Abstract
Background and Aims
Human-mediated environmental change is increasing selection pressure for the capacity in plants to colonize new areas. Habitat fragmentation combined with climate change, in general, forces species to colonize areas over longer distances. Mating systems and genetic load are important determinants of the establishment and long-term survival of new populations. Here, the mating system of Asplenium scolopendrium, a diploid homosporous fern species, is examined in relation to colonization processes.
Methods
A common environment experiment was conducted with 13 pairs of sporophytes, each from a different site. Together they constitute at least nine distinct genotypes, representing an estimated approx. 95 % of the non-private intraspecific genetic variation in Europe. Sporophyte production was recorded for gametophytes derived from each parent sporophyte. Gametophytes were grown in vitro in three different ways: (I) in isolation, (II) with a gametophyte from a different sporophyte within the same site or (III) with a partner from a different site.
Key Results
Sporophyte production was highest in among-site crosses (III), intermediate in within-site crosses (II) and was lowest in isolated gametophytes (I), strongly indicating inbreeding depression. However, intragametophytic selfing was observed in most of the genotypes tested (eight out of nine).
Conclusions
The results imply a mixed mating system in A. scolopendrium, with outcrossing when possible and occasional selfing when needed. Occasional intragametophytic selfing facilitates the successful colonization of new sites from a single spore. The resulting sporophyte, which will be completely homozygous, will shed large amounts of spores over time. Each year this creates a bed of gametophytes in the vicinity of the parent. Any unrelated spore which arrives is then selectively favoured to reproduce and contribute its genes to the new population. Thus, while selfing facilitates initial colonization success, inbreeding depression promotes genetically diverse populations through outcrossing. The results provide further evidence against the overly simple dichotomous distinction of fern species as either selfing or outcrossing.
Keywords: Asplenium scolopendrium, colonization, gametophyte, homosporous fern, inbreeding depression, life history, mixed mating system, selfing, sporophyte
INTRODUCTION
Ecosystems are increasingly under stress from human-induced environmental change and species survival is becoming more and more dependent on the ability to colonize new areas (Cain et al., 2000; Thomas et al., 2004). Habitat fragmentation increases the average distance among patches of suitable habitat, while on a larger spatial scale, climate change simultaneously forces species to shift their natural ranges (Svenning and Condit, 2008; Svenning et al., 2008, 2009). Besides the issues of dispersal to new areas and local establishment, the long-term success of new colonies is dependent on the reproductive success of its colonists (e.g. Soons and Heil, 2002). Increases in local population size upon initial colonization are necessary to reduce the risk of local extinction, while increases in genetic diversity are often also important in this respect (e.g. Spielman et al., 2004; Crawford and Whitney, 2010).
The likelihood of finding a mate upon reaching sexual maturity declines rapidly as propagules disperse further from their parents (Peck et al., 1990; Nathan, 2001). Colonizing plants are therefore more reliant on self-fertilization and will experience prolonged periods of mating among relatives. In many species, selfing, or inbreeding due to small population size, may result in a reduction in fitness of inbred relative to outbred progeny, i.e. inbreeding depression (Crnokrak and Roff, 1999; Keller and Waller, 2002, but see Pujol et al., 2009). Experimental breeding studies provide information about the extent to which inbreeding depression will affect individual fitness, thus allowing assessment of the genetic risks that environmental changes pose to survival.
Ferns represent a relatively understudied group in the Plant Kingdom and for this group there is a general lack of knowledge about colonization processes and the role of mating systems therein (Haufler, 2009). Ferns are distinct from seed plants in that dispersal occurs via haploid spores and their life-cycle features an independent gametophyte generation. Gametophytes are generally thought to be short-lived in modern ferns, although there are few experimental data (but see Sato, 1982; Peck et al., 1990) and notable exceptions exist (e.g. Osmundaceae, and grammitids in the Polypodiaceae). Sporophytes are diploid (or polyploid derivatives in certain taxa), and mature individuals produce several million spores each year. Relative to seeds, spores tend to be dispersed much further by wind, due to their small size. However, as in seed plants, the majority of propagules land in the immediate vicinity of the parent (Peck et al., 1990). Male gamete dispersal distance tends to be very limited as spermatozoids require transport in water. Although ferns produce large amounts of spores and dispersal by wind is relatively easy, successful mating after long-distance dispersal [defined as >1 km by Peck et al. (1990)], requiring two gametophytes to establish simultaneously within a few centimetres, is considered to an unlikely event (Peck et al., 1990; Flinn, 2006). However, the dispersal distance frequency distribution of ferns is still largely unknown and hence it is unclear how often such events do occur.
Homosporous ferns produce a single type of spore, which may give rise to gametophytes with the potential to become bisexual. Self-fertilization of a haploid gametophyte, i.e. intragametophytic selfing, presents an extreme case of inbreeding and leads to completely homozygous sporophytes (Vogel et al., 1999b). In the absence of genetic load only a single spore is therefore required for successful colonization of new sites (Lloyd, 1974). Besides intragametophytic selfing, which has no analogue in seed plants, intergametophytic selfing is possible in ferns when gametes from two gametophytes originating from the same sporophyte unite; this is equivalent to self-fertilization in seed plants. Outcrossing occurs through union of gametes of two gametophytes originating from different sporophytes, although this can still effectively constitute inbreeding when the parent sporophytes are closely related (i.e. biparental inbreeding). Based on breeding experiments and analyses of genetic variability homosporous ferns have in the past been classified as either predominantly outcrossing or selfing species (Soltis and Soltis, 1992). Compared with diploids, polyploid fern species tend to be less susceptible to inbreeding depression and sustain higher selfing rates (Masuyama, 1979; Soltis and Soltis, 2000).
