by bees. In this study, only native honeybees (Apis mellifera scutellata) were effective pollinators. As with many pollen-reward flowers (Bernhardt, 1996), most C. breviflorus flowers did not produce nectar, or produced only minute amounts of dilute nectar. It was demonstrated here that pollen was the floral reward by emasculating flowers, causing visits by honeybees to decrease markedly compared with intact controls. Snijman (2007) observed that C. aureolinus also has yellow flowers, produces little or no nectar and is visited by pollen-collecting honeybees (see also Snijman and Meerow, 2010). Of the 42 species considered by Snijman and Meerow (2010) in a recent phylogenetic analysis, 37 were putatively pollinated by sunbirds, butterflies, sphingid moths, noctuid moths or long-proboscid flies. Contrasting with C. breviflorus, available data show that Cyrtanthus species pollinated by butterflies and sunbirds produce moderate-to-large amounts of nectar (Johnson and Bond, 1997).
Flowers specialized for active pollen collection have simple bowl-shaped perianths or short floral tubes, allowing anthers to be displayed prominently. Such flowers often have specialized porose anthers or pseudantherous structures such as staminodes (Bernhardt, 1996). In C. breviflorus, flowers are campanulate and erect and all anthers are exserted beyond the short floral tube, although specialized structures are absent. The flowers produce only trace amounts (<10 ng flower−1 h−1) of floral volatiles (S. D. Johnson, unpubl. res.) and thus it is suspected that floral visual cues attract bees. Besides reflecting long wavelengths, tepals also strongly reflected in the ultraviolet (UV) part of the spectrum, whereas anthers and pollen only reflected long wavelengths. Because honeybees have trichromatic colour vision with UV-, blue- and green-receptors (Chittka et al., 1994), they would perceive C. breviflorus tepals as UV-green, but anthers and pollen as green. Contrasting spectra of perianth parts and anthers have been documented in other species and are thought to function to increase attractiveness of anthers to pollen-collecting insects (Bernhardt, 1996; Lunau, 2000).
Self-incompatibility
It was found that C. breviflorus was largely self-sterile (ISI < 0·1) which is consistent with earlier reports (Gordon-Gray and Wright, 1969; Ising, 1969). However, the present study provides the first evidence that this self-sterility is caused by LSI. Self- and cross-pollen tubes penetrated equally well to the top of the ovary and, based on a smaller sample size, were equally capable of penetrating ovules (Fig. 4). LSI rather than early-acting inbreeding depression is inferred because abscission of selfed flowers occurred within a few days of pollination and selfed seed set was uniformly low for all plants (Seavey and Bawa, 1986). To determine whether the self-sterility barrier in C. breviflorus is pre- or post-zygotic, histological analysis of post-pollination events at different time intervals after self- and cross-pollinations are now required (Bittencourt et al., 2003). Further, experiments such as those conducted by Lipow and Wyatt (2000) would be necessary to elucidate the genetic control of LSI (i.e. within family diallels). LSI has been reported in two other species of Amaryllidaceae, Narcissus tazetta (Dulberger, 1964) and N. triandrus (Sage et al., 1999). In N. tazetta, self-rejection was associated with a failure in ovule development after pollen tubes entered the micropyle and penetrated the embryo sac. By contrast, in N. triandrus the presence of self-pollen tubes in the style was found to inhibit the maturation of a proportion of ovules in the ovary, possibly as a result of long-distance signalling between pollen tubes and ovarian tissues.
Although LSI is expected to ensure a highly outcrossed mating system, opportunities for self-fertilization were not totally precluded in C. breviflorus. As in N. triandrus (Barrett et al., 1997), a sizable percentage of self-pollinated flowers produced fruits (approx. 40 %), but each contained few seeds. In N. triandrus, self-pollen tubes are able to penetrate ovules that are fertile, which results in about 12 % of seeds being self-fertilized following mixed pollinations with self- and cross-pollen (Sage et al., 1999). Genetic marker studies would be useful to determine the parentage of seeds following mixed pollinations in C. breviflorus, and to assess mating patterns of open-pollinated plants. The leaky nature of LSI in C. breviflorus and N. triandrus, contrasts with reports of LSI in Bombacaceae and Bignoniaceae species, in which virtually no seeds are produced following selfing (Gibbs and Bianchi, 1999; Gribel and Gibbs, 2002; Bittencourt et al., 2003).
