Abstract
Background and Aims
Insectivorous plants frequently display their flowers on the ends of long racemes. Conventional wisdom is that long racemes in insectivorous plants have evolved to provide spatial separation between flowers and traps, which consequently prevents pollinators from being captured. However, it is also possible that long racemes evolved for better seed dispersal or to make flowers more visible to pollinators.
Methods
Two sympatric insectivorous plants with identical pollinators were studied: Drosera cistiflora, with an upright growth form but a short raceme; and Drosera pauciflora, with a basal rosette of traps and a very long raceme. If long racemes evolved to protect their pollinators then D. cistiflora should capture more pollinators than D. pauciflora. However, if long racemes evolved to attract pollinators then taller flowers should receive more pollination visits than shorter flowers.
Key Results
Examination of D. pauciflora and D. cistiflora traps revealed that no pollinators were captured by either species, suggesting that long racemes did not evolve to protect pollinators from being captured. Experimental manipulations of flower height in D. cistiflora showed that experimentally shortened plants received significantly fewer pollination visits than plants which were taller in stature.
Conclusions
Long scapes in Drosera and non-insectivorous plants probably evolved due to similar selective pressures such as pollinator attraction.
Keywords: Carnivorous plant, Drosera cistiflora, Drosera pauciflora, food or sex, inflorescence height, insectivorous plant, pollination, pollinator attraction, pollinator–prey conflict, pollinator protection, prey-capture
INTRODUCTION
Inflorescence height is frequently viewed as being a trade-off between pollinators selecting for greater inflorescence height and herbivores selecting for reduced inflorescence height. Multiple studies have shown that plants with longer scapes have greater reproductive success than shorter-scaped plants (e.g. Hainsworth et al., 1984; Ehrlen et al., 2002; Agren et al., 2006; Vanhoenacker et al., 2006; Vandewoestijne et al., 2009), although it is sometimes not clear whether this is because longer-scaped plants simply have more resources than shorter-scaped plants or whether they are more attractive to pollinators. Other studies (e.g. Peakall and Handel, 1993; Donnelly et al., 1998; O'Connell and Johnston, 1998; Lortie and Aarssen, 1999) actually examine the visitation rates (directly or through pollen or pollinaria deposition/removal) of short- and long-scaped plants and show that long-scaped plants are often actively favoured by pollinators over short-scaped plants, perhaps because they are more visible. However, much longer inflorescences may be selected against if pollinators search for flowers within a particular height range, e.g. Peakall and Handel (1993). It has also been shown that longer-scaped inflorescences are more likely to be grazed than shorter-scaped inflorescences (e.g. Ehrlen, 1997; Torang et al., 2006), probably because they are also more visible to grazers. Seed predators and pathogens may also have negative effects on inflorescence height (Galen and Cuba, 2001; Collin et al., 2002; Leimu et al., 2002; Cariveau et al., 2004; Giles et al., 2006) for the same reason. In addition to pollinator attractiveness, some plants exhibit post-floral elongation of their flower stalks, possibly in order to increase seed dispersal distances (Verbeek and Boasson, 1995), so elongated scapes may also be interpreted in this context. Very few studies have investigated the effects of scape length on seed dispersal ability.
Interestingly, the long scape lengths frequently observed for insectivorous plants are usually interpreted not as adaptations to attract pollinators or disperse seeds but rather as adaptations to protect pollinators from becoming ensnared (Juniper et al., 1989; Givnish, 1989; Ellison and Gotelli, 2001; Ellison et al., 2003). This so-called pollinator–prey conflict is hinted at by Wickler (1968) and Wiens (1978), who point out that the pitfall traps of Sarracenia look and smell similar to flowers, and may in fact mimic them in order to trap pollinators. This is also supported by Jurgens et al. (2009), who found that the traps of some Sarracenia plants produced scent volatiles which were similar to the volatiles produced by flowers, thus potentially attracting would-be pollinators to their traps. However, actual evidence for this pollinator–prey conflict is very scarce and is supported by a single study (Zamora, 1999), who showed that in marginal habitats which were shady, very small pollinators such as thrips and tiny beetles contributed significantly to seed set in Pinguicula vallisneriifolia. These pollinators were frequently captured by the Pinguicula plants, which were pollen limited and may have been consuming potential pollinators.
