Abstract
Background and Aims
Nutritional changes associated with the evolution of achlorophyllous, mycoheterotrophic plants have not previously been inferred with robust phylogenetic hypotheses. Variations in heterotrophy in accordance with the evolution of leaflessness were examined using a chlorophyllous–achlorophyllous species pair in Cymbidium (Orchidaceae), within a well studied phylogenetic background.
Methods
To estimate the level of mycoheterotrophy in chlorophyllous and achlorophyllous Cymbidium, natural 13C and 15N contents (a proxy for the level of heterotrophy) were measured in four Cymbidium species and co-existing autotrophic and mycoheterotrophic plants and ectomycorrhizal fungi from two Japanese sites.
Key Results
δ13C and δ15N values of the achlorophyllous C. macrorhizon and C. aberrans indicated that they are full mycoheterotrophs. δ13C and δ15N values of the chlorophyllous C. lancifolium and C. goeringii were intermediate between those of reference autotrophic and mycoheterotrophic plants; thus, they probably gain 30–50 % of their carbon resources from fungi. These data suggest that some chlorophyllous Cymbidium exhibit partial mycoheterotrophy (= mixotrophy).
Conclusions
It is demonstrated for the first time that mycoheterotrophy evolved after the establishment of mixotrophy rather than through direct shifts from autotrophy to mycoheterotrophy. This may be one of the principal patterns in the evolution of mycoheterotrophy. The results also suggest that the establishment of symbiosis with ectomycorrhizal fungi in the lineage leading to mixotrophic Cymbidium served as pre-adaptation to the evolution of the mycoheterotrophic species. Similar processes of nutritional innovations probably occurred in several independent orchid groups, allowing niche expansion and radiation in Orchidaceae, probably the largest plant family.
Keywords: Mycoheterotrophy; nutritional mode; evolution; Cymbidium; Orchidaceae; symbiosis; mycorrhizal fungi; δ15N, δ13C
INTRODUCTION
Unlike the autotrophic nutritional mode of chlorophyllous and leafy plants, which use atmospheric CO2 as their sole carbon source, some achlorophyllous and leafless plant species obtain carbon from mycorrhizal fungi (Björkman, 1960; McKendrick et al., 2000; Smith and Read, 2008). This fully heterotrophic nutrition, so-called mycoheterotrophy (MH), occurs in >400 species belonging to 87 genera in 11 families (Leake, 1994, 2005).
MH nutrition has evolved repeatedly from autotrophy (AT) in various plant lineages, but detailed evolutionary processes that lead to MH remain unclear. Recent studies demonstrated that several chlorophyllous species in Orchidaceae and Ericaceae obtain carbon not only from their photosynthetic activity, but also from mycorrhizal fungi (Gebauer and Meyer, 2003; Bidartondo et al., 2004; Selosse et al., 2004; Julou et al., 2005; Cameron et al., 2006; Tedersoo et al., 2007; Zimmer et al., 2007, 2008). This nutritional mode, called mixotrophy (MX), has been suggested to be a pre-adaptation in the evolution of MH nutrition (Bidartondo et al., 2004; Selosse et al., 2004, 2006; Abadie et al., 2006). In orchids, both MX and MH species tend to associate with unusual mycorrhizal fungi: instead of the usual saprobic or parasitic Rhizoctonia fungi that are the almost exclusive associates of autotrophic orchids (Rasmussen, 1995), most MX and MH orchids recruit various ascomycetes and basidiomycetes, which are ectomycorrhizal fungi on surrounding tree roots (Taylor et al., 2002). MX and MH species thus indirectly exploit tree photosynthates as a carbon source (McKendrick et al., 2000).
Nutritional changes associated with the evolution of achlorophyllous plant species have to date been inferred from assumptions without using clades in which leaflessness appears (Bidartondo et al., 2004; Tedersoo et al., 2007; Roy et al., 2009) or from comparisons between green plants and albino mutants that exist in some species (Julou et al., 2005; Abadie et al., 2006). For these reasons, it is necessary to clarify fluctuations of heterotrophy in accordance with the evolution of leaflessness using a relationship between chlorophyllous and achlorophyllous species based on credible phylogenetic hypotheses.
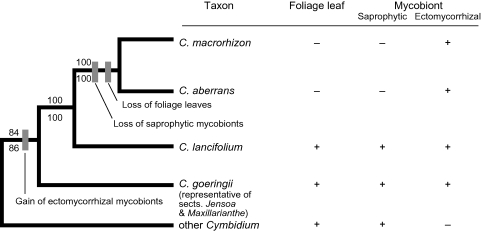
Cymbidium, an orchid genus distributed from east and south-east Asia to Australia, comprises about 52 species (DuPuy and Cribb, 2007). This genus exhibits distinctive ecological diversification (Motomura et al., 2008) and occurs in terrestrial, epiphytic and lithophytic life forms. Two species, C. macrorhizon and C. aberrans, lack foliage leaves and are thus assumed to be MH (Fig. 1).
Fig. 1.
Cymbidium lancifolium, a leafy and chlorophyllous species (A), and C. macrorhizon, a leafless and achlorophyllous species (B). Cymbidium lancifolium is the closest relative of the achlorophyllous Cymbidium species (see Fig. 2). Reproduced from Maekawa (1971), del. Yoai Ohta.
