Abstract
OBJECTIVE
To compare the diagnostic characteristics of tests used for a prompt diagnosis of chronic osteomyelitis in the diabetic foot, using bone histology as the criterion standard. The tests assessed were probe-to-bone (PTB), clinical signs of infection, radiography signs of osteomyelitis, and ulcer specimen culture.
RESEARCH DESIGN AND METHODS
A prospective study was performed on patients with foot ulcers referred to our diabetic foot clinic. Ulcer infection was diagnosed by recording clinical signs of infection and taking specimens for culture. The presumptive diagnosis of osteomyelitis was based on these results and the findings of a plain X-ray and PTB test. All patients with a clinical suspicion of bone infection were subjected to surgical treatment of the affected bone. During surgery, bone specimens were obtained for a histological diagnosis of osteomyelitis.
RESULTS
Over 2.5 years, 210 foot lesions were consecutively examined and 132 of these wounds with clinical suspicion of infection selected as the study sample. Of these, 105 (79.5%) lesions were diagnosed as osteomyelitis. Among the tests compared, the best results were yielded by the PTB test including an efficiency of 94%, sensitivity of 98%, specificity of 78%, positive predictive value of 95%, and negative predictive value of 91% (P < 0.001, κ 0.803); the positive likelihood ratio was 4.41, and the negative likelihood ratio was 0.02 (95% CI).
CONCLUSIONS
In our outpatient population with a high prevalence of osteomyelitis, the PTB test was of greatest diagnostic value, especially for neuropathic ulcers, and proved to be efficient for detecting osteomyelitis in the diabetic foot.
In Spain, amputations due to osteomyelitis are performed each year in 46.1 of every 100,000 individuals with diabetes (1). In diabetic patients, approximately two-thirds of all amputations are preceded by the infection of a foot ulcer (2–4). However, the diagnosis of chronic osteomyelitis in the diabetic foot continues to be a challenge, and we believe that there is a need for more studies validating the different diagnostic methods available. There is also a clear need for a safe, rapid, and efficient diagnostic protocol designed to optimize the therapeutic approach and improve the prognosis in these patients.
The early suspicion of osteomyelitis is essentially clinical and is based on detection of the presence of signs and symptoms of infection, although many patients have no typical local signs (4). Even in the absence of clinical signs, most infected chronic ulcers of the diabetic foot have underlying osteomyelitis. A lack of clinical signs can lead to a delay in diagnosing the initial stages of infection (5).
The plain X-ray is not useful for detecting bone infection in the first 2 weeks. Bone abnormalities can generally not be seen until bone mineral density drops to 35–50% of that of normal adjacent bone (6,7). Moreover, subtle changes at the onset of osteomyelitis are not easily distinguished from other changes that occur in the feet of these patients due to their neuropathy or peripheral vascular disease (8).
The probe-to-bone (PTB) test is routinely performed to detect, using a blunt instrument, palpable bone through the ulcer, indicating osteomyelitis (9). However, this test has been validated only in a few previous studies, and there is currently no consensus on a standardized protocol for the clinical diagnosis of osteomyelitis (10). The present study provides data on the validity of the clinical tests most frequently used to detect this disease.
RESEARCH DESIGN AND METHODS
This observational, descriptive study with prospective collection of data was conducted in patients with type 1 or 2 diabetes who attended the Diabetic Foot Clinic of the University Podology Clinic, Universidad Complutense de Madrid (Spain) because of a foot ulcer.
Over the period May 2006 to November 2008, we treated 210 foot lesions in diabetic patients. Of these lesions, 132 with clinical suspicion of infection were selected as the study sample, of which 105 (79.5%) were finally diagnosed as osteomyelitis. Infection was recorded according to the presence of clinical signs and symptoms and a positive soft tissue culture result. Once infection of the ulcer had been established, presumptive osteomyelitis was diagnosed by plain radiography and a clinical examination.