In this paper, the breeding system of Asplenium scolopendrium (Aspleniaceae), a diploid (2n = 72) homosporous fern species (Tutin et al., 1993) that occurs throughout western Europe (Jalas and Suominen, 1988), is examined. Observations of gametophyte ontogeny for some Scottish populations suggested an outcrossing strategy for A. scolopendrium (Pangua et al., 1994). This species has successfully colonized a number of forests in a recently embanked (1942) polder in The Netherlands (Bremer and Jongejans 2010). With an estimated population size of >13 000 sporophytes in 2002, it is among the most abundant fern species in one of those forests – the ‘Kuinderbos’ (Bremer, 2007). We hypothesized that outcrossing should be more successful in this species than selfing. First because, at least in Europe, A. scolopendrium is a diploid taxon and second because its gametophyte ontogeny suggests an outcrossing strategy (Pangua et al., 1994). Additionally, given the apparent colonization success in the Dutch polders and also throughout Europe (Vogel et al., 1999b), its potential for intragametophytic selfing was of interest.
MATERIALS AND METHODS
Study area and field sampling
The Kuinderbos (52·8°N, 5·8°E), The Netherlands, is a recently planted forest (1949–1954) on land that was reclaimed from a former sea inlet, the ‘Zuiderzee’, in 1942. The vegetation consists of evenly aged stands of trees (Fagus sylvatica, Fraxinus excelsior, Picea abies, P. sitchensis and Quercus robur) and various understorey species. Amongst the latter are, for The Netherlands, remarkably high numbers of ferns (Bremer, 1980, 2007) and bryophytes (Bremer and Ott, 1990). The soil is composed of a layer of calcareous sands (pH ≥ 7·2) over peat. The top sand layer is intersected by numerous drainage trenches that cut well into the peat layer below (Bremer, 2007). This has created an excellent environment for ferns, and, locally, Asplenium scolopendrium is strongly associated with these trenches (Bremer, 1980, 2007). Detailed inventory data have been collected in the area over the past 30 years (Bremer, 2007), which allow the delineation of individual colonization sites. Colonization sites were defined as groups of individuals occupying the same trench, with the distribution of individuals within sites not interspersed by more than a few metres.
Entire fertile fronds were collected from 13 A. scolopendrium sites across the Kuinderbos, taking fronds of two spore-bearing plants at each site. The sampled sporophytes were analysed for allozyme variation at 11 loci following standard methods (see Vogel et al., 1999a). Vouchers from the investigated colonization sites have been deposited at the Natural History Museum London (BM). The following 11 enzyme systems were resolved: 6-phosphogluconate dehydrogenase (6-PGD, EC 1·1·1·44), aspartate aminotransferase (AAT, EC 2·6·1·1), acid phosphatase (ACP, EC 3·1·3·2), glutamate dehydrogenase (GDH, EC 1·4·1·2), isocitrate dehydrogenase (IDH, EC 1·1·1·42), leucine aminopeptidase (LAP, EC 3·4·11·1), malate dehydrogenase (MDH, EC 1·1·1·37), phosphoglucose isomerase (PGI, EC 5·3·1·9), shikimate dehydrogenase (SkDH, EC 1·1·1·25), triose-phosphate isomerase (TPI, EC 5·3·1·1) and utp-glucose pyrophosphorylase (UGPP, EC 2·7·9). Samples from seven populations across Europe (England, n = 8 and 24; France, 30; Germany, 10; Greece, 14; Scotland, 21; Wales, 32) were also analysed for allozyme variation at these loci to estimate the genetic variability included in the experimental part of this study (see below). These data are part of a larger population genetic study of this species (J. C. Vogel et al., unpubl. res.).
Experimental set-up
Fronds were air dried and spores were subsequently sown onto Petri dishes containing a medium consisting of Parker's macronutrients and Thompson's micronutrients (following Klekowski, 1969) solidified with Gelrite® (5·0 g L−1), and the mixture was autoclaved before solidifying. Spores of each sporophyte were sown onto separate Petri dishes, which were sealed with Parafilm® to prevent moisture loss. The dishes were placed in a growth cabinet at 20 °C, with 16 h light, 8 h dark, 134·8 (± 8·3) μmol m−2 s−1 PAR. Upon reaching sufficient size (6 weeks after sowing) the gametophytes were transplanted to their respective experimental treatments.
For each site, one parent sporophyte was chosen as the focal plant and experimental crosses were established in three treatments. The first treatment (I) consisted of isolated gametophytes, the second treatment (II) received one gametophyte from the focal plant and one from the other sporophyte originating from the same site (Table 1). The third treatment group (III) received one gametophyte from the focal sporophyte and one from a different sporophyte, in this treatment originating from a different site. These treatments will collectively be referred to as levels of inbreeding in this paper. Each treatment was replicated 20 times for the 13 focal sporophytes (n = 3 × 13 × 20 = 780). The colonization site which supplied the ‘foreign’ gametophyte in treatment III was chosen randomly for each of the 20 replicate crosses for all focal plants.
Table 1.