Yukawa et al. (2002) analysed phylogenetic relationships among 36 Cymbidium spp. using nucleotide variation in the nuclear and plastid genomes and found that these achlorophyllous species are sister species, in a clade successively connecting with Cymbidium lancifolium and the clade including Cymbidium goeringii (Fig. 2). Cymbidium lancifolium and C. goeringii develop foliage leaves and appear to be capable of AT nutrition. On the other hand, the evolution of characters related to nutritional traits has been well analysed in Cymbidium: in an investigation of the vegetative anatomy of the genus, Yukawa and Stern (2002) found degeneration of stomata in C. macrorhizon, indicating a lack of CO2 exchange in this species. Yokoyama et al. (2002) and Y. Ogura-Tsujita and T. Yukawa (unpubl. res.) found a shift of mycobionts between chlorophyllous and achlorophyllous Cymbidium. Chlorophyllous C. lancifolium and C. goeringii both harbour saprobic (Tulasnellaceae) and tree ectomycorrhizal (Sebacinales, Russulaceae, Thelephoraceae, etc.) fungi, whereas achlorophyllous C. macrorhizon and C. aberrans establish symbiosis exclusively with ectomycorrhizal fungi. Furthermore, Motomura et al. (2008) demonstrated a large diversification of photosynthetic modes in Cymbidium. Therefore, Cymbidium is an ideal model taxon to test contributions of carbon from mycorrhizal fungi, in accordance with the evolution of leaflessness and associated characters related to nutritional innovations.
Fig. 2.
Reconstruction of character evolution related to nutritional properties in Cymbidium. The phylogram of Cymbidium is summarized from the results of molecular phylogenetic analyses by Yukawa et al. (2002). Numbers above and below internodes indicate bootstrap values from 1000 replicates of Fitch parsimony analysis and neighbor-joining analysis, respectively.
MH plants are highly enriched in 13C and 15N relative to AT plants (Gebauer and Meyer, 2003; Trudell et al., 2003). Their 13C contents are similar to, or slightly more elevated than, those of associated mycorrhizal fungi, whereas their 15N contents tend to be higher (Trudell et al., 2003; Selosse and Roy, 2009). Fractionation against heavy isotopes occurs commonly in physical and metabolic processes, and thus analysis of the natural abundance of stable isotopes allows tracking of nutrient sources and fluxes in ecosystems (Dawson et al., 2002; Post, 2002). Isotopic abundance in MH plants correlates with the use of resources derived from their mycorrhizal fungi (and ultimately from nearby AT plants). As expected, 13C and 15N abundances in MX orchid species range between those of AT and MH species (Gebauer and Meyer, 2003; Bidartondo et al., 2004; Julou et al., 2005; Roy et al. 2009), and are indicative of variable levels of heterotrophy from one species or one site to another.
In this study, spontaneous stable isotopic contents (13C and 15N) of aerial parts from the aforementioned Cymbidium species are used to estimate their level of heterotrophy and to draw conclusions about the evolution of MH, comparing an achlorophyllous clade with its closest chlorophyllous relatives.
MATERIALS AND METHODS
Sampling
Samples were collected in August 2006 from two forest sites in the eastern part of Honshû island, Japan: Minamibouso, Chiba (site A: 35°03′22″N, 140°01′35″E) and Mitaka, Tokyo (site B: 35°41′55″N, 139°34′22″E). At site A, the Cymbidium populations were growing under warm-temperate evergreen broadleaved forest in which Castanopsis sieboldii (Fagaceae), Cinnamomum tenuifolium (Lauraceae), Lithocarpus edulis (Fagaceae), Neolitsea sericea (Lauraceae) and Machilus thunbergii (Lauraceae) dominate. Site B harbours warm-temperate deciduous broadleaved forest in which Aphananthe aspera (Ulmaceae) and Carpinus tschonoskii (Betulaceae) dominate. The canopy begins to develop in May and leaves are shed in November.
In areas adjacent to site B, the mean annual precipitation from 1977 to 2004 was 1496 mm and mean temperature ranged from 13·9 °C to 16·7 °C. More humid conditions are observed in areas adjacent to site A where the mean annual precipitation from 1977 to 2004 was 1809 mm and mean temperature ranged from 14·4 °C to 16·6 °C (data from the Japan Meteorological Agency).
Shoots of achlorophyllous orchids (Cyrtosia septentrionalis, Lecanorchis nigricans, Cymbidium macrorhizon and C. aberrans) and achlorophyllous Ericaceae (Monotropa uniflora), and leaves of chlorophyllous C. lancifolium and C. goeringii (Table 1) were collected. As references, at site A, leaves of 40 non-orchid chlorophyllous species belonging to 26 plant families and four chlorophyllous orchids (Cephalanthera erecta, Liparis nervosa, Zeuxine agyokua-na and Goodyera schlechtendaliana) were collected. At site B, eight non-orchid chlorophyllous species belonging to seven plant families were collected. Fruit bodies of ectomycorrhizal fungi growing near the Cymbidium populations were also collected at both sites and their genera were identified using morphology and molecular identification on the basis of nucleotide sequences of internal transcribed spacer regions in ribosomal DNA as described in Selosse et al. (2002). The sequences obtained were deposited in GenBank (accession numbers GQ359817–GQ359821; Tables S1 and S2 available online).
Table 1.
