Abstract
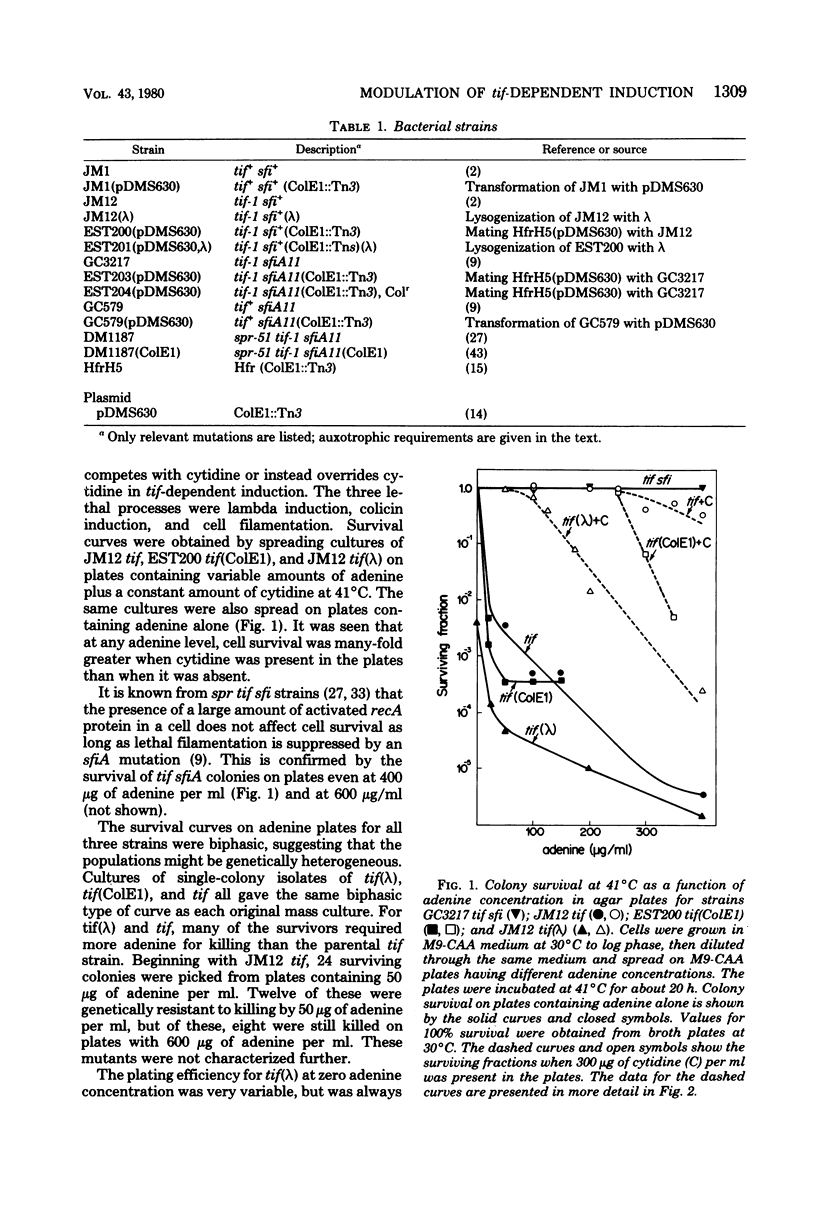
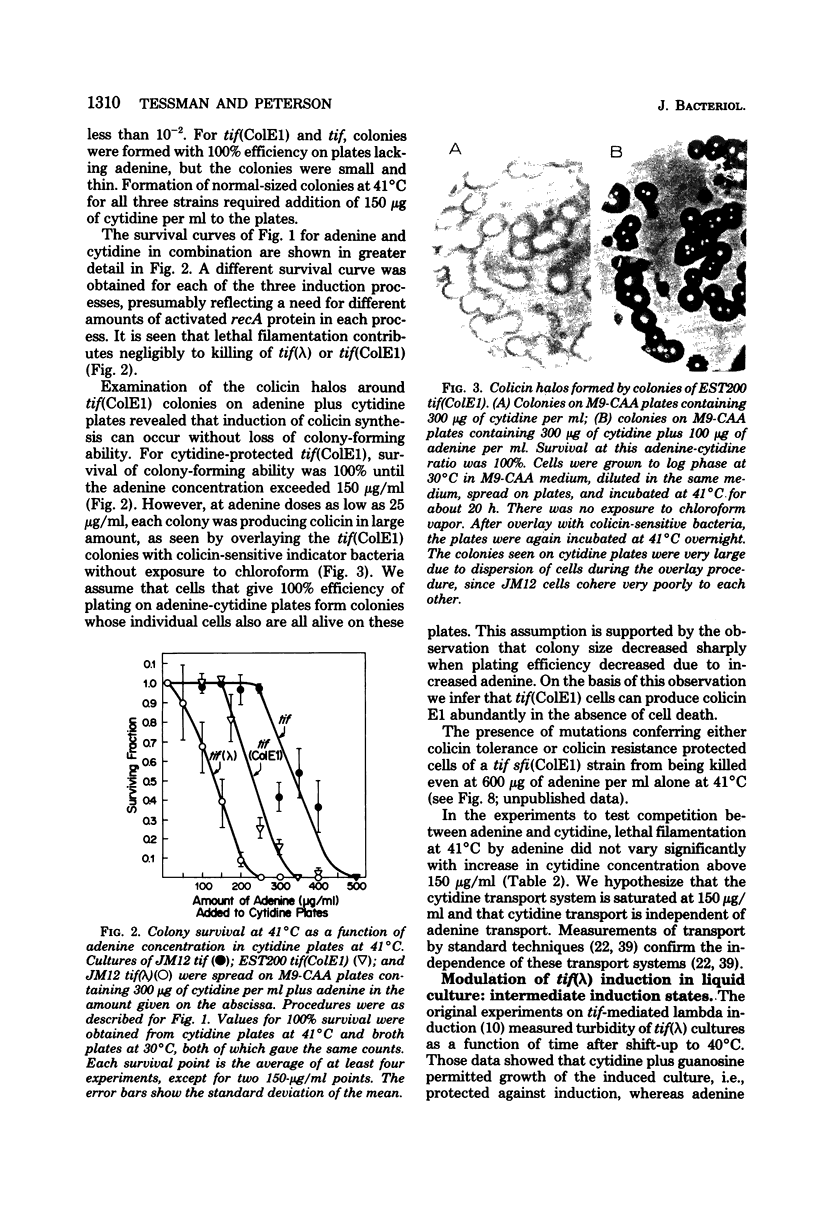
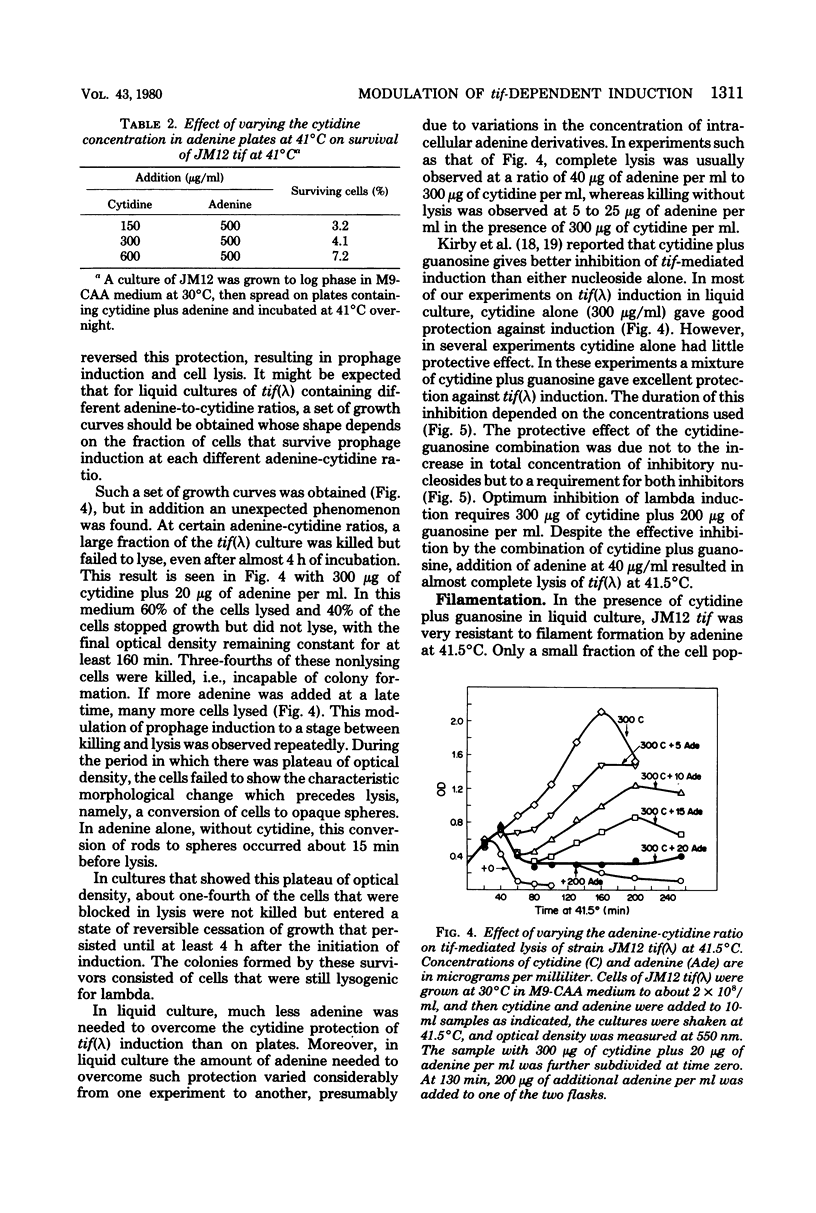
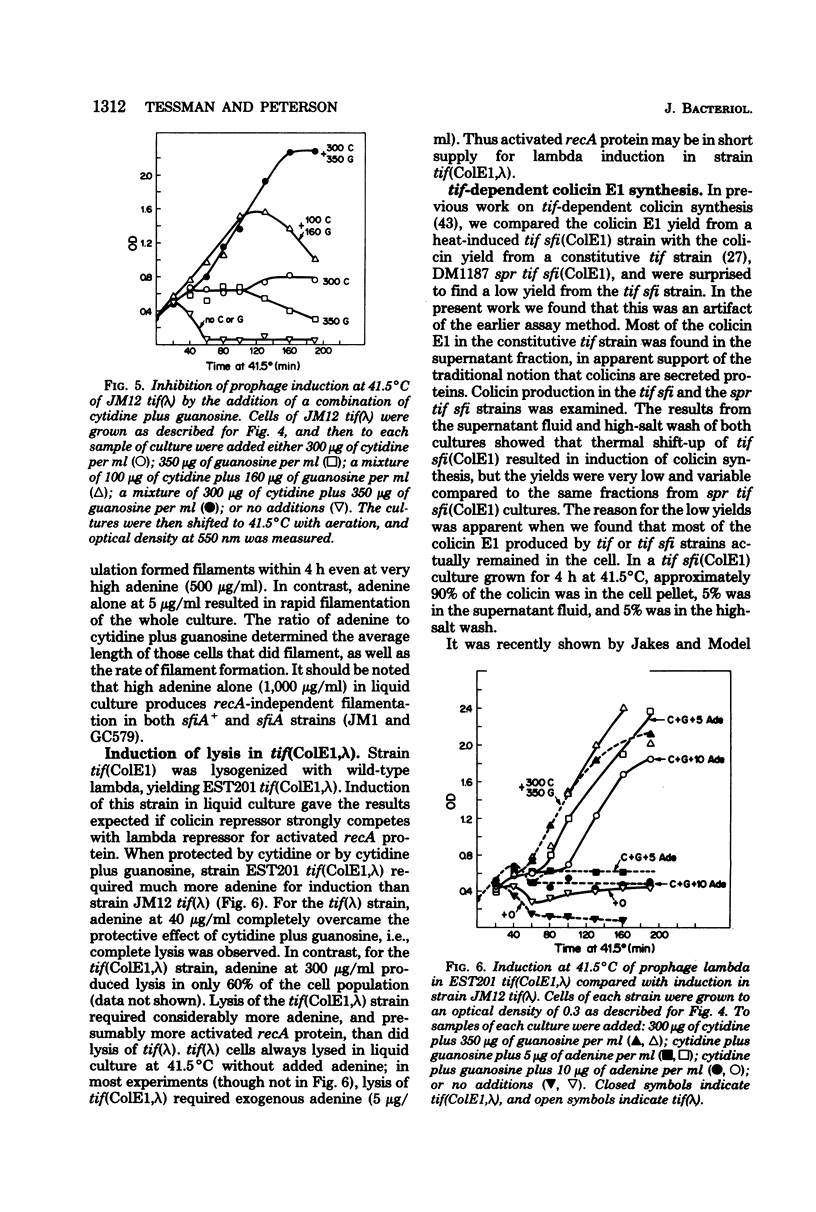
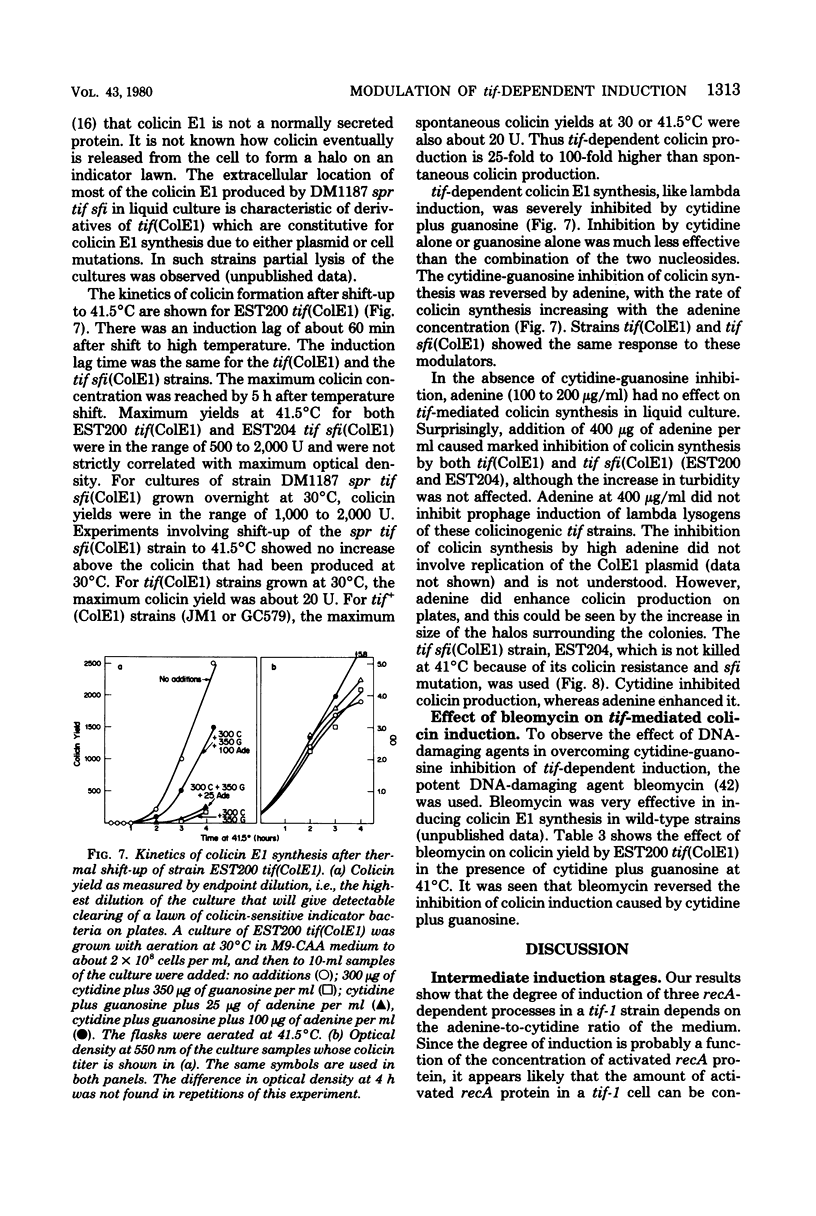
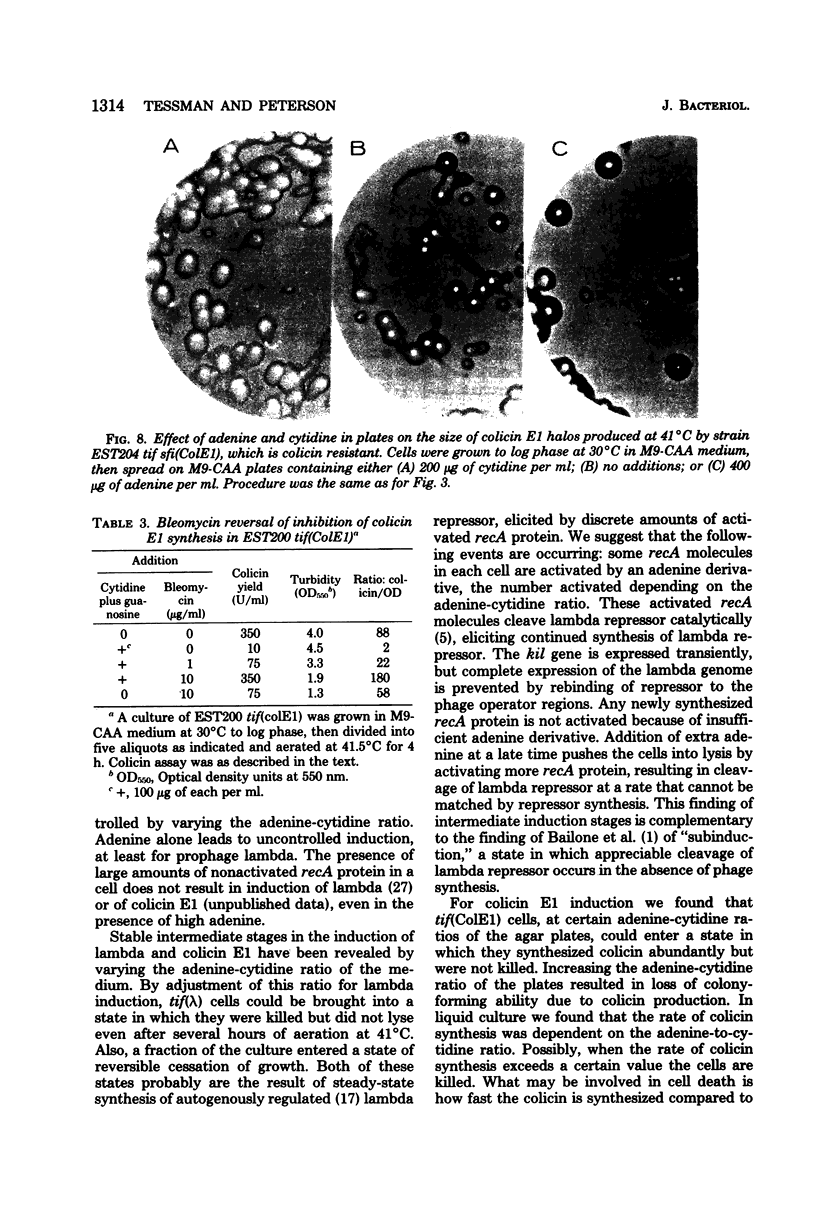
To help understand how the tif-1 mutation of the recA gene of Escherichia coli confers adenine activability on the recA protein, we used the fact that cytidine plus guanosine inhibits induction of prophage lambda and cell filamentation in a tif-1 mutant, and that adenine reverses this inhibition. We varied the amount of adenine in agar plates containing a fixed amount of cytidine and scored for survivors of three different tif-dependent lethal induction processes. Much more adenine was required for cell killing when cytidine was present than when it was absent. Therefore adenine does not override cytidine inhibition, but instead appears to compete with it for a site of action which may be on the recA protein. The competition is not at the cell transport level. Our results lead to a model in which the tif form of the recA protein is an allosteric enzyme that binds both negative and positive