Patients were enrolled if they had a single ulcer of neuropathic or neuroischemic etiology below the ankle, there was suspicion of bone infection according to clinical signs and symptoms and the diagnostic tests standardized in the protocol used at our center (ulcer specimen culture, radiography, and PTB), if they had undergone surgery for acute osteomyelitis, or if after adequate local or antibiotic treatment and rest, the ulcer did not resolve. Patients were excluded if they had critical ischemia according to the classification of Fontaine et al. (11) or were due for an operation that was unrelated to a diagnosis of osteomyelitis. The study protocol was approved by our institutional review board.
A neuropathic ulcer was defined as a full-thickness skin defect produced, in the absence of ischemia, as the consequence of loss of protective sensation or of a deformity due to a motor neuropathy, sometimes aggravated by autonomic alterations. Callus is observed covering the lesion or at its margins, and these ulcers usually bleed easily. Neuropathic ulcers often appear at points of sustained low pressure or shear over a bony prominence.
A neuroischemic ulcer is a full-thickness skin wound whose underlying cause is both peripheral neuropathy and peripheral arterial disease. Despite ischemia, symptoms may be absent. The wound base is ulcerous, sphacelated, or necrotic. Bleeding is absent or only slight. Neuroischemic ulcers often appear in the dorsal and lateral zones of the foot as the consequence of a small traumatic injury or shear.
Neuropathy was diagnosed by examining 10 sites on the foot using a Semmes-Weinstein monofilament 5.07 10 g (Sensifil-Novalab Ibérica, Madrid, Spain) and a Horwell 997 neurotensiometer (Sensifil-Novalab Ibérica) (12,13). Vascular involvement was defined as an ankle-arm index <0.8 (14), transcutaneous oxygen tension (TcPo2) (using a TCM4 transcutaneous monitor; Radiometer Medical, Brønshøj, Denmark) <30 mmHg (15), and lack of a dorsal pedal and posterior tibial pulse (14).
Wound infection was clinically defined according to the criteria of the International Working Group on the Diabetic Foot (IWGDF) (16) as the presence of two or more signs and symptoms of local inflammation or systemic signs of infection of no other apparent cause, along with a purulent exudate. In addition, we also looked for specific signs such as necrosis, delayed wound healing, foul odor, and bone exposure.
Soft tissue specimens for culture were obtained after brief cleaning of the ulcer surface with saline and sterile gauze. Samples of exudate were obtained by rubbing the surface with a sterile cotton swab, and deep tissue samples were obtained using a no. 10 or 15 scalpel blade (CE 0086; Swann-Morton, Sheffield, U.K.). Specimens were transferred to a sterile vessel containing transport medium (CE 0344; Copan Innovation, Brescia, Italy) and submitted to the microbiology laboratory for culture.
We assessed the ulcers according to the classification schemes of Wagner and Texas (16,17) to record the extension and depth of all soft tissue lesions and detect any evidence of bone infection. In addition, a PTB test was performed in all patients using a blunt, sterile, metal surgical instrument to gently explore the ulcer. The test result was scored positive when a hard substance assumed to be bone was palpated accompanied or not by deep sinus tracts. The PTB was always conducted by the same experienced podiatrist.
All of the patients with diabetic ulcers underwent plain radiography to obtain dorsoplantar, lateral, and oblique views of both feet for assessment of possible bone alterations produced by the lesion. Osteomyelitis was suspected when one or more of the following radiographic signs were observed: periosteal elevation, cortical disruption, medullary involvement, osteolysis, and sequestra (segments of necrotic bone separated from living bone by granulation tissue).
Finally, based on the results of the clinical examination, soft tissue culture, PTB, and plain X-ray, patients with a diagnostic suspicion of osteomyelitis were subjected to a bone tissue biopsy obtained by conservative surgery. During surgery, we first removed all nonviable infected soft tissue and then excised all of the affected bone, obtaining a representative bone sample for subsequent histopathological analysis. The bone biopsy specimens were introduced in a sterile recipient containing 10% buffered formalin solution and transported to the pathological anatomy laboratory within 48 h, where they were immediately processed and examined. The histological criteria considered diagnostic of osteomyelitis were inflammatory cell infiltrate mostly composed of lymphocyte cells, plasma cells, neutrophils within spongy and cortical bone; bone necrosis; and reactive bone neoformation possibly accompanied by prominent periosteal bone proliferation (18). We used the results of the bone biopsy to confirm the diagnosis of osteomyelitis.
