Abstract
Background and Aims
Endospermic legumes are abundant in tropical forests and their establishment is closely related to the mobilization of cell-wall storage polysaccharides. Endosperm cells also store large numbers of protein bodies that play an important role as a nitrogen reserve in this seed. In this work, a systems approach was adopted to evaluate some of the changes in carbohydrates and hormones during the development of seedlings of the rain forest tree Sesbania virgata during the period of establishment.
Methods
Seeds imbibed abscisic acid (ABA), glucose and sucrose in an atmosphere of ethylene, and the effects of these compounds on the protein contents, α-galactosidase activity and endogenous production of ABA and ethylene by the seeds were observed.
Key Results
The presence of exogenous ABA retarded the degradation of storage protein in the endosperm and decreased α-galactosidase activity in the same tissue during galactomannan degradation, suggesting that ABA represses enzyme action. On the other hand, exogenous ethylene increased α-galactosidase activity in both the endosperm and testa during galactomannan degradation, suggesting an inducing effect of this hormone on the hydrolytic enzymes. Furthermore, the detection of endogenous ABA and ethylene production during the period of storage mobilization and the changes observed in the production of these endogenous hormones in the presence of glucose and sucrose, suggested a correlation between the signalling pathway of these hormones and the sugars.
Conclusions
These findings suggest that ABA, ethylene and sugars play a role in the control of the hydrolytic enzyme activities in seeds of S. virgata, controlling the process of storage degradation. This is thought to ensure a balanced flow of the carbon and nitrogen for seedling development.
Keywords: Abscisic acid, α-galactosidase, ethylene, galactomannan, glucose, proteases, seeds, Sesbania virgata, storage protein, sucrose
INTRODUCTION
The Angiosperms display different strategies for seedling establishment that include accumulation of different storage compounds in their seeds (Buckeridge et al., 2000c). These polymers, which can be proteins and carbohydrates, are mobilized during development and their products are used for several purposes such as energy generation and production of raw material for building cells and tissues (Buckeridge et al., 2004). The physiological performance of the seed is essential for successful plant establishment. This phase of plant growth is thought to be the weakest in the life cycle of plants (Stebbins, 1974).
The seeds of many legumes are known to accumulate a large number of protein bodies in the cytoplasm of endosperm cells, which play a major role as a nitrogen reserve in the seed. Besides protein bodies, seeds of legumes and also from species of many other plant families can accumulate cell-wall storage polysaccharides (galactomannan, xyloglucan and arabinogalactan) (Buckeridge and Dietrich, 1996; Buckeridge et al., 2000b; Tonini et al.; 2006). To efficiently mobilize reserves, seed tissues use programmed cell death systems in which extremely complex biochemical and molecular mechanisms have been described (Alcântara et al., 2006; Brandão et al., 2009).
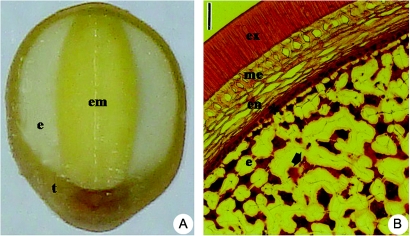
A number of species, including guar (Cyamopsis tetragonoloba), locust bean or carob (Ceratonia siliqua), fenugreek (Trigonella foenum-graecum) and the tropical fast-growing tree Sesbania virgata, have been used as a model to understand physiological and biochemical aspects of galactomannan mobilization as a cell-wall storage polysaccharide. In contrast to carob, seeds of S. virgata have a conspicuous aleurone layer as the outermost endosperm layer between the testa and the remaining living endosperm (Fig. 1) (Buckeridge and Dietrich, 1996; Potomati and Buckeridge, 2002). Sesbania virgata is a legume tree that occurs mainly in moist and flooded regions in the gallery forests of the Neotropical regions and is thought to be associated with early stages of ecological succession (Potomati and Buckeridge, 2002).
Fig. 1.
Transverse sections of seeds of S. virgata showing: (A) a resistant testa (t) and a massive endosperm (e) surrounding the embryo (em) and (B) an aleurone layer (asterisk) between the testa [divided into exotesta (ex), mesotesta (me), endotesta (en)] and the endosperm (e) with protein bodies (arrow) and thick walls. Scale bar = 84 µm. Pictures reproduced from Tonini et al. (2007) with permission.
Many legume seeds (probably >50 % of the species in the family; Buckeridge et al. 2000a) store galactomannans in their endosperms. The galactomannans are composed of a linear backbone of β-(1 → 4)-linked-d-mannose residues to which single units of d-galactose residues are attached by α-(1 → 6)-linkages, providing polymers with different mannose : galactose ratios (Reid, 1985; Buckeridge et al., 2000a). Three hydrolytic enzymes are involved in galactomannan degradation: α-galactosidase (EC 3·2.1·22), endo-β-mannanase (EC 3·2.1·78) and β-mannosidase (EC 3·2.1·25) (Reid and Meier, 1972; McCleary and Mathenson, 1976; Buckeridge and Dietrich, 1996).
Abscisic acid (ABA) is a potent inhibitor of storage protein mobilization (Garciarrubio et al., 1997) and galactomannan degradation in seeds of fenugreek (Reid and Meier, 1973; Kontos and Spyropoulos, 1995), carob (Seiler, 1977), coffee (Coffea arabica) (Giorgini and Comoli, 1996), lettuce (Lactuca sativa) (Toorop et al., 1999), tomato (Lycopersicon esculentum) (Nomaguchi et al., 1995; Nonogaki and Morohashi, 1996; Toorop et al., 1999, 2000) and S. virgata (Potomati and Buckeridge, 2002; Tonini et al., 2006). High concentrations of ABA have been detected in seeds of S. virgata (Tonini et al., 2006). These authors found that most of the ABA in quiescent seeds is present in the testa and during germination it decreases with a concomitant increase in the endosperm and embryo. Furthermore, exogenous ABA has been shown to inhibit storage mobilization in seeds of S. virgata, strongly inhibiting the appearance of α-galactosidase activity in the endosperm (Potomati and Buckeridge, 2002; Tonini et al., 2006). The testa, where most of the ABA is present in quiescent seeds and in which most of the activity of α-galactosidase and endo-β-mannanase are present during galactomannan degradation, seems to play a key role in storage mobilization (Tonini et al., 2006, 2007).
The presence of ABA in the testa of some species, has suggested that the powerful inhibitory effect of this tissue may play a role in the dormancy and germination of seeds (Van Staden et al., 1987; Bewley and Black, 1994; Frey et al., 2004). For S. virgata, it is not yet known how ABA and hydrolases are linked to each other, but it is likely that such an inhibitory system is present and involves not only the endosperm and galactomannan, but also other seed tissues and hormones.