Plants examined in this study; number of individuals collected at each site and trophic status are provided
| Collection site |
||||
|---|---|---|---|---|
| Species | Family | A | B | Trophic status* |
| Amphicarpaea bracteata (L.) Fernald subsp. edgeworthii (Benth.) H.Ohashi | Fabaceae | 2 | – | FIX |
| Aphananthe aspera (Thunb.) Planch. | Ulmaceae | – | 3 | AM/ECM |
| Arachniodes standishii (T.Moore) Ohwi | Dryopteridaceae | 3 | – | AM/NON |
| Ardisia crenata Sims | Primulaceae | – | 1 | AM |
| Ardisia japonica (Thunb.) Blume | Primulaceae | 6 | – | AM |
| Arisaema aequinoctiale Nakai & F.Maek. | Araceae | 4 | – | AM |
| Aucuba japonica Thunb. | Cornaceae | 3 | – | AM |
| Carex conica Boott | Cyperaceae | 2 | – | AM/NON |
| Carex siderosticta Hance | Cyperaceae | 3 | – | AM/NON |
| Carpinus tschonoskii Maxim. | Betulaceae | – | 3 | ECM |
| Castanea crenata Siebold et Zucc. | Fagaceae | 1 | – | ECM |
| Castanopsis sieboldii (Makino) Hatus. ex T.Yamaz. & Mashiba | Fagaceae | 6 | – | ECM |
| Cephalanthera erecta (Thunb.) Blume | Orchidaceae | 3 | – | OM |
| Cephalotaxus harringtonia (Knight ex Forbes) K.Koch | Taxaceae | 2 | – | AM |
| Chamaecyparis obtusa (Siebold & Zucc.) Endl. | Cupressaceae | 1 | – | AM |
| Cinnamomum tenuifolium (Makino) Sugim. ex H.Hara | Lauraceae | 2 | – | AM |
| Cryptomeria japonica (L.f.) D.Don | Taxodiaceae | 1 | – | AM |
| Cymbidium goeringii (Rchb.f.) Rchb.f. | Orchidaceae | 6 | 1 | OM |
| Cymbidium macrorhizon Lindl.† | Orchidaceae | 4 | 2 | OM |
| Cymbidium lancifolium Hook. | Orchidaceae | 6 | – | OM |
| Cymbidium aberrans Finet† | Orchidaceae | – | 3 | OM |
| Cyrtosia septentrionalis (Rchb.f.) Garay† | Orchidaceae | 1 | – | OM |
| Damnacanthus indicus Gaertn.f. | Rubiaceae | 6 | – | AM |
| Dendropanax trifidus (Thunb.) Makino ex H.Hara | Araliaceae | 3 | – | AM/NON |
| Desmodium laxum DC. | Fabaceae | 1 | – | FIX |
| Deutzia scabra Thunb. | Hydrangeaceae | 1 | – | AM/NON |
| Dioscorea japonica Thunb. | Dioscoreaceae | 1 | 1 | AM/NON |
| Dryopteris pacifica (Nakai) Tagawa | Dryopteridaceae | 1 | – | AM/NON |
| Elaeagnus macrophylla Thunb. | Elaeagnaceae | 1 | – | AM |
| Eriobotrya japonica (Thunb.) Lindl. | Rosaceae | 1 | – | AM |
| Eurya japonica Thunb. | Theaceae | 4 | – | AM |
| Ficus erecta Thunb. | Moraceae | 3 | – | AM |
| Goodyera schlechtendaliana Rchb.f. | Orchidaceae | 1 | – | OM |
| Hedera rhombea (Miq.) Bean | Araliaceae | 6 | – | AM/NON |
| Ilex crenata Thunb. | Aquifoliaceae | – | 1 | AM |
| Ilex serrata Thunb. f. argutidens (Miq.) Satomi | Aquifoliaceae | 3 | – | AM |
| Lecanorchis nigricans Honda† | Orchidaceae | 3 | – | OM |
| Ligustrum lucidum Aiton | Oleaceae | – | 2 | AM |
| Lilium auratum Lindl. | Liliaceae | 1 | – | AM |
| Liparis nervosa (Thunb.) Lindl. | Orchidaceae | 3 | – | OM |
| Lithocarpus edulis (Makino) Nakai | Fagaceae | 6 | – | ECM |
| Machilus thunbergii Siebold & Zucc. | Lauraceae | 2 | – | AM |
| Monotropa uniflora L.† | Ericaceae | 3 | – | ECM |
| Neolitsea sericea (Blume) Koidz. | Lauraceae | 2 | – | AM |
| Ophiopogon japonicus (Thunb.) Ker Gawl. | Asparagaceae | 2 | – | AM |
| Ophiopogon japonicus (Thunb.) Ker Gawl. var. umbrosus Maxim. | Asparagaceae | 3 | – | AM |
| Oplismenus undulatifolius (Ard.) Roem. & Schult. | Poaceae | 3 | 1 | AM |
| Padus grayana (Maxim.) C.K.Schneid. | Rosaceae | 1 | – | AM |
| Piper kadsura (Choisy) Ohwi | Piperaceae | 3 | – | AM/NON |
| Pleioblastus chino (Franch. & Sav.) Makino | Poaceae | 6 | 2 | AM |
| Pteris cretica L. | Pteridaceae | 1 | – | AM/NON |
| Smilax china L. | Smilacaceae | 1 | – | AM |
| Stegnogramma pozoi (Lag.) K.Iwats. subsp. mollissima (Fisch. ex Kunze) K.Iwats. | Thelypteridaceae | 3 | – | AM/NON |
| Trachelospermum asiaticum (Siebold & Zucc.) Nakai | Apocynaceae | 6 | – | AM/NON |
| Wisteria floribunda (Willd.) DC. | Fabaceae | 1 | – | FIX |
| Zeuxine agyokuana Fukuy. | Orchidaceae | 1 | – | OM |
* Trophic status based on general assumption: AM, arbuscular mycorrhizal plants; AM/NON: arbuscular mycorrhizal or non-mycorrhizal plants; ECM, ectomycorrhizal plants; FIX, nitrogen-fixing plants; OM, orchid mycorrhizal plants.