Pollen limitation and ovule discounting
As is common in other SI species (Larson and Barrett, 2000), reproductive success of C. breviflorus in both populations was pollen-limited as evidenced by lower seed set of open-pollinated flowers compared with that of cross-pollinated flowers. Pollen limitation, however, was not due to a scarcity of pollinators or the quantity of pollen deposited on stigmas. It was found that newly opened flowers received 9–14 honeybee visits h−1 and open-pollinated flowers had average stigmatic pollen loads that were almost 6-fold greater than ovule number per flower (i.e. 206 grains versus 36 ovules). Indeed, it was estimated that this deposition exceeded the number of compatible cross grains required for full seed set by 45 %, as evidenced by the cross + dead treatment, which had similarly high seed set, but only half the cross-pollen deposition as the cross-only treatment (i.e. 284 pollen grains × 0·5 = 142).
Even if pollination is seemingly sufficient for full seed set, female fertility can still be limited in species with SI, if pollen loads comprise a large proportion of self- or incompatible cross-pollen (Aizen and Harder, 2007). In this case, quantities of compatible cross-pollen may be insufficient to fertilize all available ovules, or in species with LSI compatible pollen may be sufficient but competes for ovules with incompatible pollen, causing ovule discounting. In C. breviflorus, pollen-limited seed set of open-pollinated plants could be explained by the deposition of limited amounts of cross-pollen followed by large deposits of self-pollen. However, this is considered unlikely because honeybees frequently moved between plants and probably cause high levels of cross-pollination. It is assumed that cross- and self-pollen is deposited simultaneously in more or less equal proportions for the following reasons. Seed set of open-pollinated flowers did not differ from that of flowers pollinated with equal quantities of cross + self-pollen, and was intermediate to that of the self-only and cross-only treatments. Reduced seed set in the cross + self-pollen treatment was not an artefact of a lack of cross-pollen because flowers would have received similar amounts of cross-pollen as those in the cross + dead treatment. Owing to LSI, a pollination mix of cross- and self-pollen in C. breviflorus would cause ovule discounting and pollen limitation of female fertility under natural conditions.
Some self-pollination is probably unavoidable in C. breviflorus, firstly because anther dehiscence and stigma receptivity overlap within flowers, allowing for pollinator-mediated intra-floral self-pollination, and secondly because many plants have several flowering scapes and geitonogamy could occur between scapes. Geitonogamy within scapes, however, is less likely because only one flower per scape usually opens per day and honeybees avoid visiting older flowers. Hand-pollination experiments involving mixtures of self- and cross-pollen have also been used in other studies to infer that self-pollen deposited by pollinators reduces open-pollinated seed set (Ramsey, 1995; Gribel and Gibbs, 2002; but see Cesaro et al., 2004). To assess directly the effect of self-pollen on female fertility in Bulbine bulbosa, a species with strong early-acting inbreeding depression, flowers were emasculated and it was found that pollinator-mediated selfing reduced female fertility by 50 % (Vaughton and Ramsey, 2010). Similar experiments, however, would be difficult to perform on C. breviflorus because unlike B. bulbosa, pollinators discriminate against emasculated flowers.
In pollen-limited environments, selection is expected to favour floral traits that improve the amount and efficiency of pollen dispersal and receipt (Ashman et al., 2004). In C. breviflorus, LSI predisposes plants to pollen limitation caused by deposition of self-pollen. Yet, selection against this trait may be unlikely because SI, no matter whether self-rejection occurs in the stigma, style or ovary, ensures outcrossing, the benefits of which often outweigh the costs of producing fewer seeds. As suggested by Knight et al. (2005) the prevalence of pollen limitation may be explained, at least in part, by selection for floral and plant traits that promote outcrossing even though pollen limitation increases as a correlated response. Further, LSI has been viewed as wasteful in terms of resources invested in ovules that fail to produce seeds (Seavey and Bawa, 1986). However, by minimizing investment in low-quality selfed seeds during any one reproductive episode, perennials such as C. breviflorus may ultimately save resources that can be devoted to producing outcrossed seeds over their lifetimes (Waser and Price, 1991; Sage et al., 1999).