Ethylene, in an antagonistic way, stimulates seed germination of many species, such as Arabidopsis thaliana (Beaudoin et al., 2000), Amaranthus caudatus (Bialecka and Kepczynski, 2007), lettuce (Nascimento et al., 2004), tobacco (Nicotiana tabacum) (Khalil, 1992), rice (Oryza sativa) (Gianinetti et al., 2007), Lepidium sativum (Linkies et al., 2009) and others. In seeds of A. caudatus, ethylene can stimulate the activity of α-galactosidase (Bialecka and Kepczynski, 2007), whereas in seeds of lettuce (Cardoso, 2004; Nascimento et al., 2004, 2005) and tomato (Pirrello et al., 2006), the hormone ethylene stimulates the activity of endo-β-mananase.
In addition to the relationship between ABA and ethylene hormonal pathways, characterization of sugar-signalling mutants in Arabidopsis has demonstrated a complex signalling network that links sugar responses to ABA and ethylene in opposite ways (León and Sheen, 2003). Sugars can promote the induction of ABA biosynthesis and signalling genes of ABA pathways (Arenas-Huertero et al., 2000; Kucera et al., 2005; Chen et al., 2006), which partially antagonize ethylene biosynthesis and signalling during seedling development (Zhou et al., 1998; León and Sheen, 2003; Rognoni et al., 2007). However, sugars may also directly inhibit ethylene signalling (Rolland et al., 2002). Such a complex control system probably avoids the accumulation of an excess of reducing sugars in seed and seedling tissues, avoiding sugar leakage and ensuring an efficient flow of the carbon and nitrogen to the developing seedling.
In the present work, a systems approach was adopted to produce evidence in favour of the hypothesis that ABA, ethylene and sugars all have a role in a network system that modulates storage mobilization of galactomannan and proteins in seeds of S. virgata and to demonstrate how this complex network system affects the establishment of the seedlings in their natural environment.
MATERIALS AND METHODS
Material, seed germination and sampling
Seeds of Sesbania marginata (Cav.) Pers. (= Sesbania marginata Benth.) were collected from trees found in the urban region of São Paulo, Brazil. Seeds were scarified, placed on filter paper in Erlenmeyer flasks fitted with rubber septum caps and then incubated in 0·5 mL of solution per seed at 25 °C, under a 12-h photoperiod, for 5 d. The seeds were placed in water (control), ABA (10−4 m) (concentration established as optimal to obtain inhibitory effect according to Potomati and Buckeridge, 2002), glucose (0·05 m) and sucrose (0·025 m) (concentration established as optimal to obtain satisfactory effects according to M. A. S. Tiné and M. S. Buckeridge, unpubl. res.). Treatment with exogenous ethylene was performed by injecting different concentrations of this hormone through the septum of the rubber caps (1 µL L−1, 10 µL L−1 or 100 µL L−1). Imbibition of the seeds was complete in all solutions after 24 h. The percentage of germination and measurements of fresh weight of seeds, testa and endosperm in each experiment were recorded daily to evaluate seedling growth.
Quantification of proteins and activity of α-galactosidase
Seeds which had imbibed water and ABA and had been exposed to ethylene were harvested from the second to the fifth day after imbibition (ten seeds per plate) and dissected to separate the testa and endosperm. These tissues were crushed in 20 mm Tris–HCl, pH 7·8 and centrifuged (13 000 g for 5 min). Aliquots of the supernatants were assayed for proteins (Bradford, 1976) and α-galactosidase activity was measured using 50 mm ρ-nitrophenyl-α-d-galactopyranoside as substrate according to Tonini et al. (2007).
Measurements of endogenous ABA
Seeds incubated in water and glucose and sucrose solutions were harvested from the first to the fifth day after imbibition began (20 seeds per plate) and dissected to separate the testa and endosperm. These tissues (minimum quantity 500 mg) were individually powdered using liquid nitrogen and submitted to alcoholic extraction, by adding 80 % methanol (with 40 mg L−1 butylated hydroxytoluene) to each sample and stirring for 20 h in the dark at 4 °C. During this extraction step, ABA (1 µg) was added to each sample as an internal standard. The extracts were filtered through a Sep-Pak C-18 column, previously activated with 80 % methanol. The filtered samples were dried, methylated with ethereal diazomethane, nitrogen-dried, resuspended in methanol and analysed using a gas–liquid chromatograph (GC-6890; Agilent Technologies, Santa Clara, CA, USA), equipped with a mass spectrometer (MS-5973; Agilent Technologies) and a medium polarity column packed with HP1701 (30 m, 0·25 mm ID), in the scan ion modulation mode. Helium was used as the carrier gas at a flow rate of 1 mL min−1. The peak area detected of endogenous ABA and internal standard ions and the initial concentration of the internal standard were used to calculate the ABA concentrations in the samples, as described by Chen et al. (1988) and Santos et al. (2004).
Measurements of endogenous ethylene
Seeds which imbibed water, glucose and sucrose in sealed flasks were used from the first to the fifth day after the beginning of imbibition (ten seeds per plate) to measure endogenous ethylene. Every day, 1- or 10-mL samples of air were collected with a gas-tight syringe from the flasks and injected into a gas chromatograph (GC-6890; Hewlett-Packard Company, Palo Alto, CA, USA), equipped with a flame ionization detector and a column packed with HP-Plot Q (30 m, 0·53 mm ID). Helium was used as the carrier gas at a flow rate of 1 mL min−1 at 30 °C. Ethylene identification and quantification were based on the peak area of a C2H4 standard.
Measurements of endogenous glucose and sucrose
Seeds incubated in water, glucose and sucrose were harvested from the first to the fifth day after imbibition began (ten seeds per plate) and dissected to separate testa and endosperm. These tissues were individually powdered using liquid nitrogen, lyophilized and submitted to four extractions with 80 % ethanol for 20 min at 80 °C. After each extraction, the samples were centrifuged (13 000 g for 10 min ) and the aliquots of the supernatants were mixed, lyophilized, resuspended in water and analysed using a high performance anion-exchange liquid chromatography – HPAEC/PAD (DX500; Dionex Corporation, Sunnyvale, CA, USA), equipped with a pulsed amperometric detector. A column Carbo-Pac PA1 was used for carbohydrate separation and these were eluted isocratically with 150 mm NaOH. Glucose and sucrose identification and quantification were based on the peak area of these sugars standards.
Statistical analysis
The statistics were performed using analyses of variance (ANOVA) with the software WinSTAT for Excel, version 2001·1 (Robert Fitch, Bad Krozingen, Germany).
