Abstract
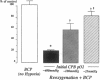
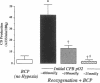
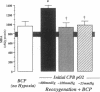
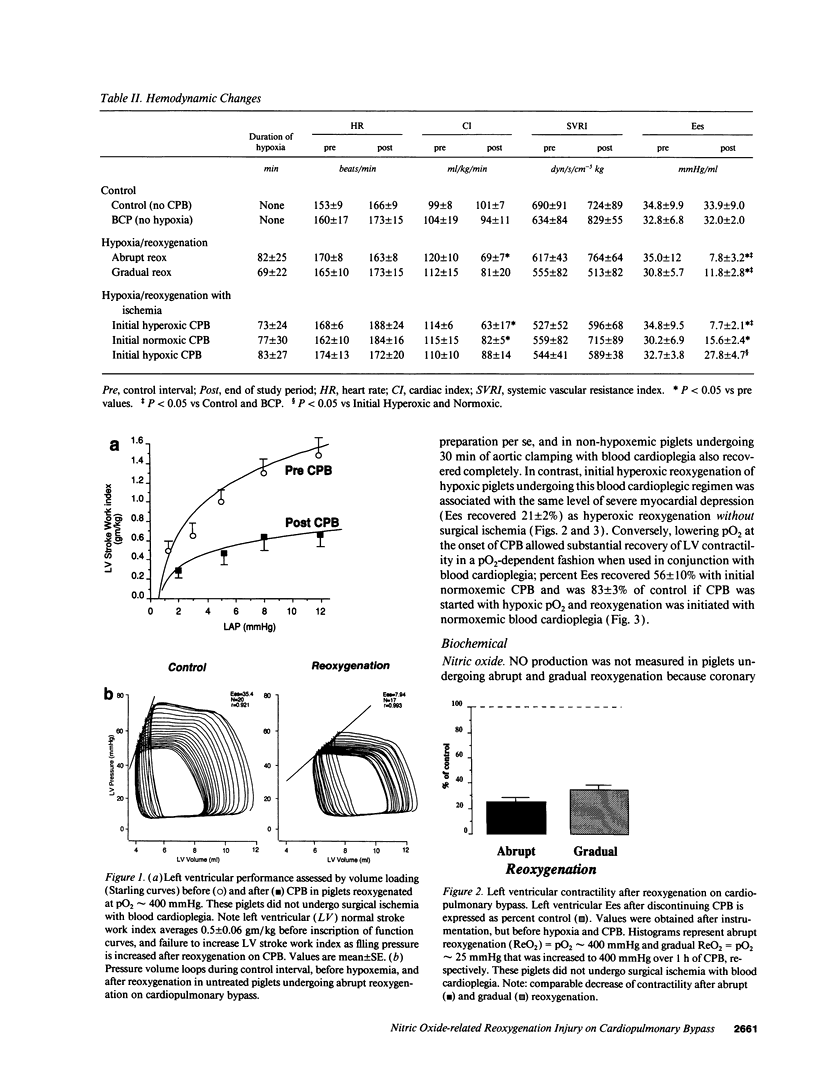
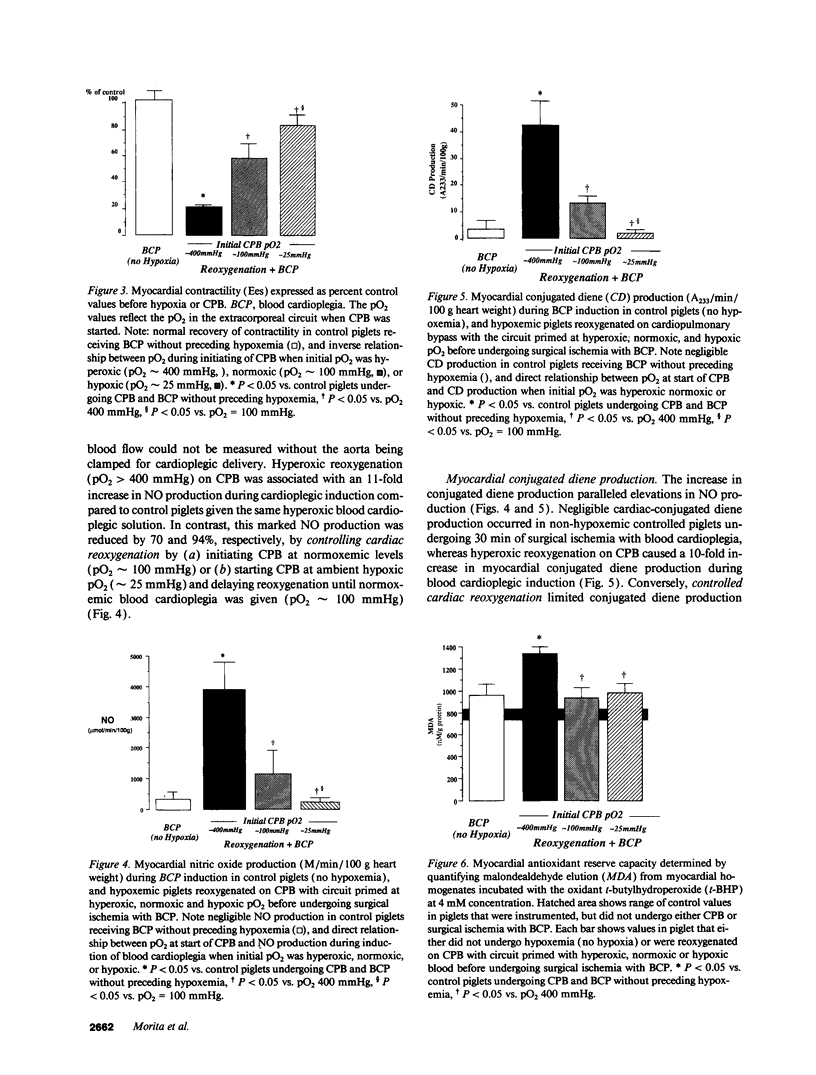
Cardiopulmonary bypass (CPB) is used increasingly to correct cyanotic heart defects during early infancy, but myocardial dysfunction is often seen after surgical repair. This study evaluates whether starting CPB at a conventional, hyperoxic pO2 causes an "unintentional" reoxygenation (ReO2) injury. We subjected 2-wk-old piglets to ventilator hypoxemia (FIO2 approximately 0.06, pO2 approximately 25 mmHg) followed by 5 min of ReO2 on CPB before instituting cardioplegia. CPB was begun in hypoxemic piglets by either abrupt ReO2 at a pO2 of 400 mmHg (standard clinical practice) or by maintaining pO2 approximately 25 mmHg on CPB until controlling ReO2 with blood cardioplegic arrest. The effects of abrupt vs. gradual ReO2 without surgical ischemia (blood cardioplegia) were also compared. Myocardial nitric oxide (NO) production (chemiluminescence measurements of NO2- + NO3-) and conjugated diene (CD) generation (spectrophotometric A233 measurements of lipid extracts) using aortic and coronary sinus blood samples were assessed during cardioplegic induction. 30 min after CPB, left ventricular end-systolic elastance (Ees, catheter conductance method) was used to determine cardiac function. CPB and blood cardioplegic arrest caused no functional or biochemical change in normoxic (control) hearts. Abrupt ReO2 caused a depression of myocardial function (Ees = 25 +/- 5% of control). Functional depression was relatively unaffected by gradual ReO2 without blood cardioplegia (34% recovery of Ees), and abrupt ReO2 immediately before blood cardioplegia caused a 10-fold rise in cardiac NO and CD production, with subsequent depression of myocardial function (Ees 21 +/- 2% of control). In contrast, controlled cardiac ReO2 reduced NO production 94%, CD did not rise, and Ees was 83 +/- 8% of normal. We conclude ReO2 injury is related to increased NO production during abrupt ReO2, nullifies the cardioprotective effects of blood cardioplegia, and that controlled cardiac ReO2 when starting CPB to correct cyanotic heart defects may reduce NO production and improve myocardial status postoperatively.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. S., Buckberg G. D., Schwaiger M., Yeatman L., Tillisch J., Kawata N., Messenger J., Lee C. Early recovery of regional wall motion in patients following surgical revascularization after eight hours of acute coronary occlusion. J Thorac Cardiovasc Surg. 1986 Sep;92(3 Pt 2):636–648. [PubMed] [Google Scholar]