In contrast, Anderson and Midgley (2001) showed that the distribution of Drosera scape lengths suggested that the evolution of elongated scapes in Drosera was driven by the same selective pressures as those that drove long scapes in non-insectivorous plants. They hypothesized that long scapes evolved for pollinator attraction (pollinator attraction hypothesis or PAH) or seed dispersal and not to protect their pollinators from sticky traps (pollinator protection hypothesis or PPH). They showed that Drosera with upright growth forms seldom produced long scapes whereas it was only rosette growth forms of Drosera which produced long scapes in order to elevate their flowers from ground level. However, another potential reason for the lack of long scapes in upright forms may simply be related to the architectural constraints associated with the lack of stability and strength when building a long scape on an already tall plant and then on top of that a large flower. Some support was found for the PAH by Murza et al. (2006), who found that there was very little overlap between the pollinators and the prey of Drosera anglica. However, in order to distinguish properly between the PAH and the PPH, it would be better to make a comparison of pollinators and prey between two sympatric species of Drosera, one with a rosette growth form and a long scape, and the other with an upright growth form and a short scape.
Such a site was found in a small private nature reserve in Darling, South Africa, where two Drosera species grow sympatrically (Fig. 1A, B). One species, Drosera pauciflora, has a rosette growth form and a long scape of about 156 mm in length which supports a large pink–purple flower (Fig. 1B). The other species, Drosera cistiflora, has a thin upright stem of about 120 mm in length which supports sticky traps along its entire length. The traps and flowers are separated by a very short peduncle of about 28 mm (Fig. 1B). The flowers of the two Drosera species are of similar height above ground. D. cistiflora flowers in this population vary from white to pink–purple, which seems very similar to that found in D. pauciflora. I expected that if PPH has driven the elongation of scapes in D. pauciflora, then this species should capture fewer pollinators than its short-scaped counterpart, D. cistiflora. If PAH has driven the elongation of D. pauciflora scapes then I would not expect to see any differences in the pollinator capture rates of the two species but I would expect to find differences in the attractiveness of tall versus short flowers to pollinators.
Fig. 1.
(A) Drosera cistiflora showing the small distance separating the traps and flower. (B) Drosera pauciflora showing with a long scape and large distance separating flowers from traps. (C) A large monkey beetle visiting a D. cistiflora flower for pollen. (D) A Tabanid fly on the petal of D. cistiflora. (E) The typical size of the prey items captured by D. cistiflora. (F) D. pauciflora with a large monkey beetle in the foreground and several smaller monkey beetles in the background.
METHODS
Similarities and differences between Drosera species
In order to compare pollinator capture rates between the two Drosera species, it is important to establish first whether they indeed have similar pollinator fauna. Similarity in pollinator fauna is likely to be directly related to how similar the flowers of the two Drosera species are. Because D. cistiflora seemed to vary in colour, I only used pink–blue D. cistiflora flowers throughout the entire study as these most closely resembled D. pauciflora to my eyes. I used an Ocean Optics (Dunedin, FL, USA) S2000 spectrometer and Ocean Optics DT-mini deuterium tungsten halogen light source (200–1100 nm) to determine the spectral reflectance over the UV–visible range (300–700 nm) for both species using representative samples from flowers used in the study. Reflectance readings were taken from the outer petals of ten flowers of each species (n = 10 per species). Readings were taken through a fibre-optic reflection probe (UV/VIS 400 µm) held at 45° and about 5 mm from the surface of the petal.
In addition to colour spectrum comparisons, the distance from ground level to the bottom of the calyx was measured for D. cistiflora (n = 21) and D. pauciflora (n = 37) in order to compare floral height of the two species. Using the same replication I also measured the scape length of each species from the point of highest leaf attachment to the bottom of the calyx. Flower–trap separation was measured as the distance between the flower (bottom of calyx) and the nearest trap. Finally flower size was measured as the distance between the widest points on the flower. Differences between the flowers were analysed using t-tests.
Similarities in pollinator fauna
Five hundred flowers of each species were randomly selected and examined for pollinators. This was done over 6 d and two seasons. Pollinators were identified to subfamily or family level. In addition to random examination of flowers I also made stationary observations on groups of ten inflorescences for each species. Each group of ten inflorescences was watched between 0900 and 1600 h during sunny, windless conditions. This was repeated over 6 d per species and took two seasons to complete. All visitors were recorded to family or subfamily level. I combined the pollinator numbers obtained from the two observation methods because there were equal observation efforts for each plant species. Pollinators were divided into groups based on their size and taxon and each group was analysed for differences in occurrence on the two Drosera species using chi-square tests, expected versus observed frequencies, where I expected to find equal numbers of pollinators on each Drosera species.