RESULTS
The prevalence of osteomyelitis in the 132 patients with clinical suspicion of infection included in this study was 79.5% (105 patients). Each of these patients had a single ulcer. Of the ulcers, 59% were classified as neuropathic and 41% were classified as neuroischemic. The mean ± SD duration of diabetes was 15.6 ± 9.5 years, blood glucose was 161.4 ± 60.3 mg/dl, and A1C was 7.9 ± 1.9%. The following complications of diabetes were recorded: diabetic retinopathy in 68 patients (51.5%), diabetic nephropathy in 27 patients (20.5%), hypertension in 94 patients (71.2%), stroke in 62 patients (47%), cardiovascular problems in 56 patients (42.4%), prior ulcers in 65 patients (49.2%), and prior conservative lower extremity amputation in 48 patients (36.4%).
The etiopathogenic characteristics of the ulcers were lack of pedal pulses in 33 patients (25%), a positive monofilament test in 100 patients (75.8%), and vibration sensitivity in 121 patients (91.7%); the ankle-arm index was 0.91 ± 0.3 and TcPo2 was 34.2 ± 14 mmHg. In 124 patients (93.9%), the ulcer was classified as Wagner grade III, in 5.3% as grade II, and in 0.8% as grade IV. According to Texas classification, the ulcers in 73 patients (55.3%) were type IIIA and in 41 patients (31.1%) they were type IIIB; types IA and IIIC were less frequently recorded, and each appeared in only 1 patient (0.8%).
In 75 patients (56.8%), the exudate from the ulcer was serous, in 32 patients (24.2%), there was no exudate, in 18 patients (13.6%), the exudate was purulent, in 5 patients (3.8%), it was sanguinous, and in 2 patients (1.5%) it was serosanguinous. In 98.2% of the patients, the ulcers appeared on the forefoot. Most of these ulcers appeared on the middle toes (second, third, and fourth) (28.7%) or the underside of the second, third, and fourth metatarsals (27.2%). The least frequent locations were over the scaphoid (0.9%) and cuboid (0.9%) bones.
The mean time of ulcer duration was 44 weeks, the median being 16 weeks (range 1–74 weeks). When we examined ulcer duration according to the results of the different diagnostic methods, in patients in whom osteomyelitis was biopsy proven, ulcer duration was 41.67 ± 75.52 weeks, and in those with a negative biopsy result, it was 52.19 ± 104.32 weeks (P = 0.554). Corresponding ulcer durations in weeks recorded for a positive versus a negative test result, respectively, were PTB 41.15 ± 74.19 vs. 56.48 ± 112.74 (P = 0.417), ulcer specimen culture 45.83 ± 85.14 vs. 33.19 ± 62.55 (P = 0.519), radiography signs 42.08 ± 72.97 vs. 55.59 ± 129.61 (P = 0.528), and clinical signs 44.26 ± 89.51 vs. 42.83 ± 62.99 (P = 0.926).
Table 1 shows the sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios obtained for the four tests. Test efficiencies (percentage of patients correctly diagnosed as positive or negative) were PTB 93.89% (88–99.1%), ulcer specimen culture 71.97% (63.4–79.7%), radiographic signs 75.76% (67.4–82.6%), and clinical signs 59.09% (50.2–67.4%). To try to improve the capacity for diagnosing osteomyelitis, we assessed the use of two of the clinical diagnostic methods compared with bone histology and found that only when the PTB test was one of the two methods was the result significant. Thus, the pair of methods, clinical signs plus PTB, showed a sensitivity of 64.8%, specificity of 77.8%, positive predictive value (PPV) of 91.9%, and negative predictive value (NPV) of 36.2% (P < 0.001, κ 0.298); for radiography signs plus PTB the values recorded were sensitivity 88.6%, specificity 66.7%, PPV 91.2%, and NPV 60% (P < 0.001, κ 0.530); and for culture plus PTB they were sensitivity 84.8%, specificity 77.8%, PPV 93.8%, and NPV 56.8% (P < 0.001, κ 0.550).
Table 1.