Three main concluding points can be made: (1) pollination by generalist pollen-collecting insects occurs in Cyrtanthus and, in C. breviflorus, is associated with short-tubed campanulate flowers with vivid yellow tepal coloration and UV-contrasting anthers and pollen; (2) it is the first time LSI has been documented in Cyrtanthus; and (3) owing to this LSI system, the self-pollen fraction of natural pollen loads deposited by insects discounts ovules and contributes to pollen limitation of female fertility. Nevertheless, discounting of some selfed ovules ensures outcrossing, and in the long-term probably minimizes resource costs associated with self-pollination.
ACKNOWLEGEMENTS
We thank Sandy-Lynn Steenhuisen for assistance with the project and the EM unit staff at the University of KwaZulu-Natal, Pietermaritzburg for access to their facilities. Pelham Senior Primary School kindly provided access to Pelham Park.
LITERATURE CITED
- Aizen MA, Harder LD. Expanding the limits of the pollen-limitation concept: effects of pollen quality and quality. Ecology. 2007;88:271–281. doi: 10.1890/06-1017. [DOI] [PubMed] [Google Scholar]
- Ashman T-L, Morgan MT. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proceedings of the Royal Society of London Series B. 2004;271:553–559. doi: 10.1098/rspb.2003.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Barrett SCH, Lloyd DG, Arroyo J. Stylar polymorphisms and the evolution of heterostyly in Narcissus (Amaryllidaceae) In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman and Hall; 1996. pp. 339–376. [Google Scholar]
- Barrett SCH, Cole WW, Arroyo J, Cruzan MB, Lloyd DG. Sexual polymorphisms in Narcissus triandrus (Amaryllidaceae): is this species tristylous? Heredity. 1997;78:135–145. [Google Scholar]
- Beath K. GLMStat 6·0. 2004 Website http://www.kjbeath.com.au/glmstat/index.html/ (accessed 10 January 2010) [Google Scholar]
- Bernhardt P. Anther adaptation in animal pollination. In: D'Arcy WG, Keating RC, editors. The anther: form function and phylogeny. Cambridge: Cambridge University Press; 1996. pp. 192–220. [Google Scholar]
- Bittencourt NS, Jr, Gibbs PE, Semir J. Histological study of post-pollination events in Spathodea campanulata Beauv. (Bignoniaceae), a species with late-acting self-incompatibility. Annals of Botany. 2003;91:827–834. doi: 10.1093/aob/mcg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaro AC, Barrett SCH, Maurice S, Vaissiere BE, Thompson JD. An experimental evaluation of self-interference in Narcissus assoanus: functional and evolutionary implications. Journal of Evolutionary Biology. 2004;17:1367–1376. doi: 10.1111/j.1420-9101.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Chase MW. Monocot relationships: an overview. American Journal of Botany. 2004;91:1645–1655. doi: 10.3732/ajb.91.10.1645. [DOI] [PubMed] [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. Ultraviolet as a component of flower reflections, and colour perception of Hymenoptera. Vision Research. 1994;34:1489–1508. doi: 10.1016/0042-6989(94)90151-1. [DOI] [PubMed] [Google Scholar]
- De Nettancour D. Incompatibility and incongruity in wild and cultivated plants. Berlin: Springer-Verlag; 2001. [Google Scholar]
- Dulberger R. Flower dimorphism and self-compatibility in Narcissus tazetta. Evolution. 1964;18:361–363. [Google Scholar]
- Ghosh S, Shivanna KR. Structure and cytochemistry of the stigma and pollen-pistil interaction in Zephyranthes. Annals of Botany. 1984;53:91–105. [Google Scholar]
- Gibbs PE, Bianchi MB. Does late-acting self-incompatibility (LSI) show family clustering? Two species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa. Annals of Botany. 1999;84:449–457. [Google Scholar]
- Gibbs PE, Oliveira PE, Bianchi MB. Post-zygotic control of selfing Hymenaea stigonocarpa (Leguminosae-Caesalpinioideae), a bat-pollinated tree of the Brazilian cerrados. International Journal of Plant Sciences. 1999;160:72–78. [Google Scholar]
- Goldblatt P, Manning JC. The long-proboscid fly pollination system in southern Africa. Annals of the Missouri Botanical Garden. 2000;89:281–302. [Google Scholar]
- Gordon-Gray KD, Wright FB. Cyrtanthus breviflorus and Cyrtanthus luteus (Amarydillaceae): observations with particular reference to Natal populations. Journal of South African Botany. 1969;35:35–62. [Google Scholar]