RESULTS
Germination and seedling development: biomass and sugars
When seeds of S. virgata were treated with ABA, ethylene at different concentrations, glucose and sucrose, little, if any, effect was observed on germination (data not shown). All seeds germinated after 2 d in all treatments, when 100 % of the seeds showed radicle protrusion. There were small differences in germination speed, but this was not investigated further in this work since the focus was on seedling development. Thus, these experiments were performed to assure that no strong effect on germination would have influenced germination speed to the point that storage mobilization would be influenced by the germination process.
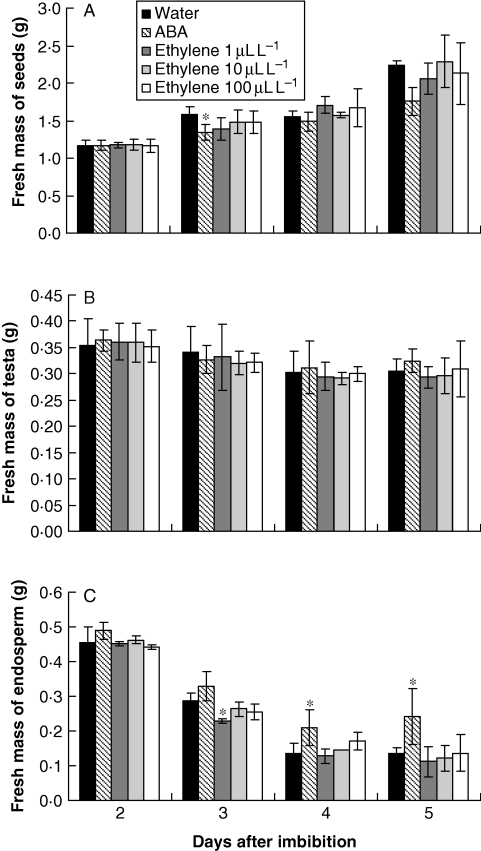
During germination and seedling establishment, an increase in the seed fresh mass was observed from the second to the fifth day, i.e after imbibition was complete, when a large increase in the fresh mass was observed (Fig. 2A). The seeds which imbibed ABA presented a proportionally lower increase in fresh mass on the second day after imbibition when compared with the seeds which imbibed water. Furthermore, the marked increase observed at the fifth day after imbibition did not occur in ABA (Fig. 2A).
Fig. 2.
Fresh weight (g) of seeds (A), testa (B) and endosperm (C) of S. virgata seeds, from the second to the fifth day after imbibing water and ABA (10−4 m) and exposure to ethylene (1 µL L−1, 10 µL L−1 or 100 µL L−1). Data are means of three biological replicates (ten seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
Changes in the fresh mass of the testa were neither influenced by ABA nor by ethylene (Fig. 2B). However, ABA clearly retarded storage mobilization in the endosperm, whereas little effect was observed after application of ethylene, which promoted storage mobilization only on the third day after imbibition at 1 µL L−1 of the hormone (Fig. 2C).
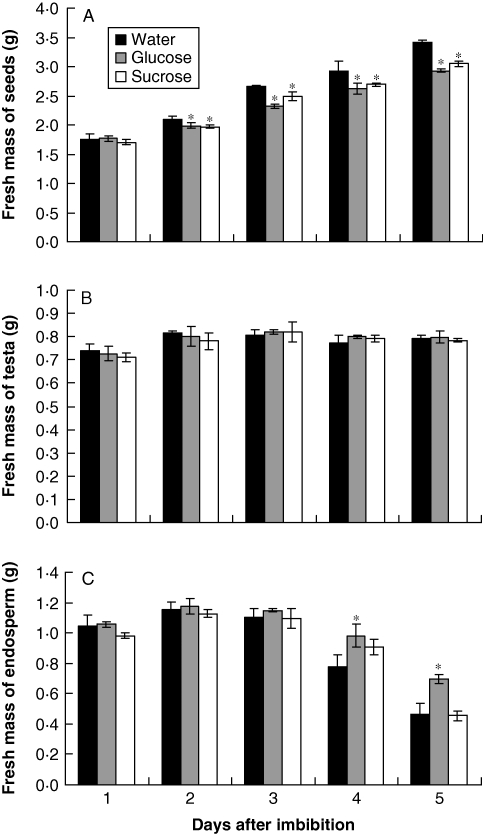
Seeds which imbibed sugars had a lower fresh mass from the second to the fifth day after imbibition compared with seeds which imbibed water (Fig. 3A). The effects of glucose or sucrose were not distinguishable. The fresh mass of the testa was not influenced by any of the sugars (Fig. 3B). In the presence of sucrose, the pace of storage mobilization, indirectly observed by changes in the fresh mass of endosperm, was similar to the seeds which imbibed water, whereas glucose significantly inhibited the reduction in fresh mass of the endosperm (Fig. 3C).
Fig. 3.
Fresh weight (g) of seeds (A), testa (B) and endosperm (C) of S. virgata seeds, from the second to the fifth day after imbibing water, glucose (0·05 m) and sucrose (0·025 m). Data are means of three biological replicates (20 seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
Storage protein mobilization
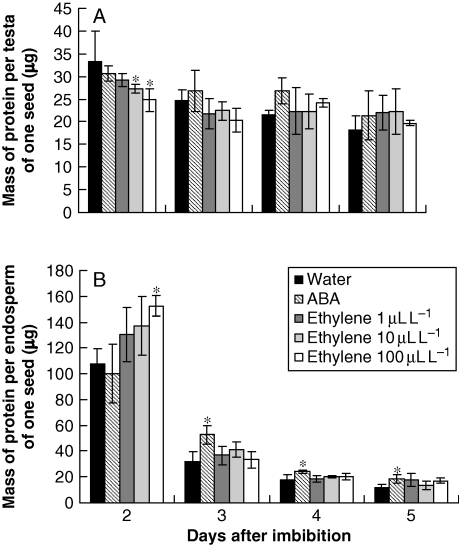
To determine the influence of ABA and ethylene on storage protein degradation and to measure indirectly the influence of these hormones in the production and action of proteases, the contents of soluble proteins were followed during and after germination in seeds which had imbibed and were exposed to ethylene.
The level of buffer-extractable proteins in both the testa and endosperm of seeds which had imbibed water decreased from the second to the fifth day after imbibition (Fig. 4). Similar changes were seen for the testa in response to ABA and ethylene (Fig. 4A). However, the patterns of changes in soluble proteins in the endosperm showed that ABA slowed down protein degradation (Fig. 4B). In the treatments with ethylene, the hormone kept the levels of soluble proteins relatively high in the endosperm, principally on the first day after imbibition, when compared with seeds which had imbibed water (Fig. 4B).
Fig. 4.