Validity indicators of the clinical diagnostic tests used to diagnose chronic osteomyelitis in diabetic foot ulcers
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | κ | |
|---|---|---|---|---|---|---|---|
| Infection signs* | 68 (67.9–5.7) | 26 (7.7–2.6) | 78.02 (57.7–6.2) | 17.07 (11.9–46.6) | 0.91 (0.79–1.12) | 1.25 (0.59–2.80) | −0.054 (P = 0.518) |
| Radiography signs* | 89.52 (81.6–4.4) | 22.22 (9.4–42.7) | 81.74 (73.2–88.1) | 35.29 (15.3–61.4) | 1.15 (0.93–1.42) | 0.47 (0.19–1.16) | 0.136 (P = 0.104) |
| Ulcer culture | 85.71 (77.2–91.5) | 18.52 (7–38.7) | 80.36 (71.7–87) | 25 (9.6–49.4) | 1.05 (0.86–1.28) | 0.77 (0.31–1.93) | 0.036 (P = 0.678) |
| PTB test | 98.10 (92.6–99.7) | 77.78 (57.3–90.6) | 94.50 (87.9–97.7) | 91.30 (70.5–98.5) | 4.45 (2.18–8.94) | 0.02 (0.01–0.10) | 0.803 (P < 0.001) |
Data in the first four columns are % (95% CI).
*See text for details of the infection and radiographic signs considered. PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Likewise, when we assessed the combined use of three or four of the methods, a significant result was only obtained when the PTB test was included. Thus, the methods clinical signs plus radiography signs plus PTB showed a sensitivity of 60.9%, specificity of 85.2, PPV of 94.1%, and NPV of 35.9% (P < 0.001, κ 0.306); for clinical signs plus culture plus PTB, the values recorded were sensitivity 61%, specificity 85.1%, PPV 94.1%, and NPV 35.8% (P < 0.001, κ 0.300), and for clinical signs plus radiography signs plus culture plus PTB, they were sensitivity 52.4%, specificity 85.2%, PPV 93.2%, and NPV 31.5% (P < 0.001, κ 0.230).
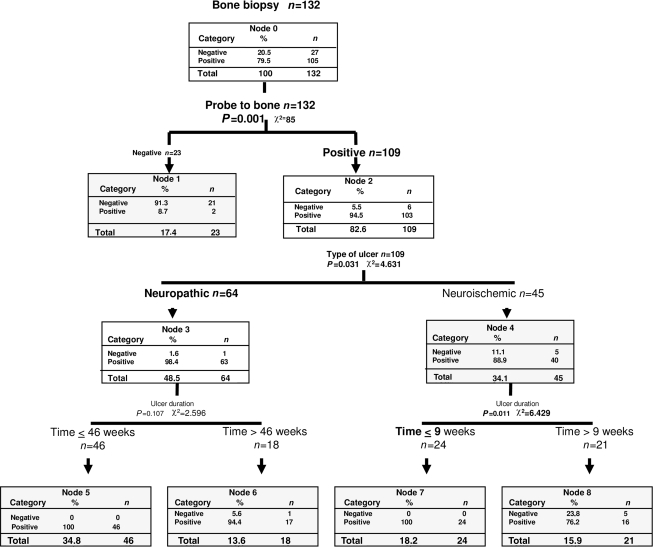
Finally, a multivariate analysis was performed to see whether we could improve the diagnosis of osteomyelitis of the 132 ulcers including the six variables: clinical signs of infection, radiographic signs of osteomyelitis, ulcer specimen culture, PTB test, and ulcer type (neuroischemic/neuropathic) and duration. The results of this analysis are presented as a decision tree (Fig. 1).
Figure 1.
The decision tree displays the results of a probabilistic analysis in a way that helps the clinician choose between the different diagnostic tests. The variables entered in the analysis were bone histology as the dependent variable and clinical signs of infection, ulcer culture, X-ray, PTB test, neuropathic/neuroischemic ulcer, and ulcer duration as independent variables. The tests or conditions providing a significant result in relation to another test or condition appear in bold.
The test revealed by this analysis as most accurate was the PTB with a PPV of 94.5%. According to the decision tree, a correct diagnosis was made in 98.4% of the neuropathic ulcers vs. 88% of the neuroischemic ulcers. The correlation between ulcer duration and risk of having osteomyelitis was only significant for the neuroischemic ulcers such that 100% of ulcers <9 weeks old were positive for osteomyelitis.
