Abstract
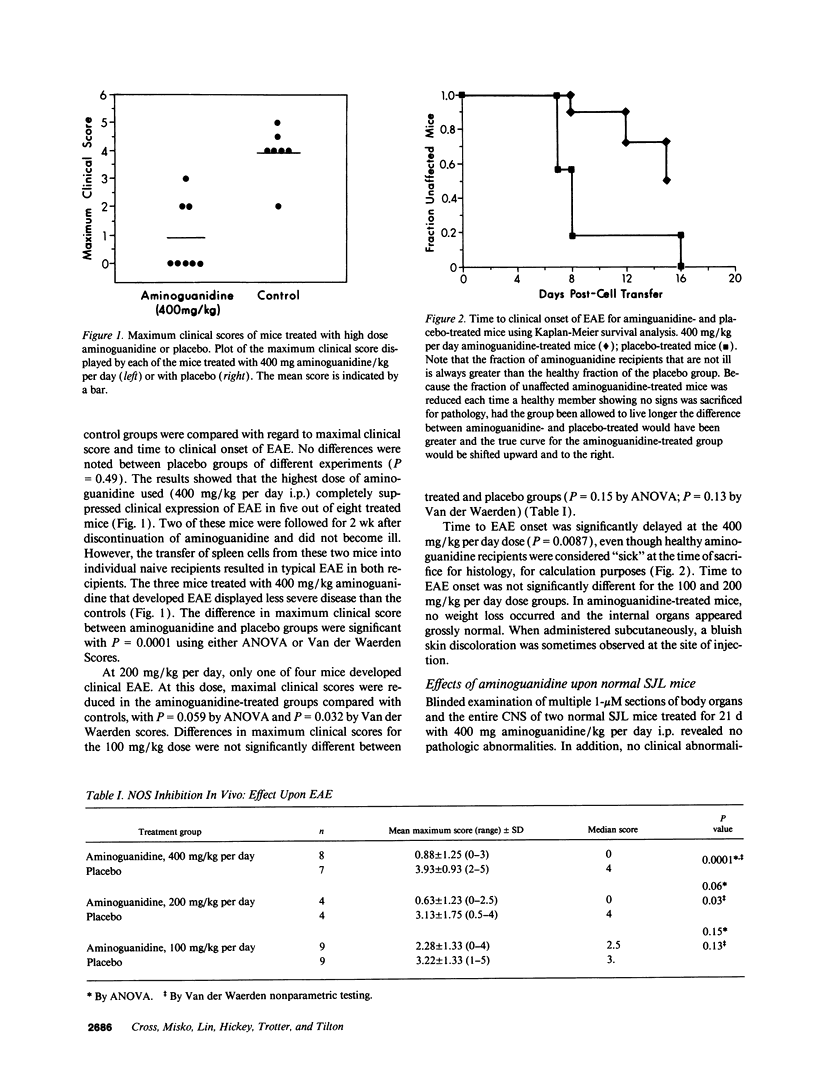
Previous work from our laboratory localized nitric oxide to the affected spinal cords of mice with experimental autoimmune encephalomyelitis, a prime model for the human disease multiple sclerosis. The present study shows that activated lymphocytes sensitized to the central nervous system encephalitogen, myelin basic protein, can induce nitric oxide production by a murine macrophage cell line. Induction was inhibited by amino-guanidine, a preferential inhibitor of the inducible nitric oxide synthase isoform, and by NG-monomethyl-L-arginine. Aminoguanidine, when administered to mice sensitized to develop experimental autoimmune encephalomyelitis, inhibited disease expression in a dose-related manner. At 400 mg aminoguanidine/kg per day, disease onset was delayed and the mean maximum clinical score was 0.9 +/- 1.2 in aminoguanidine versus 3.9 +/- 0.9 in placebo-treated mice. Histologic scoring of the spinal cords for inflammation, demyelination, and axonal necrosis revealed significantly less pathology in the aminoguanidine-treated group. The present study implicates excessive nitric oxide production in the pathogenesis of murine inflammatory central nervous system demyelination, and perhaps in the human disease multiple sclerosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Vlassara H., Kooney A., Ulrich P., Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986 Jun 27;232(4758):1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Cross A. H., McCarron R., McFarlin D. E., Raine C. S. Adoptively transferred acute and chronic relapsing autoimmune encephalomyelitis in the PL/J mouse and observations on altered pathology by intercurrent virus infection. Lab Invest. 1987 Nov;57(5):499–512. [PubMed] [Google Scholar]
- Cross A. H., Raine C. S. Serial adoptive transfer of murine experimental allergic encephalomyelitis: successful transfer is dependent on active disease in the donor. J Neuroimmunol. 1990 Jun;28(1):27–37. doi: 10.1016/0165-5728(90)90038-o. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Experimental allergic encephalomyelitis, a useful model for multiple sclerosis. A satellite conference of the International Society of Neurochemists. Seattle, Washington, July 16-19, 1983. Prog Clin Biol Res. 1984;146:1–554. [PubMed] [Google Scholar]
- Galea E., Feinstein D. L., Reis D. J. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. L., Bhan A. K., Gilles F., Kemp M., Kerr C., Weiner H. L. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986 Jun;19(6):578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- Henry Y., Ducrocq C., Drapier J. C., Servent D., Pellat C., Guissani A. Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur Biophys J. 1991;20(1):1–15. doi: 10.1007/BF00183275. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Zheng Y. M., Heber-Katz E., Fraser N., Rorke L., Fu Z. F., Hanlon C., Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari K., Umar S., Bansal V., Sahib M. K. Monoaminoguanidine inhibits aldose reductase. Biochem Pharmacol. 1991 May 15;41(10):1527–1528. doi: 10.1016/0006-2952(91)90571-l. [DOI] [PubMed] [Google Scholar]