† Achlorophyllous species.
Except for fungi, all samples were collected at 10–30 cm above the soil and in the same light conditions to avoid carbon isotope distortion due, respectively, to CO2 resulting from soil respiration and different photosynthetic rates (slow rates enhance higher 13C discrimination during CO2 assimilation; Julou et al., 2005). To ensure independence of the data, all samples were from different individuals; for each species, the number of replicates was up to six whenever possible, but in some cases the number of available individuals limited the repetition number.
Isotopic analysis
Samples were dried at 60 °C for 4 d before grinding with a steel ball mill (Wig-L-Bug Model 30; International Crystal Laboratories, Garfield, NJ, USA). 14N and 15N contents were measured using 4 mg of each ground sample and 12C and 13C contents using 2 mg. Stable isotope ratios were analysed using a combined system of an elemental analyser (NC-2500; CE Instruments, Milan, Italy) and an isotope ratio mass spectrometer (MAT-252; Thermo Electron, Bremen, Germany), as described by Motomura et al. (2008). The isotope ratios in the delta notation in per mil units (‰) were expressed using Pee Dee belemnite and atmospheric N2 as standards:
| 1 |
where R is the molar ratio, i.e. 15N/14N or 13C/12C. The standard deviations for replicate combustions of the internal standards (DL-alanine) were 0·11 ‰ for δ15N and 0·07 ‰ for δ13C.
13C and 15N values were tested for normality and for homogeneity of variances using a Shapiro–Wilk test and a Levene test, respectively.
Nutritional modes of the Cymbidium species were tested in comparison with other AT and MH plants using δ13C and δ15N values and one-way ANOVA performed for each variable and each site, followed by pairwise t-tests (Bonferroni correction) to calculate pairwise comparisons between group levels at α = 0·01. The percentage of carbon acquired in a MH way from fungi was estimated by using a linear two-source mixing model (Phillips and Gregg, 2001; Gebauer and Meyer, 2003; Tedersoo et al., 2007):
| 2 |
where δCR and δCMH are the mean values of AT and MH references, respectively, and δCMX is the mean value of the putative MX species. All chlorophyllous plants except Cymbidium goeringii, C. lancifolium and Cephalanthera erecta were assumed to be AT (see below). At site A, the mean values for the chlorophyllous non-orchids and the chlorophyllous orchids were used as references for AT; since chlorophyllous orchid species were absent from site B, the mean value for all chlorophyllous non-orchids was used as the reference for AT. The relative contribution of carbon derived from fungi was estimated from mean values, with approximate standard errors and 95 % confidence intervals as used in Phillips and Gregg (2001). Statistical analyses and graphics were computed using R 2·7·1 (R Foundation, Vienna, Austria).
RESULTS
Tables S1 and S2 (available online) show the δ15N and δ13C values in plants and fungi collected at sites A and B and morphological and molecular identifications of fungal samples collected at these sites that proved to belong to the ectomycorrhizal genera Amanita, Boletus, Lactarius and Russula (GenBank accession numbers GQ359817–GQ359821; Tables S1 and S2).
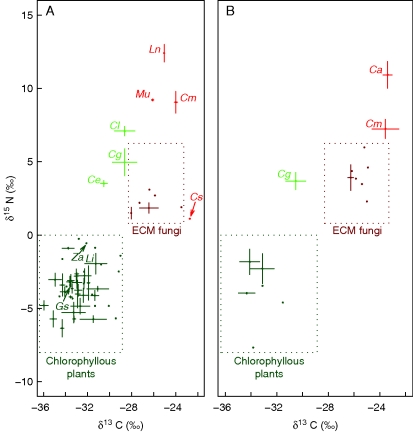
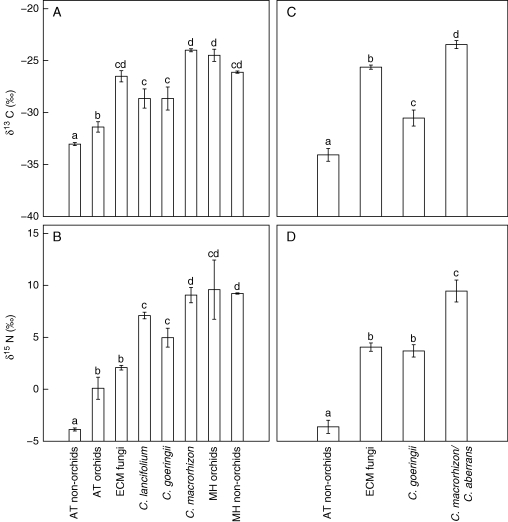
Figure 3 shows that high δ15N and δ13C values were recorded in all Cymbidium species (C. macrorhizon, C. aberrans, C. lancifolium and C. goeringii) at both sites and achlorophyllous plants at site A (Orchidaceae: Cyrtosia septentrionalis and Lecanorchis nigricans; Ericaceae: Monotropa uniflora). At site A, C. macrorhizon did not significantly differ from the other MH species in δ13C and δ15N (Fig. 4A and B). At sites A and B, C. macrorhizon and C. aberrans had higher δ15N but identical δ13C values compared with co-occurring ectomycorrhizal fungi (Fig. 4), including Russulaceae which are mycorrhizal with these Cymbidium species. At site A, C. lancifolium and C. goeringii had significantly higher δ13C and δ15N values than the AT plants (orchids or non-orchids; Fig. 4A and B), and the same trend was observed for C. goeringii at site B (Fig. 4C and D). At both sites, δ13C values of the chlorophyllous Cymbidium species were significantly lower than for the achlorophyllous Cymbidium species; similarly, δ15N values of the chlorophyllous Cymbidium species tended to be lower, although this was not significant at site A (Fig. 4). Among the remaining chlorophyllous species, Cephalanthera erecta at site A showed significantly higher δ15N and non-significantly higher δ13C values as compared with the other AT species (P < 0·0001 and P = 0·014, respectively, according to a pairwise t-test).