modulators. By varying the adenine-cytidine ratio of the medium it is possible to control the degree of induction in a tif-1 cell. For the three different tif-dependent inductions studied here, least adenine was required for lambda induction and most for lethal filamentation, presumably reflecting requirements for different amounts of activated recA protein in each process. Varying the adenine-cytidine ratio revealed two stable intermediate stages in lambda induction, as well as a stage of colicin E1 induction in which the cells produced colicin without cell death. The rate of filament formation could be similarly controlled. Experiments with tif (ColE1, lambda) gave evidence of a competition between colicin repressor and lambda repressor for activated recA protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- D'Ari R., George J., Huisman O. Suppression of tif-mediated induction of SOS functions in Escherichia coli by an altered dnaB protein. J Bacteriol. 1979 Nov;140(2):381–387. doi: 10.1128/jb.140.2.381-387.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M, Fano U. Bacteriophage-Resistant Mutants in Escherichia Coli. Genetics. 1945 Mar;30(2):119–136. doi: 10.1093/genetics/30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Isolation, mapping, and examination of effects of TnA insertions in ColE1 plasmids. J Bacteriol. 1977 Jan;129(1):482–491. doi: 10.1128/jb.129.1.482-491.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inselburg J. Studies of colicin induction with an imm- col+ mutant of the plasmid colicin E1. Mol Gen Genet. 1979 Jun 20;173(3):333–338. doi: 10.1007/BF00268644. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Model P. Mechanism of export of colicin E1 and colicin E3. J Bacteriol. 1979 Jun;138(3):770–778. doi: 10.1128/jb.138.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. P., Ruff W. L., Goldthwait D. A. Cell division and prophage induction in Escherichia coli: effects of pantoyl lactone and various furan derivatives. J Bacteriol. 1972 Aug;111(2):447–453. doi: 10.1128/jb.111.2.447-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of a copy mutant of the bacteriocinogenic plasmid Clo DF13. J Bacteriol. 1974 Nov;120(2):569–578. doi: 10.1128/jb.120.2.569-578.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. K., Visser D. W. Uridine and cytidine transport in Escherichia coli B and transport-deficient mutants. J Biol Chem. 1977 Apr 25;252(8):2492–2497. [PubMed] [Google Scholar]