Captured prey
The traps of 350 flowering individuals from each of the two Drosera species were examined for pollinators observed in prior pollinator observations. In addition to this, the traps of 15 individuals from each species were thoroughly examined and all prey recorded to order level and total body length was measured using a steel ruler. It was not possible to identify most prey items beyond order level as they tended to be very small and in poor condition.
Does display height affect visitation rate?
Drosera pauciflora flowers were cut and placed in test tubes and these test tubes were arranged in ten pairs. One tube in each pair was placed so that the flower was about 150 mm above ground level, simulating a normal D. pauciflora plant with an average scape length. The other test tube was buried in the ground so that the flower was about 10 mm above ground level, simulating a D. pauciflora plant that had not evolved a long scape. The ten pairs were watched and pollinators were recorded when they landed on the flowers. Data were not normally distributed and so the numbers of pollinators landing on the two treatments were compared using a Wilcoxon matched-pairs test.
RESULTS
Similarities and differences between Drosera species
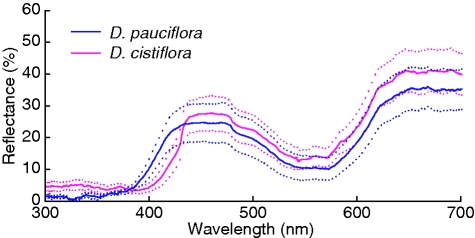
Drosera cistiflora flowers (171·00 ± 25·12 mm, mean ± s.d.) were very slightly taller than D. pauciflora flowers (156·38 ± 25·42 mm, t = –2·11, P = 0·0386). The flowers of D. cistiflora were held upright by a very short scape (49·19 ± 13·25 mm) whereas the D. pauciflora scape was considerably longer (156·38 ± 25·42 mm, t = 17·94, P < 0·00001, Fig. 1). This translated into a small flower–trap separation distance for D. cistiflora (27·71 ± 16·16 mm) compared with a large separation distance in D. pauciflora (156·38 ± 25·42 mm, t = 20·88, P < 0·00001). The flowers of D. cistiflora and D. pauciflora were of the same size (23·76 ± 2·84 and 22·89 ± 2·75 mm, respectively, t = –1·14, P = 0·2572, Fig. 1). In addition to similarities in plant height and flower size, the colours of the flowers as measured by spectrophotometry were also very similar (Fig. 2).
Fig. 2.
The spectral reflectance of D. pauciflora and D. cistiflora, as indicated, where solid lines are averages and dotted lines are the standard deviations.
Similarities in pollinator fauna
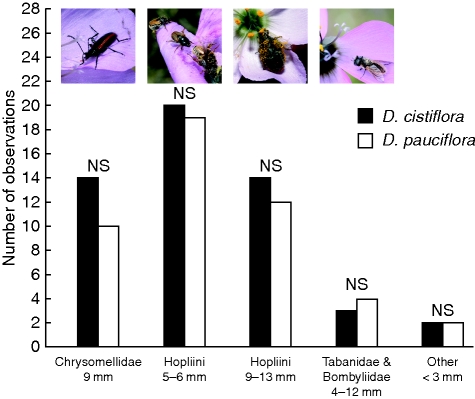
Large, robust monkey beetles (Hopliini) made up most of the pollinators (in excess of 58 %) for both Drosera species (Fig. 3). A single species of Chrysomellid beetle was also very commonly found on the flowers of both species, making up more than 18 % of visitors on the flowers (Fig. 3). None of the pollinator groups was significantly more numerous on either of the Drosera species (χ2 < 0·08, P > 0·75 for all taxa) and the size of all insects observed touching either the stigmas or the anthers tended to be large and in excess of 5 mm in length (Fig. 3).
Fig. 3.
The number of insects observed from various functional groups on D. cistiflora and D. pauciflora, as indicated. NS, non-significant at P > 0·05.
Captured prey
Insects captured by the traps of the plants tended to be tiny, averaging less than 2·0 mm in length for both plant species. Only a single pollinator was observed in the traps of D. pauciflora (a Chrysomellid beetle) and none was observed in the traps of D. cistiflora. Of the prey items captured by D. cistiflora, over 97 % were diptera compared with 72 % for D. pauciflora. Crawling invertebrates such as beetles, arachnids and ants made up the bulk of the additional prey items captured by D. pauciflora.