Fig. 3.
δ13C and δ15N values in plants and ectomycorrhizal (ECM) fungi collected at site A (A) and site B (B). Bars indicate standard errors, and squares surrounded by dotted lines indicate values for chlorophyllous plants and ECM fungi (see Tables S1 and S2). Chlorophyllous plants, achlorophyllous plants, ECM fungi and green, mixotrophic plants are shown dark green, red, brown–red and light green, respectively. Abbreviations for Orchidaceae: Ca, Cymbidium aberrans; Ce, Cephalanthera erecta; Cm, Cymbidium macrorhizon; Cl, Cymbidium lancifolium; Cs, Cyrtosia septentrionalis; Gs, Goodyera schlechtendaliana; Ln, Lecanorchis nigricans; Li, Liparis nervosa; Za, Zeuxine agyokuana. Abbreviation for Ericaceae: Mu, Monotropa uniflora.
Fig. 4.
δ13C and δ15N values (mean ± s.e.) of plants and ectomycorrhizal (ECM) fungi grouped in different species and/or nutritional modes at site A (A, B) and site B (C, D). Different letters denote significant differences between species and functional groups according to Bonferroni corrected pairwise t-tests (P < 0·01). Cymbidium goeringii and C. lancifolium are chlorophyllous; C. macrorhizon and C. aberrans are achlorophyllous. Mycoheterotrophic (MH) orchids include Cyrtosia septentrionalis and Lecanorchis nigricans; and MH non-orchids are represented by Monotropa uniflora. For details on autotrophic (AT) non-orchids, AT orchids and ECM fungi, see Tables 1, S1 and S2.
To estimate the percentage of carbon acquired heterotrophically from fungi, references for δ13C were determined in full MH and full AT nutrition, focusing on phylogenetically close lineages. Given the results above, C. macrorhizon and C. aberrans were hypothesized to be full MH reference. At site A, the mean values for the chlorophyllous non-orchids and the chlorophyllous orchids were used as a reference for AT; the possibly MX Cephalanthera erecta was omitted from baseline calculations. Since chlorophyllous orchid species were absent from site B, only the mean value for all chlorophyllous non-orchids was available as reference for AT. Table 2 showed that a significant contribution of fungal C was found in Cymbidium lancifolium at site A (between 41·2 % and 48·3 % in the mean value among the different references for AT) and C. goeringii at the two sites (between 33·4 % and 48·3 % in the mean value among the sites or the different references for AT). This result was not much changed by using non-Cymbidium achlorophyllous orchids as full MH reference at site A (not shown), since these orchids showed δ13C values similar to those of the achlorophyllous Cymbidium, whereas using the ericaceous Monotropa uniflora (which has lower δ13C) and all chlorophyllous plants as baselines, fungal carbon contribution reached 63 % both for C. lancifolium and C. goeringii. A two-way analysis of variance showed no effect of either the species (P = 0·88) or the site for C. goeringii (P = 0·27) on the fungal contribution. For Cephalanthera erecta at site A, the carbon gain from fungi was lower (17–27 %) and only significant when estimated with the chlorophyllous orchids as baseline (Table 2).
Table 2.
Net carbon gain from fungi in Cymbidium species and Cephalanthera erecta at sites A and B (mean ± s.e.), based on a linear mixing model
| Species | Carbon gain, using all autotrophic plants as 0 % baseline† | Carbon gain, using all non-Cymbidium autotrophic orchids as 0 % baseline‡ |
|---|---|---|
| At site A | ||
| Cymbidium macrorhizon | 100 % (baseline) | 100 % (baseline) |
| C.goeringii | 48·3 ± 12·3 %* | 41·1 ± 14·0 %* |
| C.lancifolium | 48·3 ± 10·2 %* | 41·2 ± 11·7 %* |
| Cephalanthera erecta | 27·3 ± 3·4 % | 17·3 ± 3·8 %* |
| At site B | ||
| C. macrorhizon + C. aberrans | 100 % (baseline) | − |
| C. goeringii | 33·4 ± 7·3 %* | − |
* Significant difference based on 95 % confidence intervals following Phillips and Gregg (2001).
† In this calculation, the δ13C of autotrophic plants (0 % gain from fungi) is supposed to be the mean δ13C value of all chlorophyllous plants (including non-Cymbidium orchids, with the exception of the possible mixotrophic Cephalanthera erecta).
‡ In this calculation, the δ13C of autotrophic plants (0 % gain from fungi) is supposed to be the mean δ13C value of all chlorophyllous orchids (with the exception of Cymbidium and the possible mixotrophic Cephalanthera erecta).