Concentrations of proteins (μg of protein specific tissue−1 of one seed) in the testa (A) and endosperm (B) of S. virgata seeds, from the second to the fifth day after imbibing water and ABA (10−4 m) and exposure to ethylene (1 µL L−1, 10 µL L−1 or 100 µL L−1). Data are means of three biological replicates (ten seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
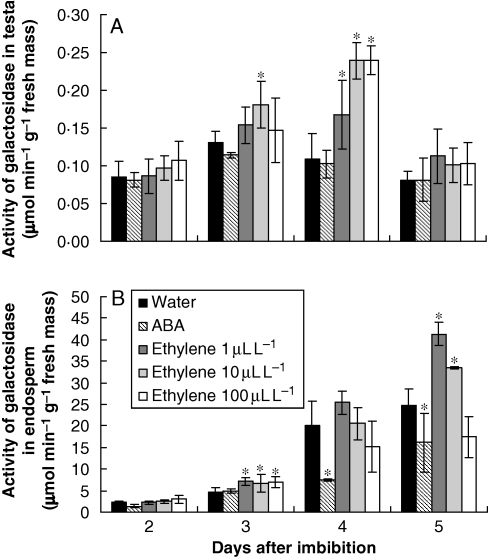
Activity of α-galactosidase (a marker of galactomannan mobilization)
To test the effects of ethylene and ABA on galactomannan mobilization, the activity of α-galactosidase, which is well characterized and a rate-limiting enzyme that is present during the degradation process, was used.
In the testa, a strong inductive effect of ethylene was observed at the third (10 µL L−1 only) and fourth day after imbibition (1, 10 and 100 µL L−1). ABA slightly inhibited the production of the enzyme (Fig. 5A). Approximately the same pattern was observed for α-galactosidase activity in the endosperm (Fig. 5B). In this instance, the effect of ABA was significantly inhibitory, whereas the lower concentration of ethylene (1 µL L−1) had a proportionally higher stimulating effect on the third and fifth day after imbibition, inducing a peak of activity at the fifth day that was almost twice the one observed for the seeds which had imbibed water (Fig. 5B).
Fig. 5.
Activity of α-galactosidase (μmol of galactose min−1 g−1 of fresh mass) in the testa (A) and endosperm (B) of S. virgata seeds, from the second to the fifth day after imbibing water and ABA (10−4 m) and exposure to ethylene (1 µL L−1, 10 µL L−1 or 100 µL L−1). Data are means of three biological replicates (ten seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
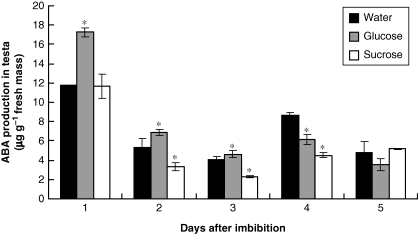
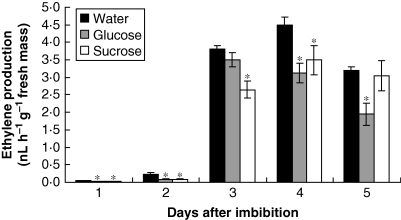
Endogenous ABA and ethylene
To understand the influence of sugars on the levels of endogenous ABA and ethylene and, indirectly, the influence of these sugars on storage mobilization, the effects of exogenous applications of sugars on the levels of endogenous hormones (ABA and ethylene) were followed during germination and seedling establishment in seeds which had imbibed glucose and sucrose.
ABA concentration decreased from the first to the third day after imbibition began, increased again on the fourth day and then decreased (Fig. 6). ABA was measured only in the testa, as it had been established that the highest concentration of this hormone occurs in this tissue (Tonini et al., 2006). Exogenous glucose slowed down the reduction in ABA and led to a lower peak of ABA on the fourth day after imbibition, whereas sucrose kept ABA concentration lower than in seeds which had imbibed water (Fig. 6).
Fig. 6.
Production of endogenous ABA in the testa of S. virgata seeds from the first to the fifth day after starting to imbibe water, glucose (0·05 m) and sucrose (0·025 m). Imbibition was complete after day 1. Data are means of three biological replicates (20 seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
In the case of ethylene, a strong increase in concentration was observed on the third day, reaching a maximum on the fourth day after imbibition and then decreased. Both sugars interfered in the production of ethylene, being inhibitory to ethylene synthesis (Fig. 7).
Fig. 7.
Production of endogenous ethylene in S. virgata seeds, from the first to the fifth day after starting to imbibe water, glucose (0·05 m) and sucrose (0·025 m). Imbibition was complete after day 1. Data are means of three biological replicates (ten seeds per replicate). *The treatments are significantly different from the control according to LSD test (0·5 %, P = 0·05).
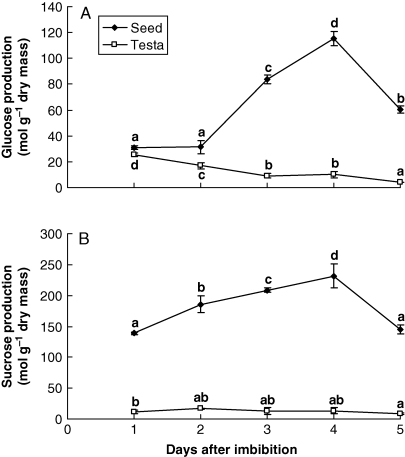
Endogenous sugars
To verify the presence of endogenous sugars during the period of ABA and ethylene increase and to corroborate the hypothesis that sugars have an influence on the production of these hormones, the measurements of glucose and sucrose were followed during germination and seedling development.
Glucose increased linearly after germination, on the second day, peaking on the fourth day after imbibition and then decreasing. On the other hand, sucrose started to increase linearly from the first up to the fourth day after imbibition. In the testa, glucose decreased up to the third day, stabilized up to the fourth day and then decreased. Sucrose increased slightly on the second day and decreased from the fourth to the fifth day after imbibition (Fig. 8).
Fig. 8.
Production of endogenous glucose (A) and sucrose (B) in S. virgata seeds, from the first to the fifth day after starting to imbibe water. Imbibition was complete after day 1. Data are means of three biological replicates (ten seeds per replicate). Means with the same letter are not significantly different according to LSD test (0·5 %, P = 0·05).
DISCUSSION
When an endospermic legume seedling establishes in the rain forest, different systems have to interact in a concerted fashion so that seed germination leads to a robust seedling that can photosynthesize and defend itself against pathogens. The developmental process that takes place from seed germination to seedling establishment requires intercommunication among organs, so that the storage compounds containing carbon and nitrogen will be mobilized and delivered to the developing organs. In the present work, events related to storage metabolism and hormones are reported that can be used as evidence that a systems network is present in seedlings of the galactomannan-containing species S. virgata. In order to develop efficiently and reach the highest fitness possible, this network system involves hormones, sugars and other compounds.