Asplenium scolopendrium genotypes (focal individuals) used in the experiment, as inferred from analysis of 11 allozyme loci, and their colonization site of origin
| Genotypes of focal individuals |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Locus | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| SkDH | aa | bb | bb | aa | aa | bb | bb | bb | ab |
| PGI | cc | aa | cc | aa | cc | cc | bc | cc | bc |
| UGPP | bb | aa | aa | aa | aa | bb | bb | ab | aa |
| IDH | bb | bb | bb | bb | bb | bb | bb | bb | ab |
| LAP | ac | aa | aa | aa | bb | aa | aa | aa | aa |
| % Ho | 9 | 0 | 0 | 0 | 0 | 0 | 9 | 9 | 27 |
| KB (site) | 1 | 2,3 | 4 | 5,13 | 7 | 8,11 | 9 | 10 | 12 |
| NF | 1 | 0,0 | 2 | 0,0 | 1 | 1,?* | 4 | 1 | 3 |
Only polymorphic loci are shown.
Different alleles are designated by different letters; bold case indicates loci in the heterozygous state.
Ho, Observed heterozygozity (% loci); KB (site), number of the colonization sites in the Kuinderbos (KB) for which the focal individual in the experiment carries this genotype; NF, number of allelic copies in which the non-focal plant differs from the focal plant in the same colonization site (e.g. 0 is an identical genotype).
*Focal plant KB-6 and non-focal plant KB-11 could not be genotyped for all loci and their genotype could therefore not be identified, non-focal plant KB-6 carries genotype 4.
Crosses were established in 6-cm Petri dishes on the same medium as before. All experimental units were transferred to a greenhouse (Botanical Gardens Utrecht) upon transplantation (March 2008). In addition to sealing all Petri dishes with Parafilm®, moisture loss was further prevented by setting the relative air humidity of the greenhouse to 90 %. Three 400-W sodium lamps were suspended approx. 2 m over the experiment to extend the natural photoperiod to 16 h per day. The greenhouse was heated to 18 °C during the day and 16 °C at night. There were no facilities to cool the greenhouse, so higher temperatures were easily reached, especially during summer. Shade cloth was suspended around the set-up to prevent direct sunlight from harming the gametophytes. Light measurements showed considerable heterogeneity in light intensities across the site of the experiment (data not shown) so the entire set-up was randomized fortnightly. Upon observing the first gametangia (12 weeks after sowing) all experimental units were watered every other week, using sterilized water. Water was applied to both gametophytes (where applicable) so as to connect them by a single water film, thereby facilitating free movement of spermatozoids among them. Fortnightly, all gametophytes of focal plants were checked for sporophyte production, and mortality of gametophytes was recorded. After transplantation the experiment was monitored for 32 more weeks (i.e. until 38 weeks after sowing).
Statistical analysis
Binary logistic regression analyses were used to assess the effects of level of inbreeding and parent sporophyte on sporophyte production at representative time points during the experiment. In the course of the experiment mortality among gametophytes was monitored. Dead gametophytes cannot produce new sporophytes, yet may have produced one in the past. To account for the reduction in opportunities for fertilization that the death of a gametophyte represents, the realized fertilization time was entered as a covariate into the analyses. Realized fertilization time was defined as the number of weeks that the gametophyte had been alive since the first gametangia were observed (12 weeks after sowing) for each point of observation. Both level of inbreeding and parent sporophyte were entered as categorical variables and non-significant interactions were removed from the final analyses. Analyses with genotype (Table 1) as the categorical variable instead of parent sporophyte led to the same conclusions. Pairwise differences among levels of inbreeding were tested using a repeated contrast, comparing, first, treatment I and II and, then, treatments II and III in the overall analysis. P-values for pairwise comparisons were Bonferroni corrected. All analyses were performed with SPSS (v16; SPSS Inc., Chicago, IL, USA).
RESULTS
The 11 enzyme systems screened revealed eight polymorphic loci within the eight European populations sampled, while five of these loci were polymorphic in the Kuinderbos. The eight polymorphic loci revealed a total of 20 alleles, of which five were private to single populations. The Kuinderbos population had no private alleles. The individuals from the Kuinderbos together represent approx. 95 % of all non-private alleles (75 % of total) found in the seven reference populations from across Europe. Hence, a large proportion of the species' genetic variation was represented in the experiment. The individuals that were used as focal plants in the experiment represent nine distinct genotypes (Table 1).
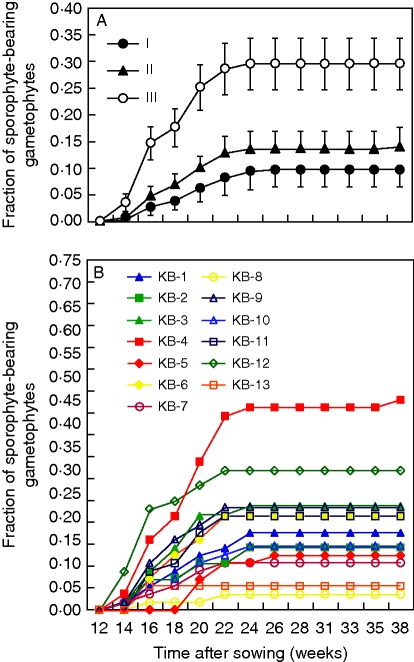
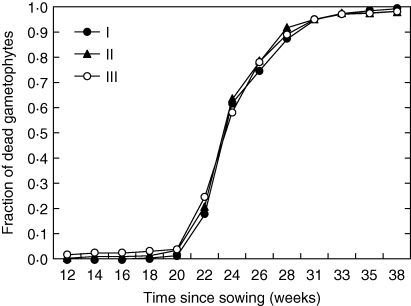
The first sporophytes were recorded 14 weeks after sowing and their number grew steadily until week 22 (Fig. 1A). By that time gametophyte mortality effectively limited opportunities for further fertilizations (Appendix 1). Due to a lack of cooling facilities in the greenhouse, daily maximum air temperatures regularly exceeded 30 °C from week 14 onward (data not shown). At the end of the experiment (week 38) nearly 18 % of the focal gametophytes had produced a sporophyte.