Does display height affect visitation rate
Twenty beetles (mean 2 ± 1·15) visited the flowers of the tall Drosera plants and only one (mean 0·1 ± 0·32) visited the shortened Drosera plants (Z = 2·666, P = 0·008).
DISCUSSION
To the human eye, the flower colour of the D. cistiflora specimens used for this study was very similar to that of the D. pauciflora specimens. This was confirmed using spectrophotometry, which indicated an overlap in the reflectance spectra of these two species across most of the colour range investigated. The similarities between these two species also included similar heights of the flowers from ground level and almost identical flower size. Probably due to the similarity in floral colour, size and height above ground, pink–purple coloured D. cistiflora and D. pauciflora also had very similar pollinator faunas that comprising mostly large beetles, including several monkey beetle species and one Chrysomellid. Bombyliid and Tabanid flies were also seen visiting both of these species occasionally. The similarity of the pollinator fauna allowed me to compare pollinator capture rates without the capture rates being biased by the two Drosera species having vastly different sets of pollinator species.
After extensive surveys of the insects captured by the long-scaped D. pauciflora, it appears that there is no overlap between the pollinator fauna and the fauna trapped by the plant. Drosera pauciflora traps mostly very small, delicate flies and minute crawling insects but is pollinated by robust beetles and occasionally large flies. Exactly the same non-overlapping trend was observed for D. cistiflora except that this species seemed to trap fewer crawling insects and more minute flies. The lack of overlap between pollinator and prey species may simply reflect the fact that flying pollinators are not motivated to land on the traps of plants, especially when there are showy flowers present. These results clearly suggest that the long scapes in D. pauciflora did not evolve to protect pollinators from being captured by plants (PPH). If the PPH was valid, then pollinators would have been captured in the traps of the short-scaped D. cistiflora but not in the traps of the long-scaped D. pauciflora.
To explain the presence of long scapes in D. pauciflora, I examined a competing hypothesis, the PAH. I found that pollinators were strongly attracted to tall flowers but not to flowers at ground level. This suggests that D. pauciflora is similar to non-insectivorous plants (e.g. Peakall and Handel, 1993; Agren et al., 2006; Vanhoenacker et al., 2006) in that pollinator attraction can account for the long scapes. These results experimentally support the earlier results of Anderson and Midgley (2001), who used the relationship between floral–trap separation and plant height to infer that the PPH was not important in selecting for long scapes.
These results suggest that the idea of pollinator protection has been over-emphasized in the literature, without being properly tested. Long scapes are very common in other plant species, especially bulbs or plants where the leaves are produced very close to ground level. Despite all the evidence suggesting that long scapes in non-insectivorous plants are clearly not a product of pollinator protection, biologists have strangely persisted in their support of the PPH as the selective force for long scapes in insectivorous plants (e.g. Ellison and Gotelli, 2001; Ellison et al., 2003; Giles et al., 2006), perhaps because it makes for a compelling story.
The one study which did find pollinator–prey conflict (Zamora, 1999) found that thrips were important pollinators in shady habitats and these were often captured. I suggest that thrip pollination is far less important in the habitats where Pinguicula are normally found and that thrips may in fact be detrimental in these habitats because they do not move much between flowers and may cause higher rates of self-pollination or pollen discounting. It is unlikely that the importance of thrips in marginal habitats could have driven the evolution of long scapes in Pinguicula. Further pollinator–prey studies are necessary to determine whether real conflict occurs in other insectivorous plant species or whether long scapes in plants such as Sarracenia, Nepenthes and Dionaea can also be attributed primarily to pollinator attraction.