ABA may connect protein and carbohydrate metabolisms
When seeds of S. virgata germinate, the first carbohydrate reserves mobilized are the raffinose family oligosaccharides (Buckeridge and Dietrich, 1996). As a consequence, sucrose is formed and this is thought to supply the seed with carbon and energy for germination. At the same time, the ABA, present mainly in the seed coat, displays an inhibitory effect on galactomannan degradation. Its disappearance after germination, by degradation or leakage, is compatible with the increase in α-galactosidase and other galactomannanases in the endosperm. As seemingly the effect of ABA is primarily on protein body disassembly and not directly on α-galactosidase biosynthesis, it can be hypothesized that the effect of ABA on galactomannan mobilization would be indirect, through the inhibition of the unpacking process of the protein bodies.
Although the beginning of protein mobilization in the testa and endosperm occurred around the third and fourth day after imbibition began in all treatments, the presence of relatively high concentrations of exogenous ethylene seemingly stimulated the mobilization of proteins in the testa during germination. This can be deduced from the lower content of proteins in this tissue treated with ethylene on the second day after imbibition (Fig. 4A). On the other hand, in the endosperm, exogenous ABA seemed to modulate protein degradation after germination, because in the presence of this hormone the protein level was higher than in seeds which had imbibed water (Fig. 4B). According to Tonini et al. (2006) and Garciarrubio et al. (1997), the presence of ABA could prevent protein mobilization in seeds of S. virgata and A. thaliana, respectively.
The inhibitory effect of ABA during the degradation of proteins, mainly at the beginning of storage mobilization, can be related to the de novo synthesis of proteases and/or activation of the pre-formed proteases, as observed in seeds of barley (Hordeum vulgare) (Cercos et al., 1999), apple (Malus domestica) (Ranjan and Lewak, 1995), rice (Shintani et al., 1997) and bean (Phaseolus vulgaris) (Domash et al., 2006). Thus, it is likely that protein and carbohydrate metabolism might be linked to each other through their own catalytic processes so that the production and delivery of compounds related to carbon and nitrogen metabolism would be balanced during seedling development (Tonini et al., 2010).
Carbohydrate metabolism and the strategy of seedling establishment
After the radicle starts growing, galactomannan degradation is released due to degradation of ABA, whereas sucrose and glucose increase. According to McCleary (1983), in guar seeds, mannose and galactose are quickly epimerized to glucose in the developing embryo (in the cotyledons). This pathway has been proposed for guar, but never observed for S. virgata. However, as observed in the present work (Fig. 8), and previously observed by Buckeridge and Dietrich (1996), sucrose and glucose are formed in relatively high amounts in the seed during seedling development.
The changes in glucose concentration in seeds observed during seedling development but not during germination are consistent with the hypothesis that its increase is somehow related to galactomannan mobilization. This suggests that in S. virgata, mannose and galactose are epimerized and it is likely that glucose will be used for the production of sucrose in the endosperm and embryo tissues. Furthermore, the fact that both, glucose and sucrose, decreased after the fourth day suggests that the seedling starts consumption of sugars for growth at the same time as galactomannan products had finished. It is likely that at this point photosynthesis will be established, but this remains to be investigated.
In the testa, the changes in the levels of these sugars seemed to follow the same pattern observed for the rest of the seed. This may be explained by the observation of Tonini et al. (2007) that the hydrolases and ABA may be transiently stored in the testa. It is possible that in the case of glucose and sucrose, the gradual disintegration of the endosperm (Tonini et al., 2006) releases some of these sugars to the testa, a situation that possibly avoids the leakage of sugars to the environment. Thus, when enzymes and sugars are produced near the endosperm–testa contact, it would be less likely that sugars and enzymes would be lost to the environment. Indeed, it is important for seedling establishment that mobilization of the storage compounds occurs as quickly as possible and with the lowest possible level of leakage. This tight control has been shown to be present in other seeds containing cell-wall storage polysaccharides, such as Hymenaea courbaril (Santos et al., 2004; Brandão et al., 2009) which stores xyloglucan in the cotyledon cell walls. In this instance, wall degradation and free sugar metabolism are so tightly connected that no free sugars or oligosaccharides can be detected during all the period of degradation.
In galactomannan-containing seeds, the system to metabolize its products may not seem as efficient, as both monosaccharides and sucrose are detectable outside the seed during polysaccharide degradation. With endospermic seeds, the separation of reserves and sugar processing in different tissues might be a constraint.
Seedling development modulates storage mobilization
As the endosperm becomes locked into programmed cell death, the products of degradation of proteins and polysaccharides will be available to the developing seedling. Thus, the pace of growth and development, although depending on the availability of these compounds, can also be limiting if growth is impaired in any way. This raises the question whether the seedling might be prompted by mechanisms that could control the pace of storage degradation.
In the present work, it was hypothesized that ABA, ethylene and sugars (glucose and sucrose) might play such a role by being part of an intercommunication system between endosperm and embryo. In fact, exogenous application of sugars affected seedling growth by inhibiting the degradation of storage compounds in the endosperm. This was deduced from the reduction in endosperm fresh mass (Fig. 3C). In arabidopsis, glucose and sucrose can delay storage (in this case lipids) mobilization (Lu and Hills, 2002; To et al., 2002) and also inhibit the initial development after germination (Gibson et al., 2001; Price et al., 2003; Dekkers et al., 2004). In S. virgata, besides sugars, exogenous ABA and ethylene also influenced the degradation of endosperm after germination. ABA inhibited the reduction in endosperm fresh mass, whereas ethylene stimulated the mobilization of reserves in this tissue (Fig. 3C). The direct effect of ethylene on mobilization, after germination, of compounds stored in the cell wall is reported here for the first time.
Effects of hormones and sugars on the activity of α-galactosidase (a marker of galactomannan mobilization)
The maximum activity of α-galactosidase occurred around the third and fourth day in the testa and around the fourth and fifth day after imbibition in the endosperm in all treatments (Fig. 5), confirming previous suggestions that the mobilization of the galactomannan is a post-germinative phenomenon that starts only after radicle protrusion (McCleary, 1983; Buckeridge and Dietrich, 1996; Buckeridge et al., 2000c; Tonini et al., 2006).
Testa and endosperm were influenced differently by ethylene regarding the appearance of α-galactosidase. The stronger effects on tissues of testa were observed at 10 and 100 µL L−1 of ethylene, with a peak on the fourth day after exposure, whereas in the endosperm the stronger effect was at 1 µL L−1and maximal activity was observed on the fifth day. Furthermore, the activity in the endosperm was 10-fold the ones observed in the testa.