- LINDELL S. E., NILSSON K., ROOS B. E., WESTLING H. The effect of enzyme inhibitors on histamine catabolism in man. Br J Pharmacol Chemother. 1960 Jun;15:351–355. doi: 10.1111/j.1476-5381.1960.tb01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Lee S. C., Dickson D. W., Liu W., Brosnan C. F. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993 Jul;46(1-2):19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Lin R. F., Lin T. S., Tilton R. G., Cross A. H. Nitric oxide localized to spinal cords of mice with experimental allergic encephalomyelitis: an electron paramagnetic resonance study. J Exp Med. 1993 Aug 1;178(2):643–648. doi: 10.1084/jem.178.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons C. R., Orloff G. J., Cunningham J. M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992 Mar 25;267(9):6370–6374. [PubMed] [Google Scholar]
- MacMicking J. D., Willenborg D. O., Weidemann M. J., Rockett K. A., Cowden W. B. Elevated secretion of reactive nitrogen and oxygen intermediates by inflammatory leukocytes in hyperacute experimental autoimmune encephalomyelitis: enhancement by the soluble products of encephalitogenic T cells. J Exp Med. 1992 Jul 1;176(1):303–307. doi: 10.1084/jem.176.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Ignarro L. J., Sherman M. P., Melinek J., Lane T. E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993 Aug 15;151(4):2132–2141. [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Schilling R. J., Salvemini D., Moore W. M., Currie M. G. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993 Oct;214(1):11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Traugott U., Farooq M., Norton W. T., Raine C. S. Experimental autoimmune encephalomyelitis. Augmentation of demyelination by different myelin lipids. Lab Invest. 1984 Oct;51(4):416–424. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard S., Parthasarathy S., Fruebis J., Witztum J. L. Aminoguanidine inhibits oxidative modification of low density lipoprotein protein and the subsequent increase in uptake by macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6876–6880. doi: 10.1073/pnas.89.15.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prineas J. W., Raine C. S. Electron microscopy and immunoperoxidase studies of early multiple sclerosis lesions. Neurology. 1976 Jun;26(6 Pt 2):29–32. doi: 10.1212/wnl.26.6_part_2.29. [DOI] [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Raine C. S., Mokhtarian F., McFarlin D. E. Adoptively transferred chronic relapsing experimental autoimmune encephalomyelitis in the mouse. Neuropathologic analysis. Lab Invest. 1984 Nov;51(5):534–546. [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cross A. H. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991 Nov;30(5):694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Simmons M. L., Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992 Sep;59(3):897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Traugott U., Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Ann Neurol. 1988 Aug;24(2):243–251. doi: 10.1002/ana.410240211. [DOI] [PubMed] [Google Scholar]
- Traugott U., McFarlin D. E., Raine C. S. Immunopathology of the lesion in chronic relapsing experimental autoimmune encephalomyelitis in the mouse. Cell Immunol. 1986 May;99(2):395–410. doi: 10.1016/0008-8749(86)90248-0. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]