DISCUSSION
The pattern of evolution of nutritional modes was examined in a Cymbidium clade comprising achlorophyllous C. macrorhizon and C. aberrans and chlorophyllous C. lancifolium and C. goeringii (Fig. 2). The following data showed that the two achlorophyllous Cymbidium species are MH. First, they had significantly higher δ15N but similar (to slightly higher) δ13C values compared with the co-occurring ectomycorrhizal fungi, including Russulaceae, which is mycorrhizal with these Cymbidium species (Yokoyama et al., 2002; Y. Ogura-Tsujita and T. Yukawa, unpubl. res.). Trudell et al. (2003) showed the same trend in other MH plants relative to co-existing ectomycorrhizal fungi, as expected in trophic chains in which 15N contents tend to increase from one level to another (Figs 3 and 4). Secondly, C. macrorhizon had similar δ15N and δ13C values relative to the other MH species at site A (Figs 3 and 4). Exceptionally, Cyrtosia septentrionalis had conspicuously higher δ13C than the other MH species. The divergence is probably due to the fact that C. septentrionalis forms mycorrhizae with Armillaria (Hamada, 1939), a saprophytic fungus group living on dead or living wood. Zeller et al. (2007) showed that δ13C values of fruiting bodies of Armillaria are much higher than those of ectomycorrhizal fungi, such as those with which Cymbidium and other MH species coexist (Table S1 and Fig. 3). Thirdly, 13C enrichment in C. macrorhizon and C. aberrans in comparison with surrounding AT plants (8·9 ± 0·4 ‰ at site A and 10·6 ± 0·8 ‰ at site B) was higher than the range reported for MH plants from temperate regions (6·9 ± 1·5 ‰; Zimmer et al., 2008) and for Japanese MH Gastrodia confusa (7·5 ± 0·8 ‰; Ogura-Tsujita et al., 2009). However, 13C enrichment was in the upper range observed for Thai MH orchids (6·8–9·9 ‰; Roy et al., 2009) and for the MH Gastrodia similis, a Mascarene MH orchid (11·8 ‰; Martos et al., 2009). δ15N values for C. macrorhizon and C. aberrans were also above those of surrounding AT plants (12·9 ± 1·7 ‰ at site A and 13·0 ± 2·2 ‰ at site B) and were in the range reported for other MH plants from temperate regions (11·7 ± 2·3 ‰; Zimmer et al., 2008). In accordance with the above-mentioned results, C. macrorhizon has degenerated stomata on its scale leaves, indicating a lack of gas exchange in this species (Yukawa and Stern, 2002).
Chlorophyllous Cymbidium lancifolium and C. goeringii exhibited higher δ15N and δ13C values than co-existing AT orchids and other AT plants and lower values than MH plants (Figs 3 and 4). The estimated level of MX (approx. one-third to one-half in the mean value depending on species and sites; Table 2) indicates that carbon derived from fungi is at least invested partially in aerial parts of the host plant. The values are within the wide range of previous studies (7–85 % of leaf mass depending on species and sites; Gebauer and Meyer, 2003; Bidartondo et al., 2004; Julou et al., 2005; Abadie et al., 2006; Selosse et al., 2006; Tedersoo et al., 2007). The results are congruent with the life history of MX species: after germination, they exhibit an underground phase in which the rhizomes symbiotic with mycorrhizal fungi are the sole vegetative organ for several seasons (Ogura-Tsujita and Yukawa, 2008a, b). Subsequently, the leafy shoots appear above the ground, but they still maintain mycorrhizal rhizomes (T. Yukawa, unpubl. res.). A probable MX nutrition was also found for Cephalanthera erecta, which belongs to a genus rich in MH and MX species in Europe, Asia and America (Taylor and Bruns, 1997; Julou et al., 2005; Abadie et al., 2006; Roy et al., 2009).
Some Cymbidium species operate crassulacean acid metabolism (CAM) as a photosynthetic pathway, entailing δ13C values typically above –20 ‰ (Motomura et al., 2008), i.e. higher than expected for MX and MH species. However, partial CAM photosynthesis is excluded as an explanation for the observed δ13C values, because C. lancifolium and C. goeringii have negligible diurnal malate fluctuations and low enzymatic activities relative to those associated with CAM metabolism (Motomura et al., 2008). Consequently, it is likely that δ13C values in C. lancifolium and C. goeringii are due to MX nutrition.
Orchid species ubiquitously show obligate heterotrophic associations with fungi during germination and the subsequent juvenile, underground stage (Bernard, 1899; Rasmussen, 1995; Rasmussen and Rasmussen, 2009). However, most species shift to AT with the development of photosynthetic organs, as supported by isotopic analyses (Gebauer and Meyer, 2003; Bidartondo et al., 2004; Abadie et al., 2006; Tedersoo et al., 2007; this study). It is thus reasonable to postulate that the outgroups of the studied taxa are AT, the plesiomorphic condition in the adult stage. Therefore, the MX Cymbidium lancifolium and C. goeringii evolved from AT ancestors. Further, the phylogenetic relationships showed that MH C. macrorhizon and C. aberrans appeared within this MX clade (Fig. 2). These results indicate that MH in Cymbidium evolved after the establishment of MX rather than directly from it. This pattern of evolution is also likely to exist in several plant groups that include both chlorophyllous and achlorophyllous species, including Cephalanthera (Orchidaceae: Abadie et al., 2006), the Limodorum–Aphyllorchis clade (Orchidaceae: Roy et al., 2009), the Corallorhiza–Oreorchis clade (Orchidaceae: Zimmer et al., 2008) and tribe Pyroleae (Ericaceae: Tedersoo et al., 2007; Zimmer et al., 2007). In these groups, however, evolution of MH nutrition has been inferred from assumptions without using clades in which leaflessness evolved and/or lack of data from sister groups of achlorophyllous species.