Fig. 1.
Mean sporophyte production over time for the different levels of inbreeding (A) over all parent sporophytes and for parent sporophyte (B) over all levels of inbreeding. I, Intragametophytic selfing; II, within-site crosses; III, among-site crosses. Error bars represent the standard error of the mean (n = 13).
In the course of the experiment numerous cases of aborted embryos were observed. Embryos started out as clumps of cells within archegonia, causing visible swelling within the gametophyte. The embryos were considered aborted when they did not produce any roots or leaves. Such abortions were, unfortunately, not systematically recorded. Polyembryony, i.e. the emergence of two or more sporophytes from a single gametophyte, was observed in only two crosses, both on gametophytes originating from the focal sporophyte of site KB-12.
Sporophyte production differed markedly over the three levels of inbreeding (Fig. 1A and Table 2). Overall, sporophyte production was highest in among-site crosses (III), with almost 30 % of the focal gametophytes eventually producing a sporophyte. Within-site crosses (II) were less than half as successful (approx. 14 %), while intragametophytic selfing (I) had the lowest success rate. Nevertheless, in the latter treatment (I) the average sporophyte production still amounted to nearly 10 %. Gametophytes of only three out of the total 13 parent sporophytes did not produce any sporophytes in isolation (KB-8, -11 and -13). All others produced some sporophytes.
Table 2.
Binary logistic regression analyses on sporophyte production 16, 20, 24 and 28 weeks after sowing (Nagelkerke R2 and Wald tests of significance for level of inbreeding, parent sporophyte and gametophyte mortality are shown for each analysis)
| Inbreeding level |
Parent sporophyte |
Gametophyte mortality* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week | R2 | Wald | d.f. | Sig. | Wald | d.f. | Sig. | Wald | d.f. | Sig. |
| 16 | 0·208 | 28·366 | 2 | < 0·0005 | 24·099 | 12 | 0·020 | <0·0005 | 1 | 0·999 |
| 20 | 0·217 | 42·113 | 2 | < 0·0005 | 39·591 | 12 | < 0·0005 | <0·0005 | 1 | 0·999 |
| 24 | 0·225 | 41·597 | 2 | < 0·0005 | 52·143 | 12 | < 0·0005 | 6·090 | 1 | 0·014 |
| 28 | 0·233 | 40·859 | 2 | < 0·0005 | 51·386 | 12 | < 0·0005 | 13·369 | 1 | < 0·0005 |
Significant P-values (P < 0·05) are displayed in bold face.
* Gametophyte mortality was treated as a reduction in fertilization time (see Materials and methods).
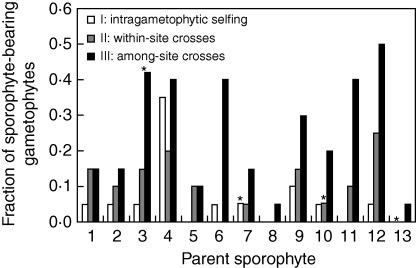
Marked differences were also observed among parent sporophytes (Fig. 1B and Table 2). In the among-site crosses (III) differences in sporophyte production among focal individuals became most interesting, as in this treatment they were paired up with gametophytes from randomly assigned colonization sites. So even though the gametophytes of each focal sporophyte were presented with the same palette of partners, marked differences in performance still arose (Fig. 2). Furthermore, the intragametophytic selfing (I) capacity of KB-4, was 3-fold higher than for any other sporophyte and it rivalled its own performance in the among-site (III) crosses (Fig. 2).
Fig. 2.
Sporophyte production 20 weeks after sowing, for all 13 parent sporophytes and all treatments. n = 20 for all combinations, except for those marked with an asterisk where n = 19.
The differences among the levels of inbreeding as well as parent sporophytes, while accounting for gametophyte mortality, were highly significant over time (Table 2). There was no significant interaction between the level of inbreeding and parent sporophyte (not shown). Pairwise comparison of the levels of inbreeding showed a significant difference between within-site (II) and among-site (III) crosses [e.g. week 20; odds ratio: 3·29; 95 % CI: (1·96 : 5·49), P < 0·001]. Differences between intragametophytic selfing (I) and within-site crosses (II) were only significant after the exclusion of parent sporophyte KB-4 [e.g. week 20; odds ratio: 2·74; 95 % CI: (1·22 : 6·15), P = 0·030; including KB-4, P = 0·168].
DISCUSSION
This study provides evidence for inbreeding depression in Asplenium scolopendrium. In the course of the experiment abortions of sporophytic embryos were frequently observed, which indicates the presence of recessive deleterious alleles (Lloyd, 1974). However, it was also found that gametophytes of the majority of parental sporophytes were capable of fertilization and sporophyte production through intragametophytic selfing (I). Thus, although this species is preferentially outcrossing, when mates are limited it can reproduce through this extreme form of selfing.