CONCLUSIONS
In this study we sought to provide data on the validity of the tests used in current clinical practice to diagnose chronic osteomyelitis in diabetic foot ulcers. There is still much controversy regarding the best way to detect bone infection in patients with diabetes, and there is also confusion about which is the most efficient treatment (19). Although most researchers consider that the histopathological study of bone specimens is the criterion standard for diagnosing osteomyelitis (20), this method is not systematically used because clinicians feel that surgically obtaining bone tissue is an aggressive procedure and puts patients at risk. Moreover, qualified medical staff are needed to undertake the surgical procedure.
Few studies have validated the use of clinical signs as a diagnostic tool for infection of the diabetic foot. Cutting and White (21) and Gardner et al. (22) performed a series of studies on chronic foot ulcers, but their patients had different systemic diseases including diabetes. Our findings indicate that assessment of clinical signs of infection in diabetic patients, although valid, provides limited information for a prompt diagnosis of osteomyelitis. There is an obvious need for a more detailed and precise definition of clinical indicators in chronic diabetic ulcers.
Our results indicate that the plain radiograph was a sensitive test because it was able to identify 89% of the unaffected patients, yet it was poorly specific, as 32 ulcers were incorrectly diagnosed. In addition, there was poor agreement between the results of this test and those of bone histology (κ 0.136, P = 0.104). These observations suggest that despite being an inexpensive and quick test, it is of little use for the prompt diagnosis of osteomyelitis in the diabetic foot. For greater diagnostic efficiency, the method would have to be combined with other imaging techniques. Prior studies have reported similar results but in very small populations such that their reliability is low (7,8).
The detection of an infected ulcer prompts the suspicion of a contiguous infection in the underlying bone. The culture of specimens from an ulcer is a valid test, but the low specificity observed here limits its use for the timely diagnosis of osteomyelitis even when combined with other tests. Microbiological cultures often induce diagnostic errors because the sample is not properly obtained or because the patient is receiving antibiotic treatment (23). In addition, the performance of microbiological culture depends on the quality of the sample and on an adequate transport medium. A further drawback of the culture method is that results are not available for 3 or 4 days, and this delay in confirming the diagnosis could lead to progression to osteomyelitis or, in more serious cases, to the need for amputation.
Since its introduction, the PTB test has been extensively used to evaluate diabetic patients with foot ulcers. Despite this, to date only three studies have validated the use of this technique for the early diagnosis of osteomyelitis in the diabetic foot (9,20,24). The different results obtained by Grayson et al. (9) and Shone et al. (24) may be attributed to the different pretest probabilities of osteomyelitis because their study populations differed and also to the fact that not all patients underwent the same test to confirm the diagnosis of osteomyelitis, making it difficult to directly compare the findings of the studies. Lavery et al. (20) conducted a microbiology study of bone specimens in each of their patients, and these authors admitted that one of the limitations of their study was that osteomyelitis was not confirmed by bone histology. Our study differs from this last study in that it was performed in the same foot clinic by the same podiatrists. The inter-rater reliability of the PTB test is also a factor to consider as there are no published data addressing this issue. The diagnostic characteristics of the PTB test reported by these authors and the values obtained here are compared in Table 2.
Table 2.
Comparing the performance indicators of the PTB test validated in the different studies including the present study
| Study | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | NLR |
|---|---|---|---|---|---|---|
| Grayson et al., 1995 (9) | 66 | 85 | 89 | 56 | 4.40* | 0.15* |
| Shone et al., 2006 (24) | 38 | 91 | 53 | 85 | 4.22* | 0.68* |
| Lavery et al., 2007 (20) | 87 | 87 | 62 | 92 | 6.59 | 0.14* |
| Present study (2010) | 98 | 78 | 95 | 91 | 4.45 | 0.02 |
*Calculated from the data provided by the authors. PLR, positive likelihood ratio; NLR, negative likelihood ratio.