- Little J. W., Harper J. E. Identification of the lexA gene product of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6147–6151. doi: 10.1073/pnas.76.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Kleid D. G. Escherichia coli protein X is the recA gene product. J Biol Chem. 1977 Sep 25;252(18):6251–6252. [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. Isolation and characterization of mutants of lambda recA which synthesize a hyperactive recA protein. Virology. 1979 Oct 30;98(2):484–488. doi: 10.1016/0042-6822(79)90574-9. [DOI] [PubMed] [Google Scholar]
- Mount D. W., Walker A. C., Kosel C. Effect of tsl mutations in decreasing radiation sensitivity of a recA- strain of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1203–1207. doi: 10.1128/jb.121.3.1203-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Walker A. C., Kosel C. Indirect suppression of radiation sensitivity of a recA- strain of Escherichia coli K12. Basic Life Sci. 1975;5A:383–388. doi: 10.1007/978-1-4684-2895-7_51. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Shimada K., Takagi Y. Isolation and characterization of a mutant ColE1 plasmid that allows constitutive colicin E1 synthesis. J Bacteriol. 1979 Jul;139(1):311–315. doi: 10.1128/jb.139.1.311-315.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacelli L. Z., Edmiston S. H., Mount D. W. Isolation and characterization of amber mutations in the lexA gene of Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):568–573. doi: 10.1128/jb.137.1.568-573.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Levine A., Bailone A. Induction of recA+-protein synthesis in Escherichia coli. Mol Gen Genet. 1978 Apr 17;160(3):267–276. doi: 10.1007/BF00332970. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Mizuuchi K., Emmerson P. T. Induction of prophage lambda by gamma-rays, mitomycin C and tif; repressor cleavage studied by immunoprecipitation. Mol Gen Genet. 1977 Sep 21;155(1):87–91. doi: 10.1007/BF00268564. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Grollman A. P., Ohtsubo E., Ohtsubo H. Interaction of bleomycin with DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5983–5987. doi: 10.1073/pnas.75.12.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Gritzmacher C. A., Peterson P. K. Derepression of colicin E1 synthesis in the constitutive tif mutant strain (spr tif sfi) and in a tif sfi mutant strain of Escherichia coli K-12. J Bacteriol. 1978 Jul;135(1):29–38. doi: 10.1128/jb.135.1.29-38.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. In vivo proteolytic cleavage of colicins requires specific receptor binding. Nature. 1979 Mar 22;278(5702):362–364. doi: 10.1038/278362a0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Persistence and decay of thermoinducible error-prone repair activity in nonfilamentous derivatives of tif-1, Escherichia coli B/r: the timing of some critical events in ultraviolet mutagenesis. Mol Gen Genet. 1975 Dec 29;142(2):87–103. doi: 10.1007/BF00266092. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The radiation sensitivity of Escherichia coli B: a hypothesis relating filament formation and prophage induction. Proc Natl Acad Sci U S A. 1967 May;57(5):1275–1279. doi: 10.1073/pnas.57.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]