Previous investigations of the gametophyte ontogeny of Scottish A. scolopendrium populations concluded that this species was predominantly outcrossing (Pangua et al., 1994). We concur with these findings, but note that the majority of genotypes investigated was able to reproduce by means of intragametophytic selfing (I) and argue that this constitutes a mixed mating system with potentially important implications for our understanding of the species' biogeography.
Intragametophytic selfing facilitates the establishment of a sporophyte from a single spore (Lloyd, 1974). When this first sporophyte grows to a certain size it will start shedding large amounts of spores every year. Most of these spores will land in proximity to the parent (Peck et al., 1990), thus locally creating an abundance of gametophytes. Any foreign spore entering and germinating within the spore shadow of the first sporophyte has a good chance of contributing its genes to the nascent population by outcrossing with the locally abundant gametophytes. Of course, population expansion through continued selfing cannot be excluded, but the present results show that outcrossing is generally far more successful. Furthermore, in ferns, inbreeding depression negatively affects not only fertilization, but also sporophyte growth and survival (Peck et al., 1990; Schneller and Holderegger, 1997). Hence, outcrossed progeny in A. scolopendrium probably stand a greater chance of producing spores and of long-term survival. In that case, outcrossed progeny will have a higher fitness in the new population and can therefore be expected to outcompete inbred progeny over time (e.g. Crnokrak and Roff, 1999; Koelewijn, 2004), except perhaps in cases where the habitat is very benign (see below). Thus, selective forces act to increase genetic diversity within newly established populations of this species. The view taken here is supported by the fact that, in spite of a very limited sampling scheme (i.e. two individuals per colonization site), two different genotypes were found within two-thirds of the colonization sites sampled (Table 1). When considering the species' colonization potential, we hypothesize that the colonization strategy just described is likely to be typical for at least the diploid populations of this species.
Establishing first colonists through intragametophytic selfing, however feeble these individuals may be, has two clear advantages. First, once the sporophyte starts releasing spores, the surface area where outcrossing can take place increases from the tiny area of a single gametophyte to potentially thousands of times that area. Second, the initial colonist's window of opportunity for outcrossing extends from the span of just a few months to many years. This suggests that within newly established populations there is strong selection for genotypes that are to some extent capable of intragametophytic selfing. Whether the proportion of genotypes capable of intragametophytic selfing found in older populations is lower than the relatively young colonies in the Kuinderbos is still an open question.
The relevance of this colonization strategy in nature is strengthened by detailed inventory data from the Kuinderbos, in which 260 isolated colonizations (roughly translating to one colonization per 900 m2 of drainage ditch) were recorded for A. scolopendrium in 1979 (Bremer, 2007). The early colonists subsequently increased their numbers, forming large groups of sporophytes, sometimes containing up to a few hundred individuals. Unfortunately, none of the initial colonizing sporophytes have survived to this day (Bremer, 2007), thus precluding genetic analysis of the mating type that resulted in these initial colonists. However, in the future we hope to assess the frequency of establishments through intragametophytic selfing of A. scolopendrium in nature, by using microsatellite (simple sequence repeat) markers that are currently under development for this species.
In the present experiment, within-site crosses (II) resulted in more sporophytes than isolated cultures (I). However, the largest increase in reproductive success occurred in the among-site crosses (III). The sampled genotypes show low levels of heterozygozity and, generally, variation was low between the two plants within each site (Table 1). Individual sites within the Kuinderbos are therefore considered to be effectively inbreeding due to a limited input of foreign spores.
Inbreeding depression under stress
In the present experiment, gametophyte mortality strongly limited fertilizations after 22 weeks. In our opinion this does, however, represent a natural situation. Mortality rates of gametophytes in the field have been shown to be very high, with the majority perishing within a few months (Peck et al., 1990). Similarly, preliminary results from a systematic study of the population dynamics of Polystichum aculeatum in the Kuinderbos indicate that the majority of gametophytes are present for <4 months (G. A. de Groot, unpubl. res.). After this time they have either managed to produce sporophytes or have vanished without apparent reproductive success. This time interval is shorter than the period between sowing and the moment that gametophyte mortality became a limiting factor in the experiment.
The low observed sporophyte production in the experiment is likely to be a consequence of the high air temperatures reached in the greenhouse (daily maximum >30 °C). This resulted in high gametophyte mortality, peaking in week 24, which effectively barred any further fertilization. Such extreme temperatures are unlikely to be reached regularly in shaded environments in most of Europe. However, many populations of A. scolopendrium in The Netherlands are wall dwelling and this environment may be more extreme in this respect. In addition, other factors, such as summer drought (Cinquemani Kuehn and Leopold, 1992), may make the environment equally stressful for A. scolopendrium gametophytes. Furthermore, inbreeding depression is expected to be most apparent under stressful conditions (Armbruster and Reed, 2005; Goodwillie et al., 2005), due to abiotic stress (Dudash, 1990) or interspecific competition (Pluess and Stöcklin, 2004). The present observations of intragametophytic selfing under high gametophyte mortality rates thus strengthen its potential importance in nature and the species' capacity to reproduce in this way under ideal conditions may well be higher.