According to some authors, the time of ulcer duration is directly related to the risk of osteomyelitis (25). However, we were unable to correlate the presence of infection with ulcer duration (P = 0.554). The number of patients in whom bone histology proved positive was 105 (79.5%), and mean ulcer duration was 42 weeks, compared with a mean of 52 weeks for a negative biopsy results. We attribute this apparent discrepancy to the wide dispersion of data, as ulcer duration ranged from 1 to 75 weeks.
In our study population, the prevalence, or pretest probability, of osteomyelitis was high at 79.5% because most patients examined at our clinic are referred from other health centers such that most have a long duration ulcer. This figure cannot be translated to general infections of the diabetic foot. Moreover, the prevalence is probably higher in an outpatient than in a hospital setting such that it is difficult to make comparisons. A further factor to consider is that almost half of our patients (49.2%) had had prior ulcers, and many had undergone prior conservative foot amputations (36.4%). These are considered risk factors for reulceration and osteomyelitis. Although we are aware that not all podiatrists direct a patient with chronic osteomyelitis to surgery, the philosophical basis to which we subscribe is early surgical treatment, prioritizing preservation so as not to alter the foot's biomechanics and avoiding major amputations.
Of all the tests examined here, the PTB test was the best at predicting the biopsy results and was especially efficient for neuropathic ulcers (98.4% correctly diagnosed vs. 88% for neuroischemic ulcers). This finding may be clearly seen in the decision tree (Fig. 1).
One of the limitations of our study was that we did not perform bone culture as well as bone histology to confirm the diagnosis of osteomyelitis. This is because specimens for culture could not always be obtained since the bone excised during surgery for histopathological analysis was insufficient in size for any further tests. However, as for the soft tissue cultures, the bone culture procedure has the drawback that false-negative results, especially in patients receiving antibiotic therapy or false-positive results (due to contamination), are sometimes produced. The shortcoming of using histological changes as the diagnostic standard for osteomyelitis is that these changes are still rather poorly defined.
We observed good agreement between the palpation of bone in the PTB test and the presence of osteomyelitis. Moreover, the early diagnostic performance of the PTB was much improved over that offered by clinical signs of infection, ulcer specimen culture, and a plain radiograph. Despite an obvious need for further validations, as part of our protocol and in our outpatient setting, we found the results of the PTB test to be valid and reliable. Moreover, its easy use, low cost, and high efficiency make it a good complementary test at any level of care for a timely diagnosis of osteomyelitis in the chronic ulcers that appear on the feet of diabetic patients.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
R.M.L. conducted the study, researched data, and wrote the manuscript. M.L.G.F. reviewed/edited the manuscript. D.M.H. researched data and contributed to discussion. S.G.J. researched data and reviewed/edited the manuscript. M.A.G.J. researched data. J.V.B.M. researched data and reviewed/edited the manuscript.
We thank the staff of the Diabetic Foot Clinic of the University Podology Clinic, Universidad Complutense de Madrid (Spain) for their cooperation. In addition, we thank Ana Burton (freelance science editor and translator) for translating the original manuscript and help with preparing the revised version.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ruiz-Ramos M, Escolar-Pujolar A, Mayoral-Sanchez E, Corral-San LF, Fernandez-Fernandez I: Mellitus diabetes in Spain: death rates, prevalence, impact, costs and inequalities. Gac Sanit 2006;20(Suppl. 1):15–24 [DOI] [PubMed] [Google Scholar]
- 2.Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA: Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288–1293 [DOI] [PubMed] [Google Scholar]