Yokoyama et al. (2002) and subsequent investigation by Y. Ogura-Tsujita and T. Yukawa (unpubl. res.) found major shifts of mycobionts in Cymbidium. The outgroup species of this study generally have only saprophytic mycobionts (mainly Tulasnellaceae, common orchid partners belonging to the Rhizoctonia assemblage; Rasmussen, 1995; Yukawa et al., 2009). The MX species (C. lancifolium and C. goeringii) harbour both Tulasnellaceae and ectomycorrhizal fungi (Russulaceae and others). The MH species C. macrorhizon and C. aberrans associate exclusively with the ectomycorrhizal fungi. These results indicate that the nutritional shift from AT to MH through MX in Cymbidium may correlate with shifts in mycobionts from saprophytic to ectomycorrhizal fungi. This scenario is in line with the evolution of considerable numbers of MH species within clades of chlorophyllous MX species associated with ectomycorrhizal fungi, such as Cephalanthera species (Taylor and Bruns, 1997; Bidartondo et al., 2004; Abadie et al., 2006) and Monotropoideae (Ericaceae; Tedersoo et al., 2007).
Light availability is a major limiting factor for plant distribution. The Cymbidium species studied here grow on floors of evergreen broadleaved forests, and C. goeringii, C. macrorhizon and C. aberrans are also distributed in warm-temperate deciduous broadleaved or Pinus forests (Maekawa, 1971; Du Puy and Cribb, 2007). Light intensities in these habitats are dark to dim in shaded sites of forests, woodland or scrub, except for during winter in deciduous broadleaved forests. MX or MH abilities of these Cymbidium species may have enabled them to survive low light conditions. Indeed, the light level at site A is lower than at site B, and MX C. goeringii tended to be more heterotrophic at site A than at site B, perhaps adapting to (or suffering from) a lower level of photosynthesis (Table 2). Gebauer (2005) reviewed the same tendencies in other MX orchids.
Adaptation to low light conditions in MX and MH species may have led to niche expansions and radiation in Orchidaceae. As mentioned above, orchid species ubiquitously show obligate MH nutrition during the juvenile stage. Among the seeds dispersed at shady sites, seedlings that extend MH in later stages of growth and operate more efficient nutritional interactions with fungal partners are expected to survive and adapt better to such environments. This process may select for MX and MH species in many independent orchid lineages. The MX and MH species pairs in Cymbidium provide an excellent model for future studies on the adaptive mechanism of plants on the forest floor.
In this study, it is demonstrated for the first time that MH plants evolved after the establishment of MX nutrition rather than directly from AT ancestors, suggesting that this course would be one of the principal patterns in the evolution of MH species. Further, the results confirm that the establishment of symbiosis with ectomycorrhizal fungi in the lineage leading to MX Cymbidium is a pre-adaptation to the evolution of the species. In addition, the MX and MH species are well-adapted to environments with low light conditions. Similar processes of nutritional innovations probably occurred in several independent orchid groups and may have contributed to niche expansions and radiation in Orchidaceae, probably the largest plant family (approx. 25000 species; Dressler, 2005).
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank K. Ohnuki, K. Suzuki and Y. Ogura-Tsujita for their help in sampling, T. Pailler for helpful discussions and D. Marsh for language corrections. This study was partly supported by a Grant-in-Aid to Scientific Research from the Japan Society for Promotion of Science (no. 21370038) to T.Y., and by the Société Française d'Orchidophilie, the Centre National de la Recherche Scientifique (CNRS) and the Région Réunion to M.-A.S. and F.M.
LITERATURE CITED
- Abadie JC, Puttsepp U, Gebauer G, Faccio A, Bonfante P, Selosse MA. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and non-photosynthetic individuals. Canadian Journal of Botany. 2006;84:1462–1477. [Google Scholar]
- Bernard N. Sur la germination du Neottia nidus-avis. Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences Paris. 1899;128:1253–1255. [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings of the Royal Society London Series B. 2004;271:1799–1806. doi: 10.1098/rspb.2004.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman E. Monotropa hypopithys L. – an epiparasite on tree roots. Physiologia Plantarum. 1960;13:308–327. [Google Scholar]
- Cameron DD, Leake JR, Read DJ. Mutualistic mycorrhiza in orchids: evidence from the plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytologist. 2006;171:405–416. doi: 10.1111/j.1469-8137.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics. 2002;33:507–559. [Google Scholar]
- Dressler RL. How many orchid species? Selbyana. 2005;26:155–158. [Google Scholar]
- Du Puy D, Cribb P. The genus Cymbidium. Richmond, UK: Kew Publishing; 2007. [Google Scholar]
- Gebauer G. Rundgespräche der Kommission für Ökologie. Vol. 30. München: Germany: Verlag Dr. Friedrich Pfeil; 2005. Partnertausch im dunklen Wald – stabile Isotope geben neue Einblicke in das Ernährungsverhalten von Orchideen. In: Bayerische Akademie der Wissenschaften. [Google Scholar]
- Gebauer G, Meyer M. 15N and 13C natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytologist. 2003;160:209–223. doi: 10.1046/j.1469-8137.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- Hamada M. Studien über die Mykorrhiza von Galeola septentrionalis Reichb. F – ein neuer Fall der Mykorrhiza-Bildung durch intraradicale Rhizomorpha. Japanese Journal of Botany. 1939;10:151–211. [Google Scholar]
- Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse M-A. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytologist. 2005;166:639–653. doi: 10.1111/j.1469-8137.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Leake JR. The biology of myco-heterotrophic ‘saprophytic’ plants. New Phytologist. 1994;127:171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Leake JR. Plants parasitic on fungi: unearthing the fungi in myco-heterotrophs and debunking the ‘saprophytic’ plant myth. Mycologist. 2005;19:113–122. [Google Scholar]
- McKendrick SL, Leake JR, Read DJ. Symbiotic germination and development of myco-heterotrophic plants in nature: transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytologist. 2000;145:539–548. doi: 10.1046/j.1469-8137.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- Maekawa F. The wild orchids of Japan in colour. Tokyo, Japan: Seibundoshinkousha [in Japanese]; 1971. [Google Scholar]
- Martos F, Dulormne M, Pailler T, et al. Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytologist. 2009;184:668–681. doi: 10.1111/j.1469-8137.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- Motomura H, Yukawa Y, Ueno O, Kagawa A. The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. Journal of Plant Research. 2008;121:163–177. doi: 10.1007/s10265-007-0144-6. [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Yukawa T. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae) American Journal of Botany. 2008a;95:93–97. doi: 10.3732/ajb.95.1.93. [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Yukawa T. In situ seed sowing techniques for the recovery of endangered orchids. Japanese Journal of Conservation Ecology. 2008b;13:121–127. [in Japanese] [Google Scholar]
- Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbon from saprotrophic Mycena. Proceedings of the Royal Society London Series B. 2009;276:761–767. doi: 10.1098/rspb.2008.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Gregg JW. Uncertainly in source partitioning using stable isotopes. Oecologia. 2001;127:171–179. doi: 10.1007/s004420000578. [DOI] [PubMed] [Google Scholar]
- Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- Rasmussen HN. Terrestrial orchids – from seed to mycotrophic plants. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Rasmussen HN, Rasmussen FN. Orchid mycorrhiza: implications of a mycophagous life style. Oikos. 2009;118:334–345. [Google Scholar]
- Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse M-A. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biology. 2009;7(51) doi: 10.1186/1741-7007-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse M-A, Roy M. Green plants that feed on fungi: facts and question about mixotrophy. Trends in Plant Science. 2009;14:64–70. doi: 10.1016/j.tplants.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Selosse M-A, Weiss M, Jany JL, Tillier A. Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) LCM Rich. and neighbouring tree ectomycorrhizae. Molecular Ecology. 2002;11:1831–1844. doi: 10.1046/j.1365-294x.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- Selosse M-A, Faccio A, Scappaticci G, Bonfante P. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microbial Ecology. 2004;47:416–426. doi: 10.1007/s00248-003-2034-3. [DOI] [PubMed] [Google Scholar]
- Selosse M-A, Richard F, Xinhua H, Simard SW. Mycorrhizal networks: des liaisons dangereuses? Trends in Ecology and Evolution. 2006;21:621–628. doi: 10.1016/j.tree.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd edn. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Taylor DL, Bruns TD. Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proceedings of the National Academy of Science of the USA. 1997;94:4510–4515. doi: 10.1073/pnas.94.9.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Bruns TD, Leake JR, Read D. Mycorrhizal specificity and function in myco-heterotrophic plants. In: Van der Heijden MGA, Sanders I, editors. Mycorrhizal ecology. Berlin, Germany: Springer-Verlag; 2002. pp. 375–413. [Google Scholar]
- Tedersoo L, Pellet P, Koljalg U, Selosse MA. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia. 2007;151:206–217. doi: 10.1007/s00442-006-0581-2. [DOI] [PubMed] [Google Scholar]
- Trudell SA, Rygiewicz PT, Edmonds RL. Nitrogen and carbon stable isotope abundances support the myco-heterotrophic nature and host-specificity of certain achlorophyllous plants. New Phytologist. 2003;160:391–401. doi: 10.1046/j.1469-8137.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama J, Fukuda T, Miyoshi K, Yukawa T. Remarkable habitat differentiation and character evolution in Cymbidium (Orchidaceae). 3. Molecular identification of endomycorrhizal fungi inhabiting in Cymbidium. Journal of Plant Research. 2002;115:42. (Suppl.) [Google Scholar]
- Yukawa T, Stern WL. Comparative vegetative anatomy and systematics of Cymbidium (Cymbidieae: Orchidaceae) Botanical Journal of the Linnean Society. 2002;138:383–419. [Google Scholar]
- Yukawa T, Miyoshi K, Yokoyama J. Molecular phylogeny and character evolution of Cymbidium (Orchidaceae) Bulletin of the National Science Museum, Series B (Botany) 2002;28:129–139. [Google Scholar]
- Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of the orchid mycorrhiza. American Journal of Botany. 2009;96:1997–2009. doi: 10.3732/ajb.0900101. [DOI] [PubMed] [Google Scholar]
- Zeller B, Brechet C, Maurice JP, Le Tacon F. 13C and 15N isotopic fractionation in trees, soils and fungi in a natural forest stand and a Norway spruce plantation. Annals of Science. 2007;64:419–429. [Google Scholar]
- Zimmer K, Hynson NA, Gebauer G, Allen EB, Allen MF, Read DJ. Wide geographical and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. New Phytologist. 2007;175:166–175. doi: 10.1111/j.1469-8137.2007.02065.x. [DOI] [PubMed] [Google Scholar]
- Zimmer K, Meyer C, Gebauer G. The ectomycorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytologist. 2008;178:395–400. doi: 10.1111/j.1469-8137.2007.02362.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.