The development and sex expression of gametophytes in laboratory-based experiments may differ from growth patterns under field conditions (Cousens, 1979; Pangua et al., 1994; Ranker and Houston, 2002). For example, under stressful conditions gametophytes tended to become male in Matteuccia struthiopteris, while relatively benign environments yielded more females [Prantl, 1881 (cited in Näf, 1979)]. However, conditions in nature also vary and patterns of sex expression are thus expected to vary across populations in the field as well (see, for example, data in Ranker and Houston, 2002). Nevertheless, the present experiment shows that, besides outcrossing, intragametophytic selfing is possible in A. scolopendrium and this is interpreted as evidence for a mixed mating system.
Species biogeography
From a biogeographical perspective, the mixed mating system, as described above, may add to our understanding of the widespread occurrence and obvious colonization potential of A. scolopendrium in Europe. Polyploids are generally less susceptible to inbreeding depression than diploids and can sustain high rates of intragametophytic selfing (e.g. Masuyama, 1979; Soltis and Soltis, 1992, 2000) and are therefore expected to be better colonizers (Baker, 1955). In line with this, it has been argued that, in Asplenium, diploid species are often geographically restricted, while polyploids are far more widespread (Vogel et al., 1999b). However, in contrast to the general pattern, A. scolopendrium is a diploid, as well as one of the most widespread Asplenium species in Europe (Jalas and Suominen, 1988; Vogel et al., 1999b). Furthermore, its recent success in colonizing the Kuinderbos (Bremer, 2007) and six other newly planted woodlands in the direct vicinity of the Kuinderbos (Bremer and Jongejans, 2010), a similar rapid colonization of a Hungarian pine forest (Szerdahelyi and Pintér, 1996), and its remarkably frequent occurrence on man-made walls (e.g. Edgington, 2007) are all testimony to the colonization potential of this species.
We hypothesize that the mixed mating system and associated colonization strategy of A. scolopendrium, as described above, is one explanation for its wide geographic distribution relative to most other diploid species in Asplenium. A prediction following from this hypothesis is that isolated colonizing sporophytes in this species result from intragametophytic selfing and should therefore be completely homozygous at all loci.
In a wider context, the four other diploid fern species reported in the literature to exhibit mixed mating systems also have geographically wide distributions: Dryopteris expansa (circumboreal; Soltis and Soltis, 1987; Page, 1997), Blechnum spicant (interruptedly circumboreal; Soltis and Soltis, 1988; Page, 1997), Hemionitis palmata (Central and South America; Ranker et al., 1989; Ranker, 1992) and Odontosoria chinensis (Indo-Pacific; Ranker et al., 2000). Furthermore, Polystichum setiferum, also a diploid fern, has been shown to be predominantly outcrossing in two Spanish populations (Pangua et al., 2003), while in the Kuinderbos it is predominantly selfing (G. A. de Groot, unpubl. res.). Hence, several diploid fern species exhibit variation in selfing rates among populations and they may rely on temporarily increased selfing rates for their colonization potential. Thus, as in seed plants (Goodwillie et al., 2005), mixed mating systems may be more common in ferns then previously realized.
Intraspecific variation in reproductive success
In the present experiment, large differences in reproductive performance among A. scolopendrium genotypes were found. Although mating systems have been studied experimentally in various fern species (e.g. Korpelainen, 1996; Lott et al., 2003; Pangua et al., 2003; Flinn, 2006), few studies have reported intraspecific variation (but see Peck et al., 1990; Suter et al., 2000). Large differences in selfing rate have been observed among populations in assays of genetic variation in ferns (e.g. Soltis and Soltis, 1987, 1992; Ranker, 1992; Ranker et al., 2000; Pryor et al., 2001), as has also been found in angiosperms (e.g. Arabis alpina; see Ansell et al., 2008) and bryophytes (e.g. Leucodon sciuroides; see Cronberg, 2000).
In the present study, the success rate of intragametophytic selfing in A. scolopendrium varied from zero to 45 %. Compared with the study by Peck et al. (1990) this range encompasses the variation in intragametophytic selfing rates among eight of the ten species they studied. As environmental conditions differ among experiments, the results are difficult to compare directly across studies. Nevertheless, this indicates that breeding system studies should include as wide a sample of the intraspecific variation as possible in order to be able to draw conclusions on the species level. Additionally, the present results challenge the overly simple dichotomous classification of fern species as predominantly selfing or outcrossing. The results call for a comprehensive experimental study involving multiple fern species and genotypes within species in order to estimate the differences in performance within species relative to differences among species.
Conclusions
Asplenium scolopendrium shows clear inbreeding depression, while simultaneously maintaining the capacity to reproduce through intragametophytic selfing. Among 13 parent sporophytes, one was found which showed far higher capacity for intragametophytic selfing, and three which did not reproduce in this way. This study adds to the increasing awareness that mixed mating systems are more prevalent in plants than has been realized previously (Goodwillie et al., 2005). In the present experiment remarkable variation in reproductive success was observed among genotypes, emphasizing the need to include sufficient intraspecific variation in breeding system studies.
ACKNOWLEDGEMENTS
We thank Fred Siesling (Botanic Gardens Utrecht) for his assistance during the experiment and Paul Westers (University Medical Centre Utrecht) for statistical advice. This work was supported by the Miquel Fund (Utrecht University).
APPENDIX
Gametophyte mortality in the experiment over time, averaged by level of inbreeding. I, Intragametophytic selfing; II, within-site crosses; III, among-site crosses.