- Gribel R, Gibbs PE. High outbreeding as a consequence of selfed ovule mortality and single vector bat pollination in the Amazonian tree Pseudobombax munguba (Bombacaceae) International Journal of Plant Sciences. 2002;163:1035–1043. [Google Scholar]
- Harder L, Aizen M. Floral adaptation and diversification under pollen limitation. Philosophical Transactions of the Royal Society Series B. 2010;365:529–543. doi: 10.1098/rstb.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for adaptation. New Phytologist. 2009;183:530–535. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Ising G. Cytogenetic studies in Cyrtanthus. IV. Chromosome morphology in Cyrtanthus luteus Baker (Anoiganthus luteus Baker) and Cyrtanthus breviflorus Harvey (Anoiganthus breviflorus Baker) Hereditas. 1969;63:352–384. [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Johnson SD, Andersson S. A simple field method for manipulating ultraviolet reflectance in flowers. Canadian Journal of Botany. 2002;80:1325–1328. [Google Scholar]
- Johnson SD, Bond WJ. Evidence for widespread pollen limitation of Cape wildflowers. Oecologia. 1997;109:530–534. doi: 10.1007/s004420050113. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: pattern and process. Annual Reviews of Ecology, Evolution and Systematics. 2005;36:467–497. [Google Scholar]
- Larson BMH, Barrett SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- Lipow SR, Wyatt R. Single gene control of post-zygotic self-incompatibility in poke milkweed, Asclepias exaltata L. Genetics. 2000;154:893–907. doi: 10.1093/genetics/154.2.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DG, Schoen DJ. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences. 1992;153:358–369. [Google Scholar]
- Lunau K. The ecology and evolution of visual pollen signals. Plant Systematics and Evolution. 2000;222:89–111. [Google Scholar]
- Manning JC, Snijman D. Hawkmoth-pollination in Crinum variabile (Amaryllidaceae) and the biogeography of sphingophily in Southern Africa. South African Journal of Botany. 2002;68:212–216. [Google Scholar]
- Ramsey M. Ovule pre-emption and pollen limitation in a self-fertile perennial herb (Blandfordia grandiflora, Liliaceae) Oecologia. 1995;103:101–108. doi: 10.1007/BF00328430. [DOI] [PubMed] [Google Scholar]
- Reid C, Dyer RA. La Jolla, CA: Botanical Research Institute, Pretoria and American Plant Life Society; 1984. A review of the southern African species of Cyrtanthus. [Google Scholar]
- Sage TL, Sampson FB. Evidence of ovarian self-incompatibility as a cause of self-sterility in the relictual woody angiosperm, Pseudowintera axillaris (Winteraceae) Annals of Botany. 2003;91:807–816. doi: 10.1093/aob/mcg085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage TL, Price MV, Waser NM. Self-sterility in Ipomopsis aggregata (Polemoniaceae) is due to prezygotic ovule degeneration. American Journal of Botany. 2006;93:254–262. doi: 10.3732/ajb.93.2.254. [DOI] [PubMed] [Google Scholar]
- Sage TL, Strumas F, Cole B, Barrett SCH. Differential ovule development following self- and cross-pollination; the basis of self-sterility in Narcissus triandrus (Amaryllidaceae) American Journal of Botany. 1999;86:855–870. [PubMed] [Google Scholar]
- Seavey SR, Bawa KS. Late-acting self-incompatibility in angiosperms. Botanical Review. 1986;52:195–218. [Google Scholar]
- Snijman DA. Notes on new and misunderstood taxa of Cyrtanthus (Amaryllidaceae: Cyrtantheae) from the Western Cape, Eastern Cape and KwaZulu-Natal, South Africa. Bothalia. 2007;37:1–8. [Google Scholar]
- Snijman DA, Meerow AW. Floral and macroecological evolution within Cyrtanthus (Amaryllidaceae): inferences from combined analyses of plastid ndhF and nrDNA ITS sequences. South African Journal of Botany. 2010;76:217–238. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Vaughton G, Ramsey M. Floral emasculation reveals pollen quality limitation of seed output in Bulbine bulbosa (Asphodelaceae) Amercian Journal of Botany. 2010;97:174–178. doi: 10.3732/ajb.0900183. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. Reproductive costs of self-pollination in Ipomopsis aggregata (Polemoniaceae): are ovules usurped? American Journal of Botany. 1991;78:1036–1043. [Google Scholar]
- Wilson P, Thomson JD. Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology. 1991;72:1503–1507. [Google Scholar]