- Allen B. S., Okamoto F., Buckberg G. D., Bugyi H., Young H., Leaf J., Beyersdorf F., Sjostrand F., Maloney J. V., Jr Immediate functional recovery after six hours of regional ischemia by careful control of conditions of reperfusion and composition of reperfusate. J Thorac Cardiovasc Surg. 1986 Sep;92(3 Pt 2):621–635. [PubMed] [Google Scholar]
- Baverel G., Martin G., Michoudet C. Glutamine synthesis from aspartate in guinea-pig renal cortex. Biochem J. 1990 Jun 1;268(2):437–442. doi: 10.1042/bj2680437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Coade S. B., Moncada S., Pearson J. D., Mann G. E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- Bolli R., Jeroudi M. O., Patel B. S., DuBose C. M., Lai E. K., Roberts R., McCay P. B. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Li X. Y., Triana J. F., Lai E. K., McCay P. B. Iron-mediated radical reactions upon reperfusion contribute to myocardial "stunning". Am J Physiol. 1990 Dec;259(6 Pt 2):H1901–H1911. doi: 10.1152/ajpheart.1990.259.6.H1901. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Poole-Wilson P. A., Harding S. E., Warren J. B. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol. 1992 Dec;263(6 Pt 2):H1963–H1966. doi: 10.1152/ajpheart.1992.263.6.H1963. [DOI] [PubMed] [Google Scholar]
- Buckberg G. D. Myocardial temperature management during aortic clamping for cardiac surgery. Protection, preoccupation, and perspective. J Thorac Cardiovasc Surg. 1991 Dec;102(6):895–903. [PubMed] [Google Scholar]
- Burton K. P., Morris A. C., Massey K. D., Buja L. M., Hagler H. K. Free radicals alter ionic calcium levels and membrane phospholipids in cultured rat ventricular myocytes. J Mol Cell Cardiol. 1990 Sep;22(9):1035–1047. doi: 10.1016/0022-2828(90)91043-7. [DOI] [PubMed] [Google Scholar]
- Bush P. A., Gonzalez N. E., Griscavage J. M., Ignarro L. J. Nitric oxide synthase from cerebellum catalyzes the formation of equimolar quantities of nitric oxide and citrulline from L-arginine. Biochem Biophys Res Commun. 1992 Jun 30;185(3):960–966. doi: 10.1016/0006-291x(92)91720-b. [DOI] [PubMed] [Google Scholar]
- Chevion M., Jiang Y., Har-El R., Berenshtein E., Uretzky G., Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1102–1106. doi: 10.1073/pnas.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J. Applications of electron spin resonance spectroscopy to the identification of radicals produced during lipid peroxidation. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):149–173. doi: 10.1016/0009-3084(87)90048-x. [DOI] [PubMed] [Google Scholar]