LITERATURE CITED
- Ansell SW, Grundmann M, Russell SJ, Schneider H, Vogel JC. Genetic discontinuity, breeding-system change and population history of Arabis alpina in the Italian Peninsula and adjacent Alps. Molecular Ecology. 2008;17:2245–2257. doi: 10.1111/j.1365-294X.2008.03739.x. [DOI] [PubMed] [Google Scholar]
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Baker HG. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- Bremer P. The ferns of the Kuinderbos (The Netherlands), the establishment of 23 species in a planted forest. Acta Botanica Neerlandica. 1980;29:351–357. [Google Scholar]
- Bremer P. The colonisation of a former sea-floor by ferns. 2007 PhD Thesis. Wageningen University, The Netherlands. [Google Scholar]
- Bremer P, Jongejans E. Frost and forest stand effects on the population dynamics of Asplenium scolopendrium. Population Ecology. 2010;52:211–222. [Google Scholar]
- Bremer P, Ott ECJ. The establishment and distribution of bryophytes in the woods of the IJsselmeerpolders, The Netherlands. Lindbergia. 1990;16:3–18. [Google Scholar]
- Cain ML, Milligan BG, Strand AE. Long-distance seed dispersal in plant populations. American Journal of Botany. 2000;87:1217–1227. [PubMed] [Google Scholar]
- Cinquemani Kuehn DM, Leopold DJ. Long-term demography of Phyllitis scolopendrium (L.) Newm. var. americana Fern. in central New York. Bulletin of the Torrey Botanical Club. 1992;119:65–76. [Google Scholar]
- Cousens MI. Gametophyte ontogeny, sex expression, and genetic load as measures of population divergence in Blechnum spicant. American Journal of Botany. 1979;66:116–132. [Google Scholar]
- Crawford KM, Whitney KD. Population genetic diversity influences colonization success. Molecular Ecology. 2010;19:1253–1263. doi: 10.1111/j.1365-294X.2010.04550.x. [DOI] [PubMed] [Google Scholar]
- Crnokrak P, Roff DA. Inbreeding depression in the wild. Heredity. 1999;83:260–270. doi: 10.1038/sj.hdy.6885530. [DOI] [PubMed] [Google Scholar]
- Cronberg N. Genetic diversity of the epiphytic bryophyte Leucodon sciuroides in formerly glaciated versus nonglaciated parts of Europe. Heredity. 2000;84:710–720. doi: 10.1046/j.1365-2540.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- Dudash MR. Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution. 1990;44:1129–1139. doi: 10.1111/j.1558-5646.1990.tb05220.x. [DOI] [PubMed] [Google Scholar]
- Edgington JA. Dynamics of long-distance dispersal: the spread of Asplenium adiantum-nigrum and Asplenium trichomanes (Aspleniaceae; Pteridophyta) on London walls. Fern Gazette. 2007;18:31–38. [Google Scholar]
- Flinn KM. Reproductive biology of three fern species may contribute to differential colonization success in post-agricultural forests. American Journal of Botany. 2006;93:1289–1294. doi: 10.3732/ajb.93.9.1289. [DOI] [PubMed] [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution and Systematics. 2005;36:47–79. [Google Scholar]
- Haufler CH. Gels and genetics: the historical impact of isozymes on paradigm shifts in hypotheses about fern evolutionary biology. American Fern Journal. 2009;99:125–128. [Google Scholar]
- Jalas J, Suominen J, editors. Atlas florae Europaeae. 2nd edn. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends in Ecology and Evolution. 2002;17:230–241. [Google Scholar]
- Klekowski EJ. Reproductive biology of the Pteridophytes. III. A study of the Blechnaceae. Botanical Journal of the Linnean Society. 1969;62:361–377. [Google Scholar]
- Koelewijn HP. Sibling competition, size variation and frequency-dependent outcrossing advantage in Plantago coronopus. Evolutionary Ecology. 2004;18:51–74. [Google Scholar]
- Korpelainen H. Intragametophytic selfing does not reduce reproduction in Dryopteris filix-max. Sexual Plant Reproduction. 1996;9:117–122. [Google Scholar]
- Lloyd RM. Reproductive biology and evolution in the Pteridophyta. Annals of the Missouri Botanical Garden. 1974;61:318–331. [Google Scholar]
- Lott MS, Volin JC, Pemberton RW, Austin DF. The reproductive biology of the invasive ferns Lygodium microphyllum and L. japonicum (Schizaeaceae): implications for invasive potential. American Journal of Botany. 2003;90:1144–1152. doi: 10.3732/ajb.90.8.1144. [DOI] [PubMed] [Google Scholar]
- Masuyama S. Reproductive biology of the fern Phegopteris decursive-pinnata. I. The dissimilar mating systems of diploids and tetraploids. The Botanical Magazine, Tokyo. 1979;92:275–289. [Google Scholar]
- Näf U. Antheridiogens and antheridial developement. In: Dyer AF, editor. The experimental biology of ferns. London: Academic Press; 1979. pp. 435–470. [Google Scholar]
- Nathan R. Dispersal biogeography. In: Levin SA, editor. Encyclopaedia of biodiversity. Vol. 2. San Diego, CA: Academic Press; 2001. pp. 127–152. [Google Scholar]
- Page CN. The ferns of Britain and Ireland. 2nd edn. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Pangua E, Lindsay S, Dyer A. Spore germination and gametophyte development in three species of Asplenium. Annals of Botany. 1994;73:587–593. [Google Scholar]