In the endosperm, the higher concentration of soluble proteins in the ethylene treatment (Fig. 4B) was especially consistent with the detection of a relatively higher peak of activity of α-galactosidase from the third to the fifth day (Fig. 5B). This brings the specific activity (activity per milligram of protein) in the tissues to about the same level in all treatments (not shown). However, to evaluate the impact of enzyme activity on storage degradation, the total enzyme activity is more relevant than specific activity. Thus, as ethylene treatment maintains a higher concentration of proteins (Fig. 4B), this leads to a much higher level of α-galactosidase (Fig. 5B) and to the acceleration of fresh mass decay (Fig. 2C).
These results suggest that ethylene plays a role as a modulator of proteins and galactomannan degradation in the endosperm of S. virgata. Indeed, ethylene can regulate the production of enzymes related to cell-wall metabolism, such as α-galactosidase during germination of A. caudatus (Bialecka and Kepczynski, 2007) and endo-β-mananase in seeds of lettuce (Cardoso, 2004; Nascimento et al., 2004; Nascimento et al., 2005) and tomato during germination (Pirrello et al., 2006).
As α-galactosidase is synthesized during seed development and probably stored in the protein bodies (Tonini et al., 2007), the present results suggest that ethylene induces the activity of α-galactosidase in seeds of S. virgata, supposedly by the activation of pre-formed α-galactosidase present in the protein bodies, probably through a proteolytic process. Similar observations had already been made in seeds of guar and lupin (Lupinus angustifolius), where α-galactosidase and exo-β-galactanase activities, respectively, were proposed to be activated through the proteolytic enzymes (Overbeeke et al., 1989; Buckeridge and Reid, 1994).
On the other hand, exogenous ABA inhibited the activation of α-galactosidase (Fig. 6). This effect on galactomannan degradation has been previously observed in S. virgata, suggesting a cause–effect relationship between ABA and activities of hydrolases (Potomati and Buckeridge, 2002; Tonini et al., 2006).
From endogenous detection (Tonini et al., 2006; Fig. 7) ABA seems to play two roles. One is played during germination and early growth of seedlings of S. virgata, where it acts as an inhibitor, holding (or delaying, depending on its concentration) the disassembly of protein bodies. Later, during seedling development (at the fourth day after imbibition), a new peak of ABA is observed. This possibly characterizes a second role of ABA that would be related to the connection with later events, possibly associated with the crosstalk among ABA, ethylene and sugars.
In this context, an ideal balance between ethylene and ABA seems to be necessary to allow storage mobilization efficiently in seeds of S. virgata, the latter controlling protease activities that are responsible for α-galactosidase activation in the testa and endosperm at the beginning of seedling development and the former as a modulator of hydrolases in the testa and the endosperm.
At the same time, glucose and sucrose are produced in the seeds, peaking on the fourth day after imbibition began. Both the endogenous concentrations of ABA and ethylene were regulated in the presence of sucrose and glucose (probably stimulation of biosynthesis of ABA and inhibition of biosynthesis of ethylene) (Figs 6 and 7); therefore it is likely that the crosstalk among ABA, ethylene and sugars plays a role during seedling development in S. virgata. The existence of a crosstalk system, where the concentrations of hormones and sugars have equalized to produce a general effect, could possibly explain why concentrations of ethylene higher than 1 µL L−1 did not present great effects in the endosperm.
Such a signalling network has been proposed to exist in a few species. This network is thought to link ABA and ethylene responses in opposite ways. The species where this aspect has been studied are seeds of A. thaliana (Beaudoin et al., 2000; Ghassemian et al., 2000; León and Sheen, 2003; Cernac et al., 2006), peanut (Arachis hypogaea) (Kepczynski and Kepczynska, 1997), Cicer arietinum (Gallardo et al., 1992), barley (Chen and An, 2006), apple (Kepczynski and Kepczynska, 1997), tobacco (Leubner-Metzger et al., 1998), Lepidium sativum (Linkies et al., 2009) and tomato (Pirrello et al., 2006).
ABA, ethylene and sugars acting in a signalling network
The idea that ABA, ethylene and sugars are involved in a signalling network in developing seedlings of S. virgata will need more experimental evidence before it can be confirmed. However, evidence in the literature is increasing. In Arabidopsis, an antagonism between ethylene and sugars pathways has been demonstrated (Zhou et al., 1998; León and Sheen, 2003; Yanagisawa et al., 2003; Gagne et al., 2004; Rognoni et al., 2007). In this context, glucose antagonizes the ethylene pathway as a consequence of the activation of the biosynthesis and ABA signalling pathway (Cheng et al., 2002), modulating the ethylene responses in a manner dependent on ABA (León and Sheen, 2003). However, it has been pointed out that glucose may inhibit the ethylene-signalling pathway directly (Rolland et al., 2002).
In S. virgata, the effects of glucose and sucrose in the ABA- and ethylene-signalling pathways are likely to occur in vivo, since these endogenous sugars increased in all parts of the seeds, inclusively in the testa, during germination and seedling development, i.e. on the fourth day after imbibition (Fig. 8).
The biological role of the hormone–sugars signalling network
The evidence presented in this work is consistent with the idea that ABA and ethylene antagonistically control storage mobilization in seeds of S. virgata and that this is under the influence of sugars. Through hydrolytic enzyme activation, the signalling-network system seems to control the processes of storage protein and galactomannan mobilization.
Such a complex control system probably avoids the accumulation of excess reducing sugars in seed and seedling tissues, having the biological roles of (a) avoiding sugar leakage and predation and therefore decreasing mortality and (b) assuring an efficient flow of carbon and nitrogen to the developing seedling. Sesbania virgata is well characterized as an early succession rainforest species, which uses the strategy of fast growth and quick establishment in its environment; therefore both roles are likely to have highly adaptive value, being involved in a general strategy of adaptation to the highly competitive rainforest environmental conditions.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science and Technology of Brazil, Coordination of Improvement of Personnel of Superior Level (CAPES), National Council for Scientific and Technological Development (CNPq) and Foundation for Research Support of the State of São Paulo (FAPESP) (98/05124-8 and 07/59708-1). This work is part of a research programme dedicated to investigate native Brazilian species and understand their importance for maintenance of biodiversity and responses to global climatic changes.