- 3.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, Landsman AS, Lavery LA, Moore JC, Schuberth JM, Wukich DK, Andersen C, Vanore JV: Diabetic foot disorders. A clinical practice guideline (2006 revision). J Foot Ankle Surg 2006;45:S1–66 [DOI] [PubMed] [Google Scholar]
- 4.Matthews PC, Berendt AR, Lipsky BA: Clinical management of diabetic foot infection: diagnostics, therapeutics and the future. Expert Rev Anti Infect Ther 2007;5:117–127 [DOI] [PubMed] [Google Scholar]
- 5.Edmonds M, Foster A:: The use of antibiotics in the diabetic foot. Am J Surg 2004;187:25S–28S [DOI] [PubMed] [Google Scholar]
- 6.Capriotti G, Chianelli M, Signore A: Nuclear medicine imaging of diabetic foot infection: results of meta-analysis. Nucl Med Commun 2006;27:757–764 [DOI] [PubMed] [Google Scholar]
- 7.Enderle MD, Coerper S, Schweizer HP, Kopp AE, Thelen MH, Meisner C, Pressler H, Becker HD, Claussen C, Häring HU, Luft D: Correlation of imaging techniques to histopathology in patients with diabetic foot syndrome and clinical suspicion of chronic osteomyelitis. The role of high-resolution ultrasound. Diabetes Care 1999;22:294–299 [DOI] [PubMed] [Google Scholar]
- 8.Yuh WT, Corson JD, Baraniewski HM, Rezai K, Shamma AR, Kathol MH, Sato Y, el-Khoury GY, Hawes DR, Platz CE: Osteomyelitis of the foot in diabetic patients: evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am J Roentgenol 1989;152:795–800 [DOI] [PubMed] [Google Scholar]
- 9.Grayson ML, Gibbons GW, Balogh K, Levin E, Karchmer AW: Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995;273:721–723 [PubMed] [Google Scholar]
- 10.Berendt AR, Peters EJ, Bakker K, Embil JM, Eneroth M, Hinchliffe RJ, Jeffcoate WJ, Lipsky BA, Senneville E, Teh J, Valk GD: Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes Metab Res Rev 2008;24(Suppl. 1):S145–S161 [DOI] [PubMed] [Google Scholar]
- 11.Fontaine R, Kim M, Kieny R: Surgical treatment of peripheral circulation disorders. Helv Chir Acta 1954;21:499–533 [PubMed] [Google Scholar]
- 12.Feng Y, Schlösser FJ, Sumpio BE: The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;50:675–682, 682.e1 [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Kim H, Choi S, Park Y, Kim Y, Cho B: Clinical usefulness of the two-site Semmes-Weinstein monofilament test for detecting diabetic peripheral neuropathy. J Korean Med Sci 2003;18:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Sufit R, Nishida T, Guralnik JM, Ferrucci L, Tian L, Liu K, Tan J, Pearce WH, Schneider JR, Sharma L, Criqui MH: Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med 2006;166:1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalani M, Brismar K, Fagrell B, Ostergren J, Jörneskog G: Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999;22:147–151 [DOI] [PubMed] [Google Scholar]
- 16.Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC: International consensus and practical guidelines on the management and the prevention of the diabetic foot. International Working Group on the Diabetic Foot Diabetes Metab Res Rev 2000;16(Suppl. 1):S84–S92 [DOI] [PubMed] [Google Scholar]
- 17.Armstrong DG, Lavery LA, Harkless LB: Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–859 [DOI] [PubMed] [Google Scholar]
- 18.Rosai J: Rosai and Ackerman's Surgical Pathology. 9th ed St. Louis, Mosby, 2004 [Google Scholar]
- 19.Jeffcoate WJ, Lipsky BA: Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis 2004;39(Suppl. 2):S115–S122 [DOI] [PubMed] [Google Scholar]
- 20.Lavery LA, Armstrong DG, Peters EJ, Lipsky BA: Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care 2007;30:270–274 [DOI] [PubMed] [Google Scholar]
- 21.Cutting KF, White RJ: Criteria for identifying wound infection—revisited. Ostomy Wound Manage 2005;51:28–34 [PubMed] [Google Scholar]
- 22.Gardner SE, Frantz RA, Park H, Scherubel M: The inter-rater reliability of the Clinical Signs and Symptoms Checklist in diabetic foot ulcers. Ostomy Wound Manage 2007;53:46–51 [PubMed] [Google Scholar]
- 23.Andersen CA, Roukis TS:: The diabetic foot. Surg Clin North Am 2007;87:1149–1177 [DOI] [PubMed] [Google Scholar]
- 24.Shone A, Burnside J, Chipchase S, Game F, Jeffcoate W: Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care 2006;29:945. [DOI] [PubMed] [Google Scholar]
- 25.Giurato L, Uccioli L: The diabetic foot: Charcot joint and osteomyelitis. Nucl Med Commun 2006;27:745–749 [DOI] [PubMed] [Google Scholar]