- Pangua E, Quintanilla LG, Sancho A, Pajarón S. A comparative study of the gametophyte generation in the Polystichum aculeatum group (Pteridophyta) International Journal of Plant Sciences. 2003;164:295–303. [Google Scholar]
- Peck JH, Peck CJ, Farrar DR. Influences of life history attributes on formation of local and distant fern populations. American Fern Journal. 1990;80:126–142. [Google Scholar]
- Pluess AR, Stöcklin J. Genetic diversity and fitness in Scabiosa columbaria in the Swiss Jura in relation to population size. Conservation Genetics. 2004;5:145–156. [Google Scholar]
- Pryor KV, Young JE, Rumsey FJ, Edwards KJ, Bruford MW, Rogers HJ. Diversity, genetic structure and evidence of outcrossing in British populations of the rock fern Adiantum capillus-veneris using microsatellites. Molecular Ecology. 2001;10:1881–1894. doi: 10.1046/j.1365-294x.2001.01343.x. [DOI] [PubMed] [Google Scholar]
- Pujol B, Zhou S-R, Sanchez Vilas J, Pannell JR. Reduced inbreeding depression after species range expansion. Proceedings of the National Academy of Sciences of the USA. 2009;106:15379–15383. doi: 10.1073/pnas.0902257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranker TA. Genetic diversity, mating systems, and interpopulation gene flow in neotropical Hemionitis palmata L. (Adiantaceae) Heredity. 1992;69:175–183. [Google Scholar]
- Ranker TA, Houston HA. Is gametophyte sexuality in the laboratory a good predictor of sexuality in nature? American Fern Journal. 2002;92:112–118. [Google Scholar]
- Ranker TA, Haufler CH, Soltis PS, Soltis DE. Genetic evidence for allopolyploidy in the neotropical fern Hemionitis pinnatifida (Adiantaceae) and the reconstruction of an ancestral genome. Systematic Botany. 1989;14:439–447. [Google Scholar]
- Ranker TA, Gemmill CEC, Trapp PG. Microevolutionary patterns and processes of the native Hawaiian colonizing fern Odontosoria chinensis (Lindsaeaceae) Evolution. 2000;54:828–839. doi: 10.1111/j.0014-3820.2000.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Sato T. Phenology and wintering capacity of sporophytes and gametophytes of ferns native to northern Japan. Oecologia. 1982;55:53–61. doi: 10.1007/BF00386718. [DOI] [PubMed] [Google Scholar]
- Schneller JJ, Holderegger R. Vigor and survival of inbred and outbred progeny of Athyrium filix-femina. International Journal of Plant Sciences. 1997;158:79–82. [Google Scholar]
- Soltis DE, Soltis PS. Breeding system of the fern Dryopteris expansa: evidence for mixed mating. American Journal of Botany. 1987;74:504–509. [Google Scholar]
- Soltis DE, Soltis PS. The distribution of selfing rates in homosporous ferns. American Journal of Botany. 1992;79:97–100. [Google Scholar]
- Soltis PS, Soltis DE. Genetic variation and population structure in the fern Blechnum spicant (Blechnaceae) from western North America. American Journal of Botany. 1988;75:37–44. [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons MB, Heil GW. Reduced colonization capacity in fragmented populations of wind-dispersed grassland forbs. Journal of Ecology. 2002;90:1033–1043. [Google Scholar]
- Spielman D, Brook BW, Frankham R. Most species are not driven to extinction before genetic factors impact them. Proceedings of the National Academy of Sciences of the USA. 2004;101:15261–15264. doi: 10.1073/pnas.0403809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Schneller JJ, Vogel JC. Investigations into the genetic variation, population structure, and breeding systems of the fern Asplenium trichomanes subsp. quadrivalens. International Journal of Plant Sciences. 2000;161:233–244. doi: 10.1086/314258. [DOI] [PubMed] [Google Scholar]
- Svenning J-C, Condit R. Biodiversity in a warmer world. Science. 2008;322:206–207. doi: 10.1126/science.1164542. [DOI] [PubMed] [Google Scholar]
- Svenning J-C, Normand S, Skov F. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography. 2008;31:316–326. [Google Scholar]
- Svenning J-C, Kerr J, Rahbek C. Predicting future shifts in species diversity. Ecography. 2009;32:3–4. [Google Scholar]
- Szerdahelyi T, Pintér I. The establishment of fern species and subsequent changes in a planted pine forest in Hungary. In: Camus JM, Gibby M, Johns RJ, editors. Pteridology in perspective. Kew: Royal Botanical Gardens; 1996. pp. 673–674. [Google Scholar]
- Thomas CD, Cameron A, Green RE, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, Valentine SH, Moore DM. Flora Europaea. 2nd edn. Vol. 1. Cambridge: Cambridge University Press; 1993. Psilotaceae to Platanaceae. [Google Scholar]
- Vogel JC, Rumsey FJ, Russell SJ, et al. Genetic structure, reproductive biology and ecology of isolated populations of Asplenium csikii (Aspleniaceae, Pteridophyta) Heredity. 1999a;83:604–612. doi: 10.1038/sj.hdy.6886120. [DOI] [PubMed] [Google Scholar]
- Vogel JC, Rumsey FJ, Schneller JJ, Barrett JA, Gibby M. Where are the glacial refugia in Europe? Evidence from pteridophytes. Biological Journal of the Linnean Society. 1999b;66:23–37. [Google Scholar]