LITERATURE CITED
- Alcântara PHN, Martin L, Silva CO, Dietrich SMC, Buckeridge MS. Purification of a beta-galactosidase from cotyledons of Hymenaea courbaril L. (Leguminosae): enzyme properties and biological function. Plant Physiology and Biochemistry. 2006;44:619–627. doi: 10.1016/j.plaphy.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Léon P. Analysis of arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes & Development. 2000;14:2085–2096. [PMC free article] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signalling cascades. The Plant Cell. 2000;12:1103–1116. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York, NY: Plenum Press; 1994. [Google Scholar]
- Bialecka EJ, Kepczynski J. Changes in concentrations of soluble carbohydrates during germination of Amaranthus caudatus L. seeds in relation to ethylene, gibberellin A3 and methyl jasmonate. Plant Growth Regulation. 2007;51:21–31. [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of proteins utilizing the principle of rotein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandão AD, Del Bem LEV, Vincentz M, Buckeridge MS. Expression pattern of four storage xyloglucan mobilisation-related genes during seedling development of the rain forest tree Hymenaea courbaril L. Journal of Experimental Botany. 2009;60:1191–1206. doi: 10.1093/jxb/erp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckeridge MS, Dietrich SMC. Mobilisation of the raffinose family oligosaccharides and galactomannan in germinating seeds of Sesbania marginata Benth. (Leguminosae-Faboideae) Plant Science. 1996;117:33–43. [Google Scholar]
- Buckeridge MS, Reid JSG. Purification and properties of a novel β-galactosidase or exo-β-(1,4)-galactanase from the cotyledons of germinated Lupinus angustifolius L. seeds. Planta. 1994;192:502–511. doi: 10.1007/BF00203588. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Dietrich SMC, Lima DU. Galactomannans as the reserve carbohydrate in legume seeds. In: Gupta AK, Kaur N, editors. Carbohydrate reserves in plants: synthesis and regulation. Amsterdam: Elsevier; 2000a. pp. 283–316. [Google Scholar]
- Buckeridge MS, Santos HP, Tiné MAS. Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiology and Biochemistry. 2000b;38:141–156. [Google Scholar]
- Buckeridge MS, Tiné MAS, Santos HP, Lima DU. Polissacarídeos de reserva de parede celular em sementes: estrutura, metabolismo, funções e aspectos ecológicos. Revista Brasileira de Fisiologia Vegetal. 2000c:137–162. Edition Especial. [Google Scholar]
- Buckeridge MS, Aidar MPM, Santos HP, Tiné MAS. Acúmulo de reservas. In: Ferreira AG, Borghetti F, editors. Germinação: do básico ao aplicado. Porto Alegre: Artmed; 2004. pp. 31–50. [Google Scholar]
- Cardoso VJM. Etileno. In: Kerbauy GB, editor. Fisiologia vegetal. Rio de Janeiro: Guanabara Koogan; 2004. pp. 386–408. [Google Scholar]
- Cercos M, Gomez-Cadenas A, Ho T-HD. Hormonal regulation of a cysteine proteinase gene, EPB-1, in barley aleurone layers: cis- and trans-acting elements involved in the co-ordinated gene expression regulated by gibberellins and abscisic acid. The Plant Journal. 1999;19:107–118. doi: 10.1046/j.1365-313x.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- Cernac A, Andre C, Hoffmann-Benning S, Benning C. WRI1 is required for seed germination and seedling establishment. Plant Physiology. 2006;141:745–757. doi: 10.1104/pp.106.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, An Y-QC. Transcriptional responses to gibberellin and abscisic acid in barley aleurone. Journal of Integrative Plant Biology. 2006;48:591–612. [Google Scholar]
- Chen K, Miller AN, Patterson GW, Cohen JD. A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiology. 1988;86:822–825. doi: 10.1104/pp.86.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ji F, Xie H, Liang J, Zhang J. The regulator of G-protein signalling proteins involved in sugar and abscisic acid signalling in Arabidopsis seed germination. Plant Physiology. 2006;140:302–310. doi: 10.1104/pp.105.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W-H, Endo A, Zhou L, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis abscisic acid biosynthesis and glucose signalling. The Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJW, Schuurmans JAMJ, Smeekens SCM. Glucose delays seed germination in Arabidopsis thaliana. Planta. 2004;218:579–588. doi: 10.1007/s00425-003-1154-9. [DOI] [PubMed] [Google Scholar]
- Domash VI, Protsko RF, Vasyuk VA, Shumikhin SV, Ermolitskaya LV, Sharpio TP. The content of abscisic acid and the activities of proteinases and trypsin inhibitory proteins, in the germinating seed of common beans under water stress conditions. Applied Biochemistry and Microbiology. 2006;42:97–100. [PubMed] [Google Scholar]
- Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta. 2004;218:958–964. doi: 10.1007/s00425-003-1180-7. [DOI] [PubMed] [Google Scholar]
- Gagne JM, Smalle J, Gingerich DJ, et al. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proceedings of the National Academy of Sciences of the USA. 2004;101:6803–6808. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo M, Matilla A, Sánchez-Calle IM. Effects of spermine, abscisic acid and temperature upon ethylene production in Cicer arietinum L. seeds. Plant Physiology and Biochemistry. 1992;30:19–27. [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA. Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta. 1997;203:182–187. doi: 10.1007/s004250050180. [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, Mccourt P. Regulation of abscisic acid signalling by the ethylene response pathway in Arabidopsis. The Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinetti A, Laarhoven LJJ, Persijn ST, Harren FJM, Petruzzelli L. Ethylene production is associated with germination but not seed dormancy in red rice. Annals of Botany. 2007;99:735–745. doi: 10.1093/aob/mcm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI, Laby RJ, Kim D. The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochemical and Biophysical Research Communications. 2001;280:196–203. doi: 10.1006/bbrc.2000.4062. [DOI] [PubMed] [Google Scholar]
- Giorgini JF, Comoli E. Effect of embryo and exogenous GA3 on endospermic endo-β-mannanase activity of Coffea arabica L. during germination and early seedling growth. Revista Brasileira de Fisiologia Vegetal. 1996;8:43–49. [Google Scholar]
- Kepczynski J, Karssen CM. Ethylene in seed dormancy and germination. Physiologia Plantarum. 1997;101:720–726. [Google Scholar]
- Khalil MK. Nature of growth regulators effects on Nicotiana tabacum seed germination. Angewandte Botanik. 1992;66:106–108. [Google Scholar]