LITERATURE CITED
- Agren J, Fortunel C, Ehrlen J. Selection on floral display in insect-pollinated Primula farinosa: effects of vegetation height and litter accumulation. Oecologia. 2006;150:225–232. doi: 10.1007/s00442-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ. Food or sex; pollinator prey conflict in carnivorous plants. Ecology Letters. 2001;4:511–513. [Google Scholar]
- Cariveau D, Irwin RE, Brody AK, Garcia-Mayeya LS, von der Ohe A. Direct and indirect effects of pollinators and seed predators to selection on plant and floral traits. Oikos. 2004;104:15–26. [Google Scholar]
- Collin CL, Pennings PS, Rueffler C, Widmer A, Shykoff JA. Natural enemies and sex: how seed predators and pathogens contribute to sex-differential reproductive success in a gynodioecious plant. Oecologia. 2002;131:94–102. doi: 10.1007/s00442-001-0854-8. [DOI] [PubMed] [Google Scholar]
- Donnelly SE, Lortie CJ, Aarssen LW. Pollination in Verbascum thapsus (Scrophulariaceae): the advantage of being tall. American Journal of Botany. 1998;85:1618–1625. [PubMed] [Google Scholar]
- Ehrlen J. Risk of grazing and flower number in a perennial plant. Oikos. 1997;80:428–434. [Google Scholar]
- Ehrlen J, Kack S, Agren J. Pollen limitation, seed predation and scape length in Primula farinosa. Oikos. 2002;97:45–51. [Google Scholar]
- Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. TREE. 2001;16:623–629. [Google Scholar]
- Ellison AM, Gotelli NJ, Brewer JS, et al. The evolutionary ecology of carnivorous plants. Advances in Ecological Research. 2003;33:1–74. [Google Scholar]
- Galen C, Cuba J. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution. 2001;55:1963–1971. doi: 10.1111/j.0014-3820.2001.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Giles BE, Pettersson TM, Carlsson-Graner U, Ingvarsson PK. Natural selection on floral traits of female Silene dioica by a sexually transmitted disease. New Phytologist. 2006;169:729–739. doi: 10.1111/j.1469-8137.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Ecology and evolution of carnivorous plants. In: Abrahamson WG, editor. Plant–animal interactions. New York: McGraw-Hill Inc; 1989. pp. 243–290. [Google Scholar]
- Hainsworth FR, Wolf LL, Mercier T. Pollination and pre-dispersal seed predation – net effects on reproduction and inflorescence characteristics in Ipomopsis aggregata. Oecologia. 1984;63:405–409. doi: 10.1007/BF00390673. [DOI] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Jurgens A, El-Sayed AM, Suckling DM. Do carnivorous plants use volatiles for attracting prey insects? Functional Ecology. 2009;23:875–887. [Google Scholar]
- Leimu R, Syrjanen K, Ehrlen J, Lehtila K. Pre-dispersal seed predation in Primula veris: among-population variation in damage intensity and selection on flower number. Oecologia. 2002;133:510–516. doi: 10.1007/s00442-002-1049-7. [DOI] [PubMed] [Google Scholar]
- Lortie CJ, Aarssen LW. The advantage of being tall: higher flowers receive more pollen in Verbascum thapsus L ( Scrophulariaceae) Ecoscience. 1999;6:68–71. [Google Scholar]
- O'Connell LM, Johnston MO. Male and female pollination success in a deceptive orchid, a selection study. Ecology. 1998;79:1246–1260. [Google Scholar]
- Murza GL, Heaver JR, Davis AR. Minor pollinator–prey conflict in the carnivorous plant. Drosera anglica. Plant Ecology. 2006;184:43–52. [Google Scholar]
- Peakall R, Handel SN. Pollinators discriminate among floral heights of a sexually deceptive orchid – implications for selection. Evolution. 1993;47:1681–1687. doi: 10.1111/j.1558-5646.1993.tb01260.x. [DOI] [PubMed] [Google Scholar]
- Torang P, Ehrlen J, Agren J. Facilitation in an insect-pollinated herb with a floral display dimorphism. Ecology. 2006;87:2113–2117. doi: 10.1890/0012-9658(2006)87[2113:fiaihw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vandewoestijne S, Rois AS, Caperta A, Baguette M, Tyteca D. Effects of individual and population parameters on reproductive success in three sexually deceptive orchid species. Plant Biology. 2009;11:454–463. doi: 10.1111/j.1438-8677.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker D, Agren J, Ehrlen J. Spatio-temporal variation in pollen limitation and reproductive success of two scape morphs in Primula farinosa. New Phytologist. 2006;169:615–621. doi: 10.1111/j.1469-8137.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- Verbeek NAM, Boasson R. Flowering height and postfloral elongation of flower stalks in 13 species of angiosperms. Canadian Journal of Botany. 1995;73:723–727. [Google Scholar]
- Wickler W. Mimicry in animals and plants. London: Weidenfeld and Nicholson; 1968. [Google Scholar]
- Wiens D. Mimicry in plants. Evolutionary Biology. 1978;6:365–401. [Google Scholar]
- Zamora R. Conditional outcomes of interactions: the pollinator–prey conflict of an insectivorous plant. Ecology. 1999;80:786–795. [Google Scholar]