- Drinkwater D. C., Jr, Cushen C. K., Laks H., Buckberg G. D. The use of combined antegrade-retrograde infusion of blood cardioplegic solution in pediatric patients undergoing heart operations. J Thorac Cardiovasc Surg. 1992 Nov;104(5):1349–1355. [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Ferreira R., Burgos M., Milei J., Llesuy S., Molteni L., Hourquebie H., Boveris A. Effect of supplementing cardioplegic solution with deferoxamine on reperfused human myocardium. J Thorac Cardiovasc Surg. 1990 Nov;100(5):708–714. [PubMed] [Google Scholar]
- Gauduel Y., Menasche P., Duvelleroy M. Enzyme release and mitochondrial activity in reoxygenated cardiac muscle: relationship with oxygen-induced lipid peroxidation. Gen Physiol Biophys. 1989 Aug;8(4):327–340. [PubMed] [Google Scholar]
- Godin D. V., Garnett M. E. Altered antioxidant status in the ischemic/reperfused rabbit myocardium: effects of allopurinol. Can J Cardiol. 1989 Oct;5(7):365–371. [PubMed] [Google Scholar]
- Guarnieri C., Flamigni F., Caldarera C. M. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J Mol Cell Cardiol. 1980 Aug;12(8):797–808. doi: 10.1016/0022-2828(80)90081-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992 Nov;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hirschl R. B., Heiss K. F., Bartlett R. H. Severe myocardial dysfunction during extracorporeal membrane oxygenation. J Pediatr Surg. 1992 Jan;27(1):48–53. doi: 10.1016/0022-3468(92)90103-e. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Jacobson R. M., Feinstein A. R. Oxygen as a cause of blindness in premature infants: "autopsy" of a decade of errors in clinical epidemiologic research. J Clin Epidemiol. 1992 Nov;45(11):1265–1287. doi: 10.1016/0895-4356(92)90168-m. [DOI] [PubMed] [Google Scholar]
- Kirklin J. K., Blackstone E. H., Kirklin J. W., McKay R., Pacifico A. D., Bargeron L. M., Jr Intracardiac surgery in infants under age 3 months: incremental risk factors for hospital mortality. Am J Cardiol. 1981 Sep;48(3):500–506. doi: 10.1016/0002-9149(81)90079-5. [DOI] [PubMed] [Google Scholar]
- Kowalski D. P., Aw T. Y., Park Y., Jones D. P. Postanoxic oxidative injury in rat hepatocytes: lactate-dependent protection against tert-butylhydroperoxide. Free Radic Biol Med. 1992;12(3):205–212. doi: 10.1016/0891-5849(92)90028-f. [DOI] [PubMed] [Google Scholar]
- Krivokapich J., Barrio J. R., Phelps M. E., Watanabe C. R., Keen R. E., Padgett H. C., Douglas A., Shine K. I. Kinetic characterization of 13NH3 and [13N]glutamine metabolism in rabbit heart. Am J Physiol. 1984 Feb;246(2 Pt 2):H267–H273. doi: 10.1152/ajpheart.1984.246.2.H267. [DOI] [PubMed] [Google Scholar]
- Kukreja R. C., Kontos H. A., Hess M. L., Ellis E. F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986 Dec;59(6):612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazar H. L., Buckberg G. D., Manganaro A. M., Becker H. Myocardial energy replenishment and reversal of ischemic damage by substrate enhancement of secondary blood cardioplegia with amino acids during reperfusion. J Thorac Cardiovasc Surg. 1980 Sep;80(3):350–359. [PubMed] [Google Scholar]
- Li R. K., Mickle D. A., Weisel R. D., Tumiati L. C., Jackowski G., Wu T. W., Williams W. G. Effect of oxygen tension on the anti-oxidant enzyme activities of tetralogy of Fallot ventricular myocytes. J Mol Cell Cardiol. 1989 Jun;21(6):567–575. doi: 10.1016/0022-2828(89)90822-5. [DOI] [PubMed] [Google Scholar]
- Littauer A., de Groot H. Release of reactive oxygen by hepatocytes on reoxygenation: three phases and role of mitochondria. Am J Physiol. 1992 Jun;262(6 Pt 1):G1015–G1020. doi: 10.1152/ajpgi.1992.262.6.G1015. [DOI] [PubMed] [Google Scholar]
- Loop F. D., Higgins T. L., Panda R., Pearce G., Estafanous F. G. Myocardial protection during cardiac operations. Decreased morbidity and lower cost with blood cardioplegia and coronary sinus perfusion. J Thorac Cardiovasc Surg. 1992 Sep;104(3):608–618. [PubMed] [Google Scholar]
- Martin G. R., Short B. L., Abbott C., O'Brien A. M. Cardiac stun in infants undergoing extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 1991 Apr;101(4):607–611. [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Ohno M., Gibbons G. H., Dzau V. J., Cooke J. P. Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation. 1993 Jul;88(1):193–197. doi: 10.1161/01.cir.88.1.193. [DOI] [PubMed] [Google Scholar]
- Reif D. W., Simmons R. D. Nitric oxide mediates iron release from ferritin. Arch Biochem Biophys. 1990 Dec;283(2):537–541. doi: 10.1016/0003-9861(90)90680-w. [DOI] [PubMed] [Google Scholar]
- Rengasamy A., Johns R. A. Characterization of endothelium-derived relaxing factor/nitric oxide synthase from bovine cerebellum and mechanism of modulation by high and low oxygen tensions. J Pharmacol Exp Ther. 1991 Oct;259(1):310–316. [PubMed] [Google Scholar]
- Rosenkranz E. R., Okamoto F., Buckberg G. D., Robertson J. M., Vinten-Johansen J., Bugyi H. I. Safety of prolonged aortic clamping with blood cardioplegia. III. Aspartate enrichment of glutamate-blood cardioplegia in energy-depleted hearts after ischemic and reperfusion injury. J Thorac Cardiovasc Surg. 1986 Mar;91(3):428–435. [PubMed] [Google Scholar]
- Rudolph W. Myocardial metabolism in cyanotic congenital heart disease. Cardiology. 1971;56(1):209–215. doi: 10.1159/000169363. [DOI] [PubMed] [Google Scholar]
- Sagawa K. The ventricular pressure-volume diagram revisited. Circ Res. 1978 Nov;43(5):677–687. doi: 10.1161/01.res.43.5.677. [DOI] [PubMed] [Google Scholar]
- Sawatari K., Kadoba K., Bergner K. A., Daitch J. A., Mayer J. E., Jr Influence of initial reperfusion pressure after hypothermic cardioplegic ischemia on endothelial modulation of coronary tone in neonatal lambs. Impaired coronary vasodilator response to acetylcholine. J Thorac Cardiovasc Surg. 1991 May;101(5):777–782. [PubMed] [Google Scholar]
- Schlüter K. D., Schwartz P., Siegmund B., Piper H. M. Prevention of the oxygen paradox in hypoxic-reoxygenated hearts. Am J Physiol. 1991 Aug;261(2 Pt 2):H416–H423. doi: 10.1152/ajpheart.1991.261.2.H416. [DOI] [PubMed] [Google Scholar]
- Tani M., Neely J. R. Mechanisms of reduced reperfusion injury by low Ca2+ and/or high K+. Am J Physiol. 1990 Apr;258(4 Pt 2):H1025–H1031. doi: 10.1152/ajpheart.1990.258.4.H1025. [DOI] [PubMed] [Google Scholar]
- Teitel D. F., Klautz R., Steendijk P., van der Velde E. T., van Bel F., Baan J. The end-systolic pressure-volume relationship in the newborn lamb: effects of loading and inotropic interventions. Pediatr Res. 1991 May;29(5):473–482. doi: 10.1203/00006450-199105010-00012. [DOI] [PubMed] [Google Scholar]
- Teoh K. H., Mickle D. A., Weisel R. D., Li R. K., Tumiati L. C., Coles J. G., Williams W. G. Effect of oxygen tension and cardiovascular operations on the myocardial antioxidant enzyme activities in patients with tetralogy of Fallot and aorta-coronary bypass. J Thorac Cardiovasc Surg. 1992 Jul;104(1):159–164. [PubMed] [Google Scholar]
- Weyrich A. S., Ma X. L., Lefer A. M. The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation. 1992 Jul;86(1):279–288. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- del Nido P. J., Mickle D. A., Wilson G. J., Benson L. N., Weisel R. D., Coles J. G., Trusler G. A., Williams W. G. Inadequate myocardial protection with cold cardioplegic arrest during repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 1988 Feb;95(2):223–229. [PubMed] [Google Scholar]