- Kontos F, Spyropoulos CG. Production and secretion of α-galactosidase and endo-β-mannanase activity by carob (Ceratonia siliqua L.) in the endosperm protoplast. Journal of Experimental Botany. 1995;46:577–583. [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- León P, Sheen J. Sugar and hormone connections. Trends in Plant Science. 2003;8:110–116. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F., Jr Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I β-1,3-glucanase during tobacco seed germination. Plant Molecular Biology. 1998;38:785–795. doi: 10.1023/a:1006040425383. [DOI] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. The Plant Cell. 2009;21:3803–3822. doi: 10.1105/tpc.109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Hills MJ. Arabidopsis mutants deficient in diacylglycerol acyltransferase display increased sensitivity to abscisic acid, sugars, and osmotic stress during germination and seedling development. Plant Physiology. 2002;129:1352–1358. doi: 10.1104/pp.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary BV. Enzymic interaction in the hydrolysis of galactomannan in germinating guar: the role of exo-β-mannanase. Phytochemistry. 1983;22:649–658. [Google Scholar]
- McCleary BV, Matheson NK. Galactomannan utilization in germinating legume seeds. Phytochemistry. 1976;15:43–47. [Google Scholar]
- Nascimento WM, Cantliffe DJ, Huber DJ. Ethylene evolution and endo-β-mannanase activity during lettuce seed germination at high temperature. Scientia Agricola. 2004;61:156–163. [Google Scholar]
- Nascimento WM, Cantliffe DJ, Huber DJ. Seed aging affects ethylene production and endo-β-mannanase activity during lettuce seed germination at high temperature. Seed Science Technology. 2005;33:11–17. [Google Scholar]
- Nomaguchi M, Nonogaki H, Yukio M. Development of galactomannan-hydrolyzing in the micropilar endosperm tip of tomato seed prior germination. Physiologia Plantarum. 1995;94:105–109. [Google Scholar]
- Nonogaki H, Morohashi Y. An endo-β-mannanase develops exclusively in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiology. 1996;110:555–559. doi: 10.1104/pp.110.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N, Fellinger AJ, Toonen MY, Van Wassenaar D, Verrips CT. Cloning and nucleotide sequence of the α-galactosidase cDNA from Cyamopsis tetragonoloba (guar) Plant Molecular Biology. 1989;13:541–550. doi: 10.1007/BF00027314. [DOI] [PubMed] [Google Scholar]
- Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, et al. Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant Cell Physiology. 2006;47:1195–1205. doi: 10.1093/pcp/pcj084. [DOI] [PubMed] [Google Scholar]
- Potomati A, Buckeridge MS. Effect of abscisic acid on the mobilisation of galactomannan and embryo development of Sesbania virgata (Cav.) Pers. (Leguminosae-Faboideae) Revista Brasileira de Botânica. 2002;25:303–310. [Google Scholar]
- Price J, Li T-C, Kang SG, Na JK, Jang J-C. Mechanisms of glucose signalling during germination of Arabidopsis. Plant Physiology. 2003;132:1424–1438. doi: 10.1104/pp.103.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Lewak S. Interaction of jasmonic and abscisic acid in the control of lipases and proteases in germinating apple embryos. Physiologia Plantarum. 1995;93:421–426. [Google Scholar]
- Reid JSG. Structure and function in legume-seed polysaccharides. In: Brett C, Hilman JR, editors. Biochemistry of plant cell walls. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Reid JSG, Meier H. The function of the aleurone layer during galactomannan mobilisation in germination seeds of fenugreek (Trigonella foenum-graecum L.), crimson clover (Trifolium incarnatum L.) and lucerne (Medicago sativa L.): a correlative biochemical and ultrastructural study. Planta. 1972;106:44–60. doi: 10.1007/BF00385472. [DOI] [PubMed] [Google Scholar]
- Reid JSG, Meier H. Enzymic activities and galactomannan mobilisation in germination seeds of fenugreek (Trigonella foenum-graecum L. Leguminosae): secretion of α-galactosidases and β-mannosidase by aleurone layer. Planta. 1973;112:301–308. doi: 10.1007/BF00390303. [DOI] [PubMed] [Google Scholar]
- Rognoni S, Teng S, Arru L, Smeekens SCM, Perata P. Sugar effects on early seedling development in Arabidopsis. Plant Growth Regulation. 2007;52:217–228. [Google Scholar]
- Rolland F, Moore B, Sheen J. Sugar sensing and signalling in plants. The Plant Cell. 2002;14:S185–S205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos HP, Purgatto E, Mercier H, Buckeridge MS. The control of storage xyloglucan mobilisation in cotyledons of Hymenaea courbaril. Plant Physiology. 2004;135:287–299. doi: 10.1104/pp.104.040220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A. Glaktomannanabbau in keimenden Johanisbrotsamen (Ceratonia siliqua L.) Planta. 1977;134:209–221. doi: 10.1007/BF00384185. [DOI] [PubMed] [Google Scholar]
- Shintani A, Kato H, Minamikawa T. Hormonal regulation of expression of two cysteine endopeptidase genes in rice seedlings. Plant Cell Physiology. 1997;38:1242–1248. doi: 10.1093/oxfordjournals.pcp.a029111. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Seeds, seedlings and the origin of angiosperms. In: Beck CB, editor. Origin and early evolution of angiosperms. New York, NY: Columbia University Press; 1974. pp. 300–311. [Google Scholar]
- To JP, Reiter WD, Gibson SI. Mobilisation of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biology. 2002;2:4. doi: 10.1186/1471-2229-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini PP, Lisboa CGS, Freschi L, Mercier H, Mazzoni-Viveiros SC, Buckeridge MS. Effect of abscisic acid on galactomannan degradation and endo-β-mannanase activity in seeds of Sesbania virgata (Cav.) Pers. (Leguminosae) Trees – Structure and Function. 2006;20:669–678. [Google Scholar]
- Tonini PP, Lisboa CGS, Silva CO, Mazzoni-Viveiros SC, Buckeridge MS. Testa is involved in the control of storage mobilisation in seeds of Sesbania virgata (Cav.) Pers., a tropical legume tree from of the Atlantic Forest. Trees – Structure and Function. 2007;21:13–21. [Google Scholar]
- Tonini PP, Carrara TB, Buckeridge MS. Storage proteins and cell wall mobilisation in seeds of Sesbania virgata (Cav.) Pers. (Leguminosae) Trees – Structure and Function. 2010;24:675–684. [Google Scholar]
- Toorop PE, Bewley JD, Abrams SR, Hilhorst HWM. Structure-activity studies with ABA analogs on germination and endo-β-mannanase activity in tomato and lettuce seeds. Journal of Plant Physiology. 1999;154:679–685. [Google Scholar]
- Toorop PE, Van AAC, Hilhorst HWM. The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germinations is under control of ABA. Journal of Experimental Botany. 2000;51:1371–1379. [PubMed] [Google Scholar]
- Van Staden J, Manning JC, Dickens CWS. A phylogenetic analysis of the role of plant hormones in the development and germination of legume seeds. In: Stirton CH, editor. Advances in legume systematics. Kew: Royal Botanic Gardens; 1987. pp. 387–432. Part 3. [Google Scholar]
- Yanagisawa S, Yoo S-D, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–525. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proceedings of the National Academy of Sciences of the USA. 1998